Abstract
Background
Toxic renal effects of myoglobin following rhabdomyolysis can cause acute kidney injury (AKI) with the necessity of kidney replacement therapy (KRT). Fast elimination of myoglobin seems notable to save kidney function and intensify kidney repair. Clinical data regarding efficacy of KRT in critical care patients with rhabdomyolysis and AKI are limited. This retrospective analysis aimed to identify differences between conservative therapy and different modalities of KRT regarding myoglobin elimination and clinical outcome.
Methods
This systematic, retrospective, single-center study analyzed 328 critical care patients with rhabdomyolysis (myoglobin > 1000 µg/l). Median reduction rate of myoglobin after starting KRT was calculated and compared for different modalities. Multivariate logistic regression models were established to identify potential confounder on hospital mortality. Filter lifetime of the various extracorporeal circuits was analyzed by Kaplan–Meier curves.
Results
From 328 included patients 171 required KRT. Health condition at admission of this group was more critical compared to patient with conservative therapy. Myoglobin reduction rate did not differ between the groups (KRT 49% [30.8%; 72.2%] vs. conservative treatment (CT) 61% [38.5%; 73.5%]; p = 0.082). Comparison between various extracorporeal procedures concerning mortality showed no significant differences. Hospital mortality was 55.6% among patients with KRT and 18.5% with CT (p < 0.001). Multivariate logistic regression model identified requirement for KRT (OR: 2.163; CI: 1.061–4.407); p = 0.034) and the SOFA Score (OR: 1.111; CI: 1.004–1.228; p = 0.041) as independent predictive factors for hospital mortality. When comparing specific KRT using multivariate regression, no benefit was demonstrated for any treatment modality. Life span of the extracorporeal circuit was shorter with CVVH compared to that of others (log-Rank p = 0.017).
Conclusions
This study emphasizes that AKI requiring KRT following rhabdomyolysis is accompanied by high mortality rate. Differences in myoglobin reduction rate between various KRTs could not be confirmed, but CVVH was associated with reduced filter lifetime compared to other KRTs, which enable myoglobin elimination, too.
Graphical Abstract

Similar content being viewed by others
Background
Acute kidney injury (AKI) is one of the leading organ dysfunctions in critically ill patients and is associated with high mortality and morbidity rates [1,2,3], high economic costs to health care systems [4], and increases the risk for chronic kidney disease (CKD) [5]. Different etiologies are involved in the development of AKI in most critically ill patients, whereby the frequent reasons are septic or cardiogenic shock, post-major surgery, hypovolemia or drug induced toxicity [3]. Rhabdomyolysis is considered as the potential cause of approximately 5–25% [6, 7].
Damaged skeletal muscle releases muscle cell contents into the circulation, e.g. myoglobin and other molecules. Myoglobin is endocytosed and oxidized by tubular cells, resulting in radical oxygen species that alter DNA and proteins. It activates an inflammatory response in the kidney and mediates vasoconstriction, which perpetuates renal damage. Myoglobin is also filtered by the glomerulus and precipitates in the renal tubules, particularly in combination with the Tamm–Horsfall proteins, forming tubular casts, which consequently result in acute tubular obstruction [6, 8].
Fast elimination of myoglobin seems essential due to its direct toxic renal effects [8]. However, clinical data regarding the efficacy of different kidney replacement therapy (KRT) modalities are limited. With normal diffusion-based hemodialysis it is usually not possible to effectively eliminate molecules with middle molecular weight, such as myoglobin (17 kDa). In critical care patients this is possible through convective transport such as continuous venovenous hemofiltration (CVVH) [9,10,11] or as well continuous veno-venous hemodiafiltration (CVVHDF) [12, 13].
Myoglobin can also be eliminated by diffusive transport by using high-cut-off filters (HCO) with a pore size larger than 0.01 μm in continuous venovenous hemodialysis (CVVHD-HCO) [12, 14,15,16,17]. The third way to eliminate myoglobin with KRT is to integrate an adsorber into the extracorporeal circuit [18,19,20,21].
Due to the paucity of clinical data that compare modalities of KRT despite the high prevalence of rhabdomyolysis induced AKI, we retrospectively analyzed patients with AKI and rhabdomyolysis admitted to our medical intensive care unit (ICU) from January 2016 to August 2020. Primary outcome measure was to identify influencing factors on the in-hospital mortality in patients with severe rhabdomyolysis. Secondary outcome measure was the evaluation of different dialysis modalities in consideration of their effectiveness.
Methods
Study design
This systematic retrospective, single-center study was approved by the local Institutional Review Board at the Medical Faculty of the University of Leipzig (312/20-ek).
