Abstract
Background
The aims of this study were to investigate the prevalence and the influence factors of gastrointestinal symptoms, and its association with the quality of life (QOL) in peritoneal dialysis (PD) patients.
Methods
Continuous ambulatory PD patients (CAPD) who followed up in our PD center between March 2016 and December 2017 were enrolled in this cross-sectional study. Gastrointestinal symptom rating scale (GSRS) was used to evaluate gastrointestinal symptoms. The related clinical data were also collected. Multiple linear regression analysis was test for the influence factors associated with score of GSRS and QOL.
Results
This study included 471 CAPD patients. The mean age was 48.5±13.9 years, 53.9% were male and 15.1% with diabetic nephropathy. The median duration of PD was 37.3 (17.5~66.5) months. The median score of GSRS was 1.2(1.1~1.3) scores. Totally 82.2% (n=387) CAPD patients had at least one gastrointestinal symptom. Higher glycosylated hemoglobin, higher score of depression, lower diastolic blood pressure, urine output, score of instrumental activities of daily living scale and more amount of pills per day were independently associated with higher score of GSRS (all P<0.05). Score of dyspepsia and eating dysfunction were independently associated with worse score of QOL and physical health (all P<0.05).
Conclusions
Gastrointestinal symptoms were common but not serious in CAPD patients. Glycemic control, depression, blood pressure, urine output, activity of daily life and amount of pills were all associated with gastrointestinal symptoms. Moreover, gastrointestinal symptoms were correlated with QOL of PD patients.
Similar content being viewed by others
End stage renal disease (ESRD) is a complex disease. Gastrointestinal symptoms related to uraemia, medications and other comorbidities are common in patients with ESRD. Endoscopic evaluation and mucosal biopsies are ideal ways to diagnose gastrointestinal diseases, but they are difficult to be widely used in peritoneal dialysis (PD) patients because of their invasiveness. There are several scales used to evaluate functional gastrointestinal symptoms in dialysis patients, such as Rome I, Rome II, Rome III and gastrointestinal symptom rating scale (GSRS). The prevalence of gastrointestinal symptoms in patients with PD evaluated by different assessment scales varied greatly, which ranged from 14.2% to 90.3% [1,2,3,4,5].
The underlying pathogenesis of gastrointestinal symptoms in PD patients has not been clearly elucidated. The evidence base of gastrointestinal symptoms in dialysis patients was limited, and further investigation of preventable causes and potential interventions were required in future research [6]. Since dextrose dialysate was indwelled into the peritoneal cavity of PD patients, the particularity of PD treatment might affect gastrointestinal function through delayed gastric emptying [7,8,9,10], increased intraperitoneal pressure and decreased lower esophageal sphincter pressure [11, 12]. It is necessary to further study the influence factors of gastrointestinal symptoms in PD patients. Moreover, gastrointestinal symptoms may play an important role on quality of life (QOL) of PD patients. Although previous studies have explored the relationship between gastrointestinal symptoms and QOL in dialysis patients, these studies only used univariate analysis that did not adjust for other confounding factors [4, 13, 14]. Therefore, the aims of this study were to investigate the prevalence and the influence factors of gastrointestinal symptoms in PD patients, and to determine the association of gastrointestinal symptoms with QOL.
Materials and methods
Participants
This cross-sectional study enrolled ESRD patients who received PD treatment and followed up in a single PD center in Southern China from March 2016 to December 2017. Inclusion criteria were as following: at least 18 years old, receiving continuous ambulatory PD (CAPD) treatment more than 3 months. Patients with severe gastrointestinal diseases (digestive tract hemorrhage, recurrent peptic ulcers, gastrointestinal perforation, intestinal obstruction, gastrointestinal malignant tumors and gastrointestinal surgery, etc.), or severe infection (refractory peritonitis, septic shock, etc.) during the past 3 months, or other complications (malignant tumor, cardiovascular disease requiring hospitalization, etc.), or inability to complete the investigation, or unwillingness to participate this study were excluded. All participants used the conventional PD solutions (Dianeal 1.5%, 2.5%, or 4.25% dextrose; Baxter Healthcare, Guangzhou, China). Y sets and twin bag systems were used in all participants. This study was approved by the Human Ethics Committee of Sun Yat-sen University [Ethics Review (2016) NO.215] and the written informed consent of patients was obtained.
Measurement of gastrointestinal symptoms
Gastrointestinal symptoms of participants were assessed by GSRS. The original GSRS included 15 items, which could be grouped into five dimensions: abdominal pain (three items), reflux (two items), dyspepsia (four items), diarrhea (three items) and constipation syndrome (three items) [15]. Eating dysfunction syndrome, which was developed by Svedlund [16], was added into the original GSRS. It included three items: early satiety, difficulties in eating normal portions, and postprandial pain. All items used a 7-grade Likert scale defined by descriptive anchors (1=none, 2=minor, 3=mild, 4=moderate, 5=moderately severe, 6=severe, 7=very severe discomfort). The questions concerned symptom severity during the previous two weeks. Each dimension score was calculated as the mean value of the items belonging to the specific syndrome with a value from 1 to 7.
Measurements of activity of daily living, depression and quality of life
Activity of daily living was assessed by Barthel index [17] and instrumental activities of daily living scale (IADLs) [18]. The Chinese version of Beck Depression Inventory-II was used to evaluate the severity of depressive symptoms [19]. QOL was evaluated by the Short Form of Medical Outcomes Study (SF-36) [20]. It was a self-administered 36-item questionnaire, which was divided into eight dimensions including physical functioning, role-physical, bodily pain, general health status, vitality, social functioning, role-emotional, and mental health. The eight dimensions could be divided into two components, which were the physical component scale including the first four dimensions, and the mental component scale including the remaining four dimensions. Each dimension was scored from 0 to 100. Higher scores of the scale indicated the better QOL. Based on the reference, total score of QOL was arithmetic averaging of the eight SF-36 domains scores [21].
Data collection
Demographic, clinical and laboratory data were obtained when participants were enrolled in the study. Demographic data included age, gender and primary renal disease. Clinical data included duration of PD, PD dose, dwell volume of dialysate, urine output, blood pressure, body mass index and total amount of pills per day. Laboratory data included hemoglobin, high-sensitivity C-reactive protein, serum albumin, serum calcium, serum phosphorus, intact parathyroid hormone, total cholesterol, triglycerides, serum sodium, serum potassium, glycosylated hemoglobin, blood urea nitrogen and serum creatinine and clearance index of urea (Kt/V). Charlson comorbidity index [22] was used to assess comorbidities of participants.
Statistical analysis
All statistical analyses were performed by SPSS Statistics 19.0. Continuous variables of approximately normally distributed were expressed as mean (standard deviation) and compared with independent t test. Skewed continuous variables were expressed as median and interquartile range and compared with Mann-Whitney test. Categorical variables were expressed as number and percentage and compared with chi-square test. Multiple linear regression analysis was test for the influence factors that associated with score of GSRS and QOL with significance level of selection entry at 0.05. Results were considered significant when double side P<0.05.
Results
Overall patient situation
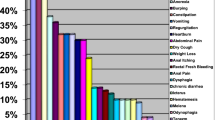
A total of 600 CAPD patients were recruited during the study period. Among them, six patients had severe gastrointestinal diseases, eight patients had severe infection or other complications, 24 patients could not complete the investigation and 91 patients did not want to participate in this study (Fig. 1). Finally, 471 CAPD patients were included in this study. The mean age was 48.5±13.9 years, and the median duration of PD was 37.3 (17.5~66.5) months. Totally 53.9% (n=254) patients were male, and 15.1% (n=71) patients were diabetic nephropathy. The median Charlson comorbidity index was 3.0 (2.0~4.0) scores. A total of 387(82.2%) CAPD patients developed at least one gastrointestinal symptom in this study. Among them, 59.9% (n=282) patients had dyspepsia, 31.0% (n=146) patients had constipation, 27.8% (n=131) patients had abdominal pain, 20.8% (n=98) patients had eating dysfunction, 20.4% (n=96) patients had reflux and 17.4% (n=82) patients had diarrhea. The severity of each dimension was showed in Fig. 2. Compared with patients without gastrointestinal symptom, patients with gastrointestinal symptoms had lower urine output, higher depression score and higher proportion of use of proton pump inhibitor drugs. (all P<0.05) (Table 1).