To address the question whether reduction in serum myoglobin concentrations by extracorporeal circuit might influence patient outcome, we planned to compare the course of myoglobin reduction rate (MRR) between different treatment modalities. Furthermore, we aimed to investigate other possible effects on clinical and kidney outcome among these patients.
Patients
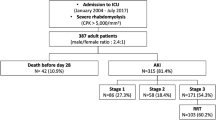
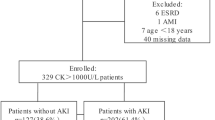
A total of 380 patients with the diagnosis of rhabdomyolysis were treated in our 28-bed medical ICU at the University Hospital Leipzig during the study period. After reviewing the medical records, we excluded 45 patients from further analysis because they did not experience severe rhabdomyolysis and seven because of preexisting dialysis dependency. Based on thresholds from published studies, rhabdomyolysis was defined as a serum myoglobin level > 1000 µg/l [22, 23]. Patients were enrolled to the study at the point of the first measured myoglobin level > 1000 µg/L following ICU admission. In the final analysis, 328 patients were considered for further evaluation (Fig. 1).
Study flow chart. *KRT during stay: KRT not at the beginning of the ICU stay, but later; CVVH continuous veno-venous hemofiltration, CVVHD-HCO continuous veno-venous hemodialysis with high cut-off filter, CVVHD-adsorber continuous veno-venous hemodialysis with “standard” high-flux filter and adsorber, CVVHDF continuous veno-venous hemodiafiltration, SLEDD sustained low efficiency daily dialysis
Firstly, we grouped these patients based on the management received to either KRT or conservative treatment group (CT). In a second step, we planned to describe the patients with KRT more precisely.
Procedures
Conservative therapy was the preferred therapeutic intervention, based on available recommendations from literature. Initially, targeted parenteral fluid therapy was carried out to compensate for a possible volume deficit and to strictly control the fluid balance [24]. Secondly, urine pH was controlled and if necessary alkalized by citrate-containing drugs three times a day. Success was monitored by urine output and urine pH with a target above 6.8 [25, 26]. Decision to start KRT and the choice of treatment modality were made by the attending intensivists according the current recommendations [27, 28].
During the observation period, patients with AKI and rhabdomyolysis received treatment with continuous kidney replacement therapy (CVVH, CVVHD; CVVHDF) with multiFiltrate® device and sustained low-efficiency daily dialysis (SLEDD; GENIUS®90) (both Fresenius Medical Care, Bad Homburg, Germany). A postdilution CVVH was applied with Ultraflux AV 600S® and CVVHDF with Ultraflux AV1000S® filter. In case of CVVHD-HCO, Ultraflux EMiC2® was applied (all Fresenius Medical Care, Bad Homburg, Germany). An adsorber (CytoSorb®, CytoSorbents Europe GmbH, Müggelseedamm 131, Berlin, Germany) was sequentially inserted into the circuit while using CVVHD with high-flux filter Ultraflux AV1000S®). SLEDD was performed with GENIUS® sleddFlux. All filters consisted of poly-sulfone with a pore size of approximately 30 kilodalton (kDa) for the high-flux filters and 45 kDa for the high cut-off filters. Anticoagulation of the extracorporeal circuit was maintained by regional citrate anticoagulation (RCA), whenever possible. Systemic anticoagulation was only performed in patients with other medical reasons justifying therapeutic anticoagulation, citrate intolerance or patients using CVVH. Hemofiltration requires a higher blood flow of the extracorporeal circuit than diffusion-based techniques due to the hemoconcentration at the filter. Necessity of higher citrate intake increases the risk of citrate accumulation in patients with severe organ dysfunction. For this reason, in our medical ICU, CVVH with RCA was not performed. Total turnover rate (TTR, dialysate and replacement fluid) in cases treated with continuous KRT was adjusted at about 25 ml/kg ideal or adjusted body weight/h [29]. SLEDD ran according to clinical requirements, mostly every 2 days.
Data collection
The following clinical characteristics and demographic data were retrieved from electronic health records of the patients at the time of ICU admission: age, sex, height, weight, BMI, kidney function and AKI stage, admission diagnosis, indication for KRT, acute physiology and chronic health evaluation II (APACHE II) and sequential organ failure assessment (SOFA) score, mean arterial blood pressure, need for mechanical ventilation, need for vasopressor, sepsis, pre-existing diseases and cause for rhabdomyolysis.
These data were collected daily during the observation period: creatinine, urea, albumin, creatine kinase (CK), myoglobin, interleukin-6 (IL-6), pH, bicarbonate, potassium, calcium, phosphate, lactate, platelet- and white blood cell count, urine output, urine pH. Duration of KRT and filter lifetime of the extracorporeal circuit were recorded.
Clinical follow-up data were monitored and retrieved from the last documented hospital or outpatient contact (length of ICU stay (days), kidney function and all-cause mortality at hospital discharge and, if available, at day 90).
Endpoints and calculations
For comparison of the different therapeutic interventions, we calculated MRR after starting therapeutic intervention or extracorporeal circuit, respectively.
In most cases, myoglobin was daily measured, so we calculated MRR between the first (Cmyoglobin T1) and the second morning (Cmyoglobin T2) after ICU admission.
Statistical analysis
Categorical variables are displayed as frequencies and percentages and tested by Chi-square (two-sided) test. Continuous variables are given as median with 25th and 75th quartile in square brackets based on test for normal distribution using the Shapiro–Wilk test. Normally distributed variables were analyzed by Student’s t-test and not normally distributed variables by Mann–Whitney U test.
The impact of different parameters on all-cause hospital mortality for the total cohort was tested by means of a logistic regression model. The following variables were included in this model: age, sex, height, weight, BMI and preexisting comorbidities (immunosuppressive therapy, CKD > stage 3a before admission, active malignancy, chronic heart failure, liver cirrhosis, chronic pulmonary disease) and the health condition at admission (AKI stage, need for KRT, non-invasive/invasive mechanical ventilation, vasopressors, sepsis/septic shock, MAP, heart rate, diuresis, urine pH, APACHE II/SOFA score and urea, creatinine, pH, HCO3−, potassium, lactate, IL-6, platelets, leukocytes, albumin, phosphate, ionized calcium, CK and myoglobin levels). This multivariate calculation had been only carried out in cases with complete data sets and for significant variables after univariate testing. Kaplan–Meier survival curves were used to depict hospital mortality and applied to the performance of extracorporeal circuits.
Additionally, the influence of the aforementioned variables and the different KRT modalities (CVVH, CVVHD-HCO, CVVHD-adsorber, CVVHDF, CVVHD/SLEDD) on hospital mortality was also tested for the KRT cohort using a logistic regression model. In this case, the specific KRT modality was tested in comparison to all other KRT modalities. Missing data and loss of follow up are addressed in detail.
Statistical analysis was performed using IBM SPSS, versions 27 and GraphPad Prism version 9. The significance level was defined as 5% for two-tailed tests.
Results
Patient characteristics
Demographic and clinical characteristics of the patients are given in Table 1. Patients on KRT were more seriously ill than those with no need for KRT illustrated by higher APACHE II and SOFA scores. Preexisting chronic heart failure (NYHA IV), preexisting immunosuppressive therapy, liver cirrhosis, chronic pulmonary disease or chronic kidney disease with eGFR below 45 ml/kg/1.72m2 did not differ between both groups. Nevertheless, active malignancy was associated with a higher percentage of need for KRT. Patients with KRT had more rhabdomyolysis triggered by shock or after cardio-pulmonary resuscitation. However, muscle hypoxia was slightly more causal in patients with CT, including crush syndrome and limb ischemia. Other triggers of rhabdomyolysis were similar.
From 328 patients with myoglobin levels of > 1000 ug/l 54 (16.5%) patients had no AKI, 155 (47.3%) mild-to-moderate AKI (AKI 1 and 2) and 119 (36.3%) severe AKI (AKI 3). KRT was performed in 171 (52.1%) patients. Indication to start KRT had been pH < 7.2 (46/171; 26.9%), bicarbonate level < 12 mmol/l (22/171; 12.9%), potassium level > 6 mmol/l (28/171; 16.4%) and pulmonary edema (14/171; 8.2%). The remaining patients (61/171; 35.7%) presented with multiple reasons for starting KRT, such as prolonged oligoanuria, uremic syndrome or others. The KRT modalities provided are shown in Fig. 1. Dialytic parameters like total turnover rate and blood flow are listed in the Suppl. Table 1. Kidney function at baseline was more impaired in the KRT group, illustrated by higher creatinine, higher potassium levels, lower HCO3− level, more acidic urine and less diuresis during the first 24 h after admission (Table 2).
Myoglobin reduction rate
Both groups showed decreasing myoglobin levels regarding MRR between the first morning after enrollment and after 24 h. For the calculation of MRR, data on myoglobin level with a 24-h period were available in 61.1% (KRT: 69% and CT: 53.5%) of the included cases (Suppl. Table 2). The MRR was 49% [30.8%; 72.2%] with KRT and 61% [38.5%; 73.5%] with CT and showed no difference between the two groups (p = 0.082) (Fig. 2A). Despite therapeutic interventions and a myoglobin reduction in the overall group, some patients showed a continued increase in myoglobin levels (KRT: 24/118 (20.3%); CT: 11/84 (13.1%)). The comparison between the various extracorporeal procedures did not demonstrate a significant advantage regarding myoglobin elimination (Table 3 and Fig. 2B).