Influence factors of gastrointestinal symptoms
Since GSRS was a continuous variable of skewness distribution, logarithmic transformation was performed for it. The unary linear regression analysis showed that age, Charlson comorbidity index, duration of PD, total amount of pills per day, PD dose, urine output, diastolic blood pressure, glycosylated hemoglobin, score of IADLs and depression were associated with the score of GSRS in PD patients (all P<0.05). These factors, gender and serum creatinine were include into the multiple linear regression analysis. The result showed that higher level of glycosylated hemoglobin, higher score of depression, lower urine output, lower diastolic blood pressure, lower score of IADLs and more amount of pills per day were independent influence factors associated with the higher score of GSRS after adjustment for confounders (all P<0.05) (Table 2).
Correlation between gastrointestinal symptoms and QOL
Using unary linear regression analysis, it was found that higher score of GSRS, reflux, dyspepsia and eating dysfunction were associated with lower total score of QOL in PD patients (all P<0.05). Moreover, advancing age [B=-1.786, 95% confidence interval (CI): -3.392~-0.181; P=0.029], longer duration of PD (B=-1.737, 95%CI: -3.343~-0.130; P=0.034), higher Charlson comorbidity index (B=-2.497, 95%CI: -4.095~-0.900; P=0.002), lower urine output (B=2.765, 95%CI: 1.171~4.359; P=0.001), diastolic blood pressure (B=2.520, 95%CI: 0.922~4.118; P=0.002), serum albumin (B=1.889, 95%CI: 0.284~3.494; P=0.021) and triglyceride (B=1.952, 95%CI: 0.349~3.556; P=0.017) were all associated with lower total score of QOL. After adjustment for confounders, it was found that higher score of dyspepsia and eating dysfunction were independently associated with lower total score of QOL (all P<0.05) (Table 3).
The unary linear regression analysis showed that higher score of GSRS, reflux, dyspepsia and eating dysfunction were significantly associated with lower score of physical component scale of PD patients (all P<0.05). Moreover, advancing age (B=-1.953, 95%CI: -3.608~-0.297; P=0.021), longer duration of PD (B=-2.004, 95%CI: -3.659~-0.348; P=0.018), higher Charlson comorbidity index (B=-2.699, 95%CI: -4.346~-1.051; P=0.001), lower urine output (B=2.976, 95%CI: 1.332~4.619; P<0.001), diastolic blood pressure (B=2.501, 95%CI: 0.851~4.151; P=0.003), serum albumin (B=2.273, 95%CI: 0.621~3.926; P=0.007) and triglyceride (B=2.034, 95%CI: 0.376~3.691; P=0.016) were significantly associated with lower score of physical component scale. After adjustment for confounders, higher score of dyspepsia and eating dysfunction were independently associated with lower score of physical component scale (all P<0.05) (Table 4).
Higher score of GSRS, dyspepsia, eating dysfunction and Charlson comorbidity index (B=-2.296, 95%CI: -4.073~-0.520; P=0.011), lower urine output (B=2.555, 95%CI: 0.781~4.328; P=0.005), diastolic blood pressure (B=2.539, 95%CI: 0.766~4.313; P=0.005) and triglyceride (B=1.871, 95%CI:0.093~3.649; P=0.039) were associated with lower score of mental component scale of PD patients by using unary linear regression analysis. After adjustment for other confounders, the score of GSRS and each dimension were not associated with lower score of mental component scale (all P>0.05) (Table 5).
Discussion
The present study showed that 82.2% CAPD patients developed gastrointestinal symptoms. The three most common gastrointestinal symptoms were dyspepsia, constipation and abdominal pain. Higher glycosylated hemoglobin, higher score of depression, lower urine output, diastolic blood pressure, score of IADLs and more amount of pills per day were independently associated with gastrointestinal symptoms of PD patients. Higher score of dyspepsia and eating dysfunction were independently associated with lower scores of QOL and physical component scale.
The prevalence of gastrointestinal symptoms in PD patients was high in this study. More than half of PD patients suffered from dyspepsia, and about a third of patients suffered from constipation and abdominal pain. These results were consistent with previous studies [2,3,4,5]. Salamon et al [2] found that 85% PD patients reported at least one gastrointestinal symptom which was defined as nausea, vomiting, bloating, early satiety, diarrhea, heartburn, fatigue, and weight changes. Dong et al [5] reported that 61.6% PD patients appeared at least one gastrointestinal symptom assessed by GSRS, and the prevalence of eating dysfunction, reflux and indigestion was 44.2%, 32.7% and 32.7%, respectively.