Myoglobin reduction rate. A Myoglobin reduction rates for kidney replacement therapy versus conservative treatment. KRT kidney replacement therapy, CT conservative treatment; B Myoglobin reduction rates for the various kidney replacement modalities. CVVH continuous veno-venous hemofiltration, CVVHD-HCO continuous veno-venous hemodialysis with high cut-off filter, CVVHD-adsorber continuous veno-venous hemodialysis with “standard” high-flux filter and adsorber, CVVHDF continuous veno-venous hemodiafiltration, CVVHD continuous veno-venous hemodialysis, SLEDD sustained low efficiency daily dialysis; C Kaplan–Meier curve for hospital survival for kidney replacement therapy versus conservative treatment. KRT kidney replacement therapy, CT conservative treatment; D Kaplan–Meier curve for hospital survival with the various kidney replacement modalities. CVVH continuous veno-venous hemofiltration, CVVHD-HCO continuous veno-venous hemodialysis with high cut-off filter, CVVHD-adsorber continuous veno-venous hemodialysis with “standard” high-flux filter and adsorber, CVVHDF continuous veno-venous hemodiafiltration, CVVHD continuous veno-venous hemodialysis, SLEDD sustained low efficiency daily dialysis
Clinical outcome
The ICU length of stay was 7 [2; 17] days in the KRT group, while it was 3 [2; 6] days for the CT group (p < 0.001). All-cause hospital mortality was 55.6% (95/171) in the KRT group and 18.5% (29/157) in the CT group (p < 0.001). Kaplan–Meier analysis illustrates Fig. 2C. Patients who died during hospital stay had significantly higher serum myoglobin levels on admission than survivors (4478 µg/ml [1824; 9794] vs. 2823 µg/ml [1590; 7107]; p = 0.036). However, admission myoglobin level was not an independent risk factor for hospital mortality in the logistic regression model. Data on kidney function on day 90 were available for only 42.6% (87/204) of survivors. The survivors did not differ in dialysis dependence (KRT: 2/33 (6.1%) versus CT: 1/54 (1.9%)) or having advanced kidney disease (eGFR < 45 ml/min/1.72m2: KRT 7/33 (21.2%) and CT 10/54 (18.5%); p = 0.76)). The eGFR was similar in both groups (KRT: 68 ml/min [51; 91], CT: 76 ml/min [50; 106]; p = 0.62). Serum myoglobin level on ICU admission was not predictive for kidney function on day 90 (CKD 1–2 after 90 days: 2700 µg/ml [1579; 6972], CKD 3–5: 3980 µg/ml [1621; 7233]; (p = 0.395).
Risk factors for hospital mortality
The following parameters were univariate associated with in-hospital mortality: need for KRT, AKI stage 3, need for invasive mechanical ventilation, sepsis, need for vasopressors, liver cirrhosis, active malignancy, high APACHE II or SOFA score, MAP, heart rate, myoglobin, urea, creatinine, IL-6, albumin, platelet count, pH, bicarbonate, potassium, lactate level and diuresis at admission. However, only the SOFA Score (OR: 1.111; CI: 1.004–1.228; p = 0.041) and need for KRT (OR: 2.163; CI: 1.061–4.407); p = 0.034) were independent predictive factors for hospital mortality according to multivariate logistic regression (Suppl. Table 3).
Hospital mortality and specific kidney replacement modalities
Survival curves of specific KRTs showed no benefit for any of the therapies (log rank p = 0.077) (Fig. 2D). Patient treated with CVVH seemed to have a lower hospital mortality compared to those with other specific therapies and lower compared to the predicted mortality based on APACHE II score (Table 4 and Fig. 3) [30, 31]. However, this supposed advantage for CVVH could not be confirmed after multivariate logistic regression (OR: 0.505 [0.208; 1.225]; p = 0.131).
Risk for all-cause hospital mortality with the various kidney replacement modalities. CVVH continuous veno-venous hemofiltration, CVVHD-HCO continuous veno-venous hemodialysis with high cut-off filter, CVVHD-adsorber continuous veno-venous hemodialysis with “standard” high-flux filter and adsorber, CVVHDF continuous veno-venous hemodiafiltration, CVVHD continuous veno-venous hemodialysis, SLEDD sustained low efficiency daily dialysis
Dialyzer lifetime
Life span of the extracorporeal circuit was shorter with CVVH compared to that of all other KRT techniques (log-Rank p = 0.017) (Fig. 4). For other modalities significant differences could not be demonstrated in this issue.
Discussion
The present study confirmed that AKI requiring KRT in rhabdomyolysis is associated with a high hospital mortality rate. Multivariate binary logistic regression showed that application of specific KRT therapy considering extracorporeal myoglobin elimination could not improve all-cause hospital mortality. Nevertheless, the trend for lower hospital mortality rates on CVVH may indicate that extracorporeal myoglobin elimination could have an influence on outcome. This hypothesis is supported by an older animal experiment of rhabdomyolysis, in which accelerated start of CVVH demonstrated improved morphological changes of renal mitochondria and less apoptosis [32].
Application of clinical scores can help to identify patients at increased risk for AKI and death from rhabdomyolysis [33]. However, it remains uncertain whether these patients benefit from early implementation of specific extracorporeal treatments.
Older studies define rhabdomyolysis on the basis of elevated serum creatinine kinase (CK) [34], but serum myoglobin concentrations above between 368 µg/l [22] and 3865 µg/l [23] predict development of AKI more accurate [22, 35] and might be a better diagnostic parameter in view of myoglobin as nephrotoxic substance. For this reasons, we defined rhabdomyolysis as myoglobin above 1000 µg/l in the range of published thresholds [22, 23].
In this study in 52,1% of the patients needed KRT, which is considerably more than in other studies [22, 23, 35, 36], but these did not only include critically ill patients with high disease severity and the definition of rhabdomyolysis differed in some cases [22, 23, 35, 36]. Higher myoglobin levels in this study were associated with an increased risk of hospital mortality but could not be confirmed as an independent risk factor for death and end stage kidney disease (ESKD). An impact on renal function at day 90 was also not detectable, although it is generally assumed that the severity of AKI influences the progression of CKD [37]. The number of cases and the relatively short follow-up period may have altered our results at this point.
Two studies could not confirm an association of initial CK-levels with mortality and renal outcome, but the stage of AKI was a strong predictor [38, 39]. Regarding the strong associations of CK and myoglobin levels [20, 22, 35], the prognostic value of myoglobin in predicting mortality and renal outcomes appears to be limited, but could still increase the risk for AKI [23, 35]. Furthermore, serum CK levels at the time of termination of KRT were not able to estimate clinical outcome if daily diuresis exceeds > 1 l/d [40].
The high hospital mortality rate of 55,6% in patients requiring KRT was similar to mortality rates observed in large multicenter trials [2, 3] and explained by a high APACHE II score in the KRT group [31, 41]. Only the SOFA score and the requirement of KRT were identified as independent risk factors for hospital mortality as already shown in large trials from critically ill patients [2, 3, 42]. These results suggest that general critical condition, rather than a single factor, determines hospital mortality in our study.
Best therapeutic approach for toxic myoglobin elimination is preservation of good kidney performance. Although not significant, the trend towards a better MRR with CT than with KRT in the present study may underline this fact. That is why keystones in treatment of rhabdomyolysis are optimization of kidney perfusion and the control of patient’s fluid balance to enhance urine production [43, 44]. Consequently, aggressive fluid replacement and probably supplementation with sodium bicarbonate had been recommended [6]. In contrast, in a recently published survey, patients with rhabdomyolysis did not benefit from high fluid load (> 3 ml/kg/h). Fluid even seems to be deleterious. Administration of sodium bicarbonate resulted in poor renal outcomes, too [45]. For this reason, recommendations for CT of rhabdomyolysis should be reconsidered. Extracorporeal treatment can not recommended in patients with working kidneys [43] as it provides the highest elimination capacity of myoglobin. If KRT is necessary, various modalities of KRT with different myoglobin elimination capacity are available [9, 11, 12, 14, 15, 17, 21, 32, 46,47,48], but good clinical data concerning outcome of patients are missing. Therefore, no recommendation for any specific method of KRT is available until now [43].
Differences in MRR between specific KRT modalities were not observed. There was only a trend towards a higher reduction rate with CVVH and CVVHD-HCO. High myoglobin reduction rate of 22 ml/min in CVVH was already observed by Amyot et al. [9]. Data from our research group already showed a better myoglobin clearance in patients treated with CVVHD-HCO compared to CVVHDF (mean difference of 5.5 (4–7) ml/min, p < 0.0005; (12.3 vs. 3.7 ml/min)) [12]. Another investigation could verify a relevant myoglobin elimination in CVVHD-HCO in rhabdomyolysis, too [48]. In a recently published study CVVHD-HCO combined with an adsorber had a particularly good elimination capacity [21]. However, there are differences to our study [12], which explain the lower MRR: firstly, the adsorber was not combined with an HCO filter but with a high-flux filter and secondly, the adsorbers were used for 24 h. As a result, the overall performance was probably weakened due to the rapid filter saturation in the first treatment hours. However, a MRR of 45% [35.3; 56.3] was similar to a retrospective analysis among 43 patients with a significant myoglobin reduction rate of 29% [8.8; 50] [− 29.4% (IQR: − 41.2, + 2.6%] within the first 24 h of treatment [20].