There might be multiple reasons for the high incidence of gastrointestinal symptoms in PD patients. To our knowledge, this study was the first one to show an association between glycosylated hemoglobin and gastrointestinal symptoms in PD patients. Schvarcz [23] and Kim [24] found that glycosylated hemoglobin level was associated with the gastrointestinal symptoms in diabetic patients. Based on large population studies, Bytzer et al [25, 26] raised the hypothesis that poor glycemic control by itself was a major cause of chronic gastrointestinal symptoms. Our results also found that depression was independently associated with gastrointestinal symptoms in PD patients. Chong et al [27] reported that psychosomatic symptoms such as anxiety, backache, depression, headache and insomnia were correlated significantly with gastrointestinal symptoms in hemodialysis patients. Kahvecioglu et al [28] found that depression was more frequent in PD patients with irritable bowel syndrome compared with those without. Lower urine output was independently associated with gastrointestinal symptoms of PD patients in this study. Dong et al [5] reported that residual renal Kt/V was negatively correlated to gastrointestinal symptoms of PD patients. It was suggested that residual renal function might play an important role on gastrointestinal symptoms in PD patients. They also [5] found that PD patients took an average of 15 pills a day, and the amount of pills daily intake was positively correlated with gastrointestinal symptoms in PD patients. Our results were similar to this report. Although we found that lower diastolic pressure and IADLs were independently associated with gastrointestinal symptoms in PD patients, the related mechanism was not yet clear. In addition, this study didn’t confirm the relationship between the dwell volume of dialysate and gastrointestinal symptoms. The main reason might be that 98.3% CAPD patients in this study were indwelled in the abdominal cavity with 2 liters of dialysate.
Only a few studies investigated the association of gastrointestinal symptoms with QOL in dialysis patients. Strid et al [4] found that gastrointestinal symptoms were negatively correlated with psychological general well-being in chronic renal failure patients. Zhang et al [14] reported that dialysis patients with constipation had significant lower mean physical component scale and mental component scale score than the non-constipation group. Although these studies found the correlation between gastrointestinal symptoms and QOL in dialysis patients, they did not perform multivariate analysis to adjust for other confounding factors. Using multiple linear regression analysis, our results confirmed that dyspepsia and eating dysfunction were independently associated with worse QOL and physical health in PD patients. It indicated that we should pay more attention to gastrointestinal symptoms to improve the QOL in PD patients.
The strength of this study was the relatively big number of samples, which may influence the power of statistical tests. But there were some limitations in this study. Firstly, the patients were enrolled in a single PD center, it was impossible to eliminate patient selection bias completely. Secondly, due to the cross-sectional nature of the study, it could not infer a causal relationship between gastrointestinal symptoms and other variables.
Conclusions
This cross-sectional study demonstrated that 82.2% CAPD patients developed at least one gastrointestinal symptom. Glycemic control, depression, blood pressure, urine output, activity of daily life and total amount of pills were associated with gastrointestinal symptoms of PD patients. Moreover, gastrointestinal symptoms were significantly associated with QOL of PD patients.
Availability of data and materials
Not applicable.
References
Kosmadakis G, Albaret J, Da Costa CE, Somda F, Aguilera D. Constipation in Peritoneal Dialysis Patients. Peritoneal Dialysis Int. 2019;39(5):399–404.
Salamon K, Woods J, Paul E, Huggins C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J Renal Nutr. 2013;23(2):114–8.
Kosmadakis G, Albaret J, da Costa CE, Somda F, Aguilera D. Gastrointestinal Disorders in Peritoneal Dialysis Patients. Am J Nephrol. 2018;48(5):319–25.
Strid H, Simren M, Johansson AC, Svedlund J, Samuelsson O, Bjornsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrology Dialysis Transpl. 2002;17(8):1434–9.
Dong R, Guo ZY. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: multivariate analysis of correlated factors. World J Gastroenterol. 2010;16(22):2812–7.
Zuvela J, Trimingham C, Le Leu R, Faull R, Clayton P, Jesudason S, et al. Gastrointestinal symptoms in patients receiving dialysis: A systematic review. Nephrology. 2018;23(8):718–27.
Strid H, Simren M, Stotzer PO, Abrahamsson H, Bjornsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39(6):516–20.