Comparison of filter lifetime in different continuous KRTs in our cohort showed shortest survival with CVVH. Maintenance of extracorporeal circuit in CVVH was ensured by systemic anticoagulation using heparin due to the need for higher blood flow rates. Filter lifetime is longer in RCA compared to systemic anticoagulation with heparin [49,50,51] and premature filter clotting seems more pronounced in convective procedures, because of more hemoconcentration along the filter and higher transmembrane pressures [52, 53].
This retrospective analysis found a significant association between an acidic urine and requirement of KRT. Data concerning this issue are rare. Aslan et al. could not demonstrate an association between urine pH and contrast-induced nephropathy [54], thus acidic urine in general sets not a bad prognosis. However an alkaline environment seems to stabilize ferry species within the myoglobin molecule [6]. In consequence, it reduces formation of reactive oxygen radicals and diminishes lipid peroxidation of tubular cells and mitochondrial membranes [43, 55]. It is comprehensible that acidic urine enhances kidney toxicity in rhabdomyolysis and therefore alkalization of urine seems a useful therapeutic option.
There are several limitations concerning this study. Firstly, it is a monocentric and retrospective analysis. Secondly, there may have been a beta error because of many small subgroups, particularly regarding the different KRT modalities. Thirdly, multiple statistical comparisons can randomly produce significant differences. Fourthly, complete data sets were not available for every patient. Fifthly, some important parameters in the evaluation of kidney function such as albuminuria, proteinuria or hematuria were not available. Sixthly we calculated only myoglobin reduction rates in patient´s serum. This should be distinguished from clearance rate due to an extracorporeal circuit. Differences in endogenous myoglobin production and residual renal myoglobin elimination could under- or overestimate myoglobin elimination of KRT modality.
Nevertheless, the data represent therapy and outcome of patients suffering from rhabdomyolysis from a daily setting in a large medical ICU over a period of more than 4.5 years and provides important information about this severe disease pattern. It can also be useful to generate hypotheses and to calculate adequate power for prospective randomized studies.
Conclusions
This study emphasizes that AKI requiring KRT following rhabdomyolysis is accompanied by high mortality rate. Differences in myoglobin reduction rate between various KRTs could not be confirmed, but CVVH was associated with reduced filter lifetime compared to other KRTs, which enable myoglobin elimination, too.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Abbreviations
- AKI:
-
Acute kidney injury
- CKD:
-
Chronic kidney disease
- KRT:
-
Kidney replacement therapy
- CVVH:
-
Continuous veno-venous hemofiltration
- CVVHDF:
-
Continuous veno-venous hemodiafiltration
- CVVHD-HCO:
-
Continuous veno-venous hemodialysis with high cut-off filter
- SLEDD:
-
Sustained low-efficiency daily dialysis
- HCO:
-
High cut-off
- CKD:
-
Chronic kidney disease
- ICU:
-
Intensive care unit
- MRR:
-
Myoglobin reduction rate
- CT:
-
Conservative treatment
- kDa:
-
Kilodaltons
- RCA:
-
Regional citrate anticoagulation
- TTR:
-
Total turnover rate
- BMI:
-
Body mass index
- APACHE II:
-
Acute physiology and chronic health evaluation II score
- SOFA:
-
Sequential organ failure assessment
- eGFR (CKD-EPI):
-
Estimated glomerular filtration according Chronic Kidney Disease Epidemiology Collaboration
- CK:
-
Creatin kinase
- IL-6:
-
Interleukin-6
- MAP:
-
Mean arterial pressure
- ESKD:
-
End-stage kidney disease
References
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet (London, England). 2019;394(10212):1949–64.
Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23.
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Gameiro J, Marques F, Lopes JA. Long-term consequences of acute kidney injury: a narrative review. Clin Kidney J. 2021;14(3):789–804.
Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72.
Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis - An overview for clinicians. Crit Care. 2005;9(2):158–69.
Panizo N, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, Moreno JA. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40(5):520–32.
Amyot SL, Leblanc M, Thibeault Y, Geadah D, Cardinal J. Myoglobin clearance and removal during continuous venovenous hemofiltration. Intensive Care Med. 1999;25(10):1169–72.
Zhang L, Kang Y, Fu P, Cao Y, Shi Y, Liu F, et al. Myoglobin clearance by continuous venous-venous haemofiltration in rhabdomyolysis with acute kidney injury: A case series. Injury. 2012;43(5):619–23.
Naka T, Jones D, Baldwin I, Fealy N, Bates S, Goehl H, et al. Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report. Crit Care. 2005;9(2):R90–5.