Schoonjans R, Van Vlem B, Vandamme W, Van Vlierberghe H, Van Heddeghem N, Van Biesen W, et al. Gastric emptying of solids in cirrhotic and peritoneal dialysis patients: influence of peritoneal volume load. Eur J Gastroenterology Hepatol. 2002;14(4):395–8.
Kim DJ, Kang WH, Kim HY, Lee BH, Kim B, Lee SK, et al. The effect of dialysate dwell on gastric emptying time in patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis Int. 1999;19(Suppl 2):S176–8.
Van V, Schoonjans RS, Struijk DG, Verbanck JJ, Vanholder RC, Van B, et al. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Peritoneal Dialysis Int. 2002;22(1):32–8.
Kim MJ, Kwon KH, Lee SW. Gastroesophageal reflux disease in CAPD patients. Adv Peritoneal Dialysis Conference Peritoneal Dialysis. 1998;14:98–101.
Hylander BI, Dalton CB, Castell DO, Burkart J, Rossner S. Effect of intraperitoneal fluid volume changes on esophageal pressures: studies in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1991;17(3):307–10.
Duncanson E, Chur-Hansen A, Jesudason S. Psychosocial consequences of gastrointestinal symptoms and dietary changes in people receiving automated peritoneal dialysis. J Renal Care. 2019;45(1):41–50.
Zhang J, Huang C, Li Y, Chen J, Shen F, Yao Q, et al. Health-related quality of life in dialysis patients with constipation: a cross-sectional study. Patient Preference Adherence. 2013;7:589–94.
Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive diseases and sciences. 1988;33(2):129–34.
Svedlund J, Sullivan M, Liedman B, Lundell L. Long term consequences of gastrectomy for patient’s quality of life: the impact of reconstructive techniques. Am J Gastroenterology. 1999;94(2):438–45.
Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Maryland State Med J. 1965;14:61–5.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Wang Z, Yuan CM, Huang J, Li ZZ, Chen J, Zhang HY, et al. Reliability and validity of the Chinese version of Beck Depression Inventory-II among depression patients. Chinese Mental Health J. 2011;25(6):476–9.
McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31(3):247–63.
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Schvarcz E, Palmer M, Ingberg CM, Aman J, Berne C. Increased prevalence of upper gastrointestinal symptoms in long-term type 1 diabetes mellitus. Diabetic Med. 1996;13(5):478–81.
Kim JH, Park HS, Ko SY, Hong SN, Sung IK, Shim CS, et al. Diabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetes. World J Gastroenterol. 2010;16(14):1782–7.
Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Int Med. 2001;161(16):1989–96.
Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97(3):604–11.
Chong VH, Tan J. Prevalence of gastrointestinal and psychosomatic symptoms among Asian patients undergoing regular hemodialysis. Nephrology. 2013;18(2):97–103.
Kahvecioglu S, Akdag I, Kiyici M, Gullulu M, Yavuz M, Ersoy A, et al. High prevalence of irritable bowel syndrome and upper gastrointestinal symptoms in patients with chronic renal failure. J Nephrol. 2005;18(1):61–6.
Acknowledgements
We thank all staffs in our PD center for their patient care and data collection.
Funding
This work was supported by the Natural Science Foundation of China (Grant no. 81774069, 81570614), Foundation of Guangdong Key Laboratory of Nephrology (Grant no. 2017B030314019), Medical Science and Technology Research Fund project of Guangdong Province (Grant no. A2019394).
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly and in keeping with the latest guidelines of the International Committee of Medical Journal Editors. X. Y. and C. Y. conceived and designed the study. C. Y. and J. L. prepared and performed data collection. C. Y., X. W. and H. Y. analyzed the data. C. Y. interpreted the results and drafted the manuscript. X. Y. coordinated the study and finally approved the version to be published. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was conducted in compliance with the ethical principles of the Helsinki Declaration and approved by the Human Ethics Committee of Sun Yat-sen University [Ethics Review (2016) NO.215]. Written informed consent was obtained from all the participants.
Consent for publication
All authors of this study were consent for publication.
Competing interests
All authors of this study declared that there was no conflict of interest in the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yi, C., Wang, X., Ye, H. et al. Patient-reported gastrointestinal symptoms in patients with peritoneal dialysis: the prevalence, influence factors and association with quality of life. BMC Nephrol 23, 99 (2022). https://doi.org/10.1186/s12882-022-02723-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02723-9