Weidhase L, de Fallois J, Haußig E, Kaiser T, Mende M, Petros S. Myoglobin clearance with continuous veno-venous hemodialysis using high cutoff dialyzer versus continuous veno-venous hemodiafiltration using high-flux dialyzer: a prospective randomized controlled trial. Crit Care. 2020;24(1):644.
Mikkelsen TS, Toft P. Prognostic value, kinetics and effect of CVVHDF on serum of the myoglobin and creatine kinase in critically ill patients with rhabdomyolysis. Acta Anaesthesiol Scand. 2005;49(6):859–64.
Heyne N, Guthoff M, Krieger J, Haap M, Häring HU. High cut-off renal replacement therapy for removal of myoglobin in severe rhabdomyolysis and acute kidney injury: a case series. Nephron Clin Pract. 2012;121(3–4):c159–64.
Weidhase L, Haussig E, Haussig S, Kaiser T, de Fallois J, Petros S. Middle molecule clearance with high cut-off dialyzer versus high-flux dialyzer using continuous veno-venous hemodialysis with regional citrate anticoagulation: A prospective randomized controlled trial. Isaka Y, editor. PLoS One. 2019;14(4):e0215823.
Goubella A, Gankam-Kengne F, Baudoux T, Fagnoul D, Husson C, Delforge ML, et al. Severe myoglobinuric acute kidney injury in a kidney recipient: rapid recovery after hemodialysis with the super high-flux membrane Theralite®. Clin Nephrol. 2017;88(12):359–63.
Premru V, Kovač J, Buturović-Ponikvar J, Ponikvar R. High cut-off membrane hemodiafiltration in myoglobinuric acute renal failure: a case series. Ther Apher Dial. 2011;15(3):287–91.
Dilken O, Ince C, van der Hoven B, Thijsse S, Ormskerk P, De Geus HRH. Successful reduction of creatine kinase and myoglobin levels in severe rhabdomyolysis using extracorporeal blood purification (CytoSorb®). Blood Purif. 2020;49:743–7.
Kousoulas L, Wittel U, Fichtner-Feigl S, Utzolino S. Hemoadsorption in a case of severe septic shock and necrotizing fasciitis caused by nontraumatic renal rupture due to pyelonephritis with obstructive uropathy. Case reports Crit care. 2018;2018:5248901.
Scharf C, Liebchen U, Paal M, Irlbeck M, Zoller M, Schroeder I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit Care. 2021;25(1):41.
Albrecht F, Schunk S, Fuchs M, Volk T, Geisel J, Fliser D, et al. Rapid and effective elimination of myoglobin with CytoSorb® hemoadsorber in patients with severe rhabdomyolysis. Blood Purif. 2023;2:1–8.
El-Abdellati E, Eyselbergs M, Sirimsi H, van Hoof V, Wouters K, Verbrugghe W, et al. An observational study on rhabdomyolysis in the intensive care unit exploring its risk factors and main complication: Acute kidney injury. Ann Intensive Care. 2013;3(1):1–8.
Kasaoka S, Todani M, Kaneko T, Kawamura Y, Oda Y, Tsuruta R, et al. Peak value of blood myoglobin predicts acute renal failure induced by rhabdomyolysis. J Crit Care. 2010;25(4):601–4.
Marx G, Schindler AW, Mosch C, Albers J, Bauer M, Gnass I, et al. Intravascular volume therapy in adults. Eur J Anaesthesiol. 2016;33:488–521.
Sever MS, Vanholder R, RDRTF of ISN Work Group on Recommendations for the Management of Crush Victims in Mass Disasters. Recommendation for the management of crush victims in mass disasters. Nephrol Dial Transplant. 2012;27(Suppl 1):i1–67.
Shigemoto T, Rinka H, Matsuo Y, Kaji A, Tsukioka K, Ukai T, et al. Blood purification for crush syndrome. Ren Fail. 1997;19(5):711–9.
KDIGO. Reference Keys. Kidney Int Suppl. 2012;2(1):4.
STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian and New Zealand Intensive Care Society Clinical Trials Group, United Kingdom Critical Care Research Group, Canadian Nephrology Trials Network, Irish Critical Care Trials Group, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(3):240–51.
Fayad AI, Buamscha DG, Ciapponi A. ntensity of continuous renal replacement therapy for acute kidney injury. In: Fayad AI, editor. Cochrane database of systematic reviews. Chichester: John Wiley & Sons, Ltd; 2013.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–8.
Beck DH, Smith GB, Pappachan JV, Millar B. External validation of the SAPS II, APACHE II and APACHE III prognostic models in South England: a multicentre study. Intensive Care Med. 2003;29(2):249–56.
Tang W, Chen Z, Wu W, Qiu H, Bo H, Zhang L, et al. Renal protective effects of early continuous venovenous hemofiltration in rhabdomyolysis: improved renal mitochondrial dysfunction and inhibited apoptosis. Artif Organs. 2013;37(4):390–400.
McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821–8.
Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20(1):135.
Vangstad M, Bjornaas MA, Jacobsen D. Rhabdomyolysis: a 10-year retrospective study of patients treated in a medical department. Eur J Emerg Med. 2019;26(3):199–204.
Candela N, Silva S, Georges B, Cartery C, Robert T, Moussi-Frances J, et al. Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10(1):27.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8.
Simpson JP, Taylor A, Sudhan N, Menon DK, Lavinio A. Rhabdomyolysis and acute kidney injury: Creatine kinase as a prognostic marker and validation of the McMahon Score in a 10-year cohort: A retrospective observational evaluation. Eur J Anaesthesiol. 2016;33(12):906–12.
Baeza-Trinidad R, Brea-Hernando A, Morera-Rodriguez S, Brito-Diaz Y, Sanchez-Hernandez S, El Bikri L, et al. Creatinine as predictor value of mortality and acute kidney injury in rhabdomyolysis. Intern Med J. 2015;45(11):1173–8.
Xiao L, Ran X, Zhong Y, Le Y, Li S. Serum creatine kinase levels are not associated with an increased need for continuous renal replacement therapy in patients with acute kidney injury following rhabdomyolysis. Ren Fail. 2022;44(1):893–901.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300.
Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224.
Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–65.
Kim HW, Kim S, Ohn JH, Kim NH, Lee J, Kim ES, et al. Role of bicarbonate and volume therapy in the prevention of acute kidney injury in rhabdomyolysis: a retrospective propensity score-matched cohort study. Kidney Res Clin Pract. 2022;41(3):310–21.
Sorrentino SA, Kielstein JT, Lukasz A, Sorrentino JN, Gohrbandt B, Haller H, et al. High permeability dialysis membrane allows effective removal of myoglobin in acute kidney injury resulting from rhabdomyolysis. Crit Care Med. 2011;39(1):184–6.
Jerman A, Andonova M, Persic V, Gubensek J. Extracorporeal removal of myoglobin in patients with rhabdomyolysis and acute kidney injury: comparison of high and medium cut-off membrane and an adsorber cartridge. Blood Purif. 2022;51(11):907–11.
Mariano F, Mella A, Rumbolo F, Holló Z, Bergamo D, Congiu G, Mengozzi G, Berardino M, Stella M, Biancone L. Clearance of NT-proBNP and procalcitonin during continuous venovenous hemodialysis with the medium cutoff filter in patients with rhabdomyolysis-associated early acute kidney injury. Blood Purif. 2023;52(5):446–54.
Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20(1):1–13.
Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41(12):2098–110.
Zarbock A, Küllmar M, Kindgen-Milles D, Wempe C, Gerss J, Brandenburger T, et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324(16):1629–39.
Ricci Z, Ronco C, Bachetoni A, D’amico G, Rossi S, Alessandri E, et al. Solute removal during continuous renal replacement therapy in critically ill patients: Convection versus diffusion. Crit Care. 2006;10(2):1–7.
Brain M, Winson E, Roodenburg O, McNeil J. Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):69.
Aslan G, Afsar B, Sag AA, Camkiran V, Erden N, Yilmaz S, et al. The effect of urine pH and urinary uric acid levels on the development of contrast nephropathy. Kidney Blood Press Res. 2020;45(1):131–41.
Reeder BJ, Wilson MT. The effects of pH on the mechanism of hydrogen peroxide and lipid hydroperoxide consumption by myoglobin: a role for the protonated ferryl species. Free Radic Biol Med. 2001;30(11):1311–8.
Acknowledgements
We thank all patients and the ICU staff for their support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JF and LW contributed to the conception and design of the study, and helped in investigation. RS wrote the first draft of the manuscript. JF, RS, LW, and SP wrote the sections of the manuscript. JF, RS, THL, CS, SP, and LW contributed to the analysis. JF helped in visualization. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local Institutional Review Board at the Medical Faculty of the University of Leipzig (312/20-ek).
Consent for publication
Every patient or the legal guardian gave consent on admission to hospital for the use of pseudonymised data for scientific research.
Competing interests
LW received funding from Fresenius Medical Care Deutschland GmbH (Else-Kröner-Straße 1, 61352 Bad Homburg, Germany) and Cytosorbents Europe GmbH (Müggelseedamm 131, 12587 Berlin, Germany). The remaining authors have disclosed that they do not have any conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Fallois, J., Scharm, R., Lindner, T.H. et al. Kidney replacement and conservative therapies in rhabdomyolysis: a retrospective analysis. BMC Nephrol 25, 96 (2024). https://doi.org/10.1186/s12882-024-03536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03536-8