Abstract
Background
KDIGO (Kidney Disease: Improving Global Outcomes) provides two sets of criteria to identify and classify acute kidney injury (AKI): serum creatinine (SCr) and urine output (UO). Inconsistencies in the application of KDIGO UO criteria, as well as collecting and classifying UO data, have prevented an accurate assessment of the role this easily available biomarker can play in the early identification of AKI.
Study goal
To assess and compare the performance of the two KDIGO criteria (SCr and UO) for identification of AKI in the intensive care unit (ICU) by comparing the standard SCr criteria to consistent, real-time, consecutive, electronic urine output measurements.
Methods
Ninety five catheterized patients in the General ICU (GICU) of Hadassah Medical Center, Israel, were connected to the RenalSense™ Clarity RMS™ device to automatically monitor UO electronically (UOelec). UOelec and SCr were recorded for 24–48 h and up to 1 week, respectively, after ICU admission.
Results
Real-time consecutive UO measurements identified significantly more AKI patients than SCr in the patient population, 57.9% (N = 55) versus 26.4% (N = 25), respectively (P < 0.0001). In 20 patients that had AKI according to both criteria, time to AKI identification was significantly earlier using the UOelec criteria as compared to the SCr criteria (P < 0.0001). Among this population, the median (interquartile range (IQR)) identification time of AKI UOelec was 12.75 (8.75, 26.25) hours from ICU admission versus 39.06 (25.8, 108.64) hours for AKI SCr.
Conclusion
Application of KDIGO criteria for AKI using continuous electronic monitoring of UO identifies more AKI patients, and identifies them earlier, than using the SCr criteria alone. This can enable the clinician to set protocol goals for earlier intervention for the prevention or treatment of AKI.
Similar content being viewed by others
Background
Studies using the KDIGO (Kidney Disease: Improving Global Outcomes) criteria have identified acute kidney injury (AKI) in up to 75% of critically ill hospitalized patients [1]. The KDIGO criteria classify AKI based on: a rise in serum creatinine (SCr), a decrease in urine output (UO) over time, or both [2,3,4].
In the intensive care unit (ICU) neither of these indicators for AKI provides timely information about kidney injury. They are dependent on the times when SCr or UO is manually measured and recorded by the medical staff. Furthermore, SCr measurements are affected by many individual, potentially confounding, factors of the hospitalized patient. Most importantly, SCr levels increase only after approximately 50% loss of renal function, and is thus recognized as a late indicator for kidney injury [5,6,7].
As for UO, KDIGO defines oliguria as a urine output of less than 0.5 ml/kg. AKI severity (stages) is established according to their guidelines for extended periods of oliguria (beyond 6 h, e.g., stage 1 for 6 h of oliguria). Unfortunately, the application of the KDIGIO UO criteria has been inconsistent [1,2,3,4]. Additionally, a recent review comparing KDIGO criteria to its predecessors (RIFLE (Risk, Injury, Failure, Loss and End stage disease) and AKIN (Acute Kidney Injury Network)), has shown that the reported incidence of AKI varies depending on how the criteria are applied [8].
There have been few prospective studies applying the AKI UO criteria, and even fewer have incorporated both SCr and UO criteria. The studies also vary in how they record and assess UO. Some studies have applied the UO criteria as an average UO over 6, 12, and 24 h intervals, using either blocks or continuous windows. Others have interpolated data by averaging UO over the missing hours in nursing records. Oftentimes, there is no indication at all of how UO measurements used in the study were recorded [1, 7, 9,10,11]. This lack of uniformity in measurement, recording, application and reporting of UO makes it difficult to draw consistent conclusions from these studies.
It is not surprising, therefore, that the vast majority of retrospective studies to explore AKI using severity scores have applied only the SCr criteria. The few studies to include UO alongside SCr have either used 24-h averages, applied UO criteria only when available, or simply excluded patients from the study group when there was no UO measurement available [8, 11,12,13,14]. The resultant inconsistencies in classifying AKI according to UO have hindered the use of this easily available biomarker for early identification of AKI.
To address this gap in the research, a prospective study was designed using a novel, real-time UO measurement tool to consistently apply the UO criteria for AKI as defined in the KDIGO guidelines [15]. The UO criteria were also compared to the more commonly-applied SCr criteria, both being used to identify AKI in the ICU patient.
Study goal
To assess and compare the performance of the two KDIGO criteria (SCr and UO) for identification of AKI in the ICU by comparing the standard SCr criteria to real-time, consecutive electronic urine output measurements.
Methods
Study design
Ninety five catheterized patients hospitalized in the General ICU (GICU) at Hadassah Medical Center, Jerusalem, Israel, were enrolled in a pilot study between August 2015 and November 2018. Local internal review board (IRB) approval was obtained and informed consent was waived.
Inclusion criteria
Patients > 18 years of age, SCr baseline within normal range prior to commencement of UOelec observations, or stable SCr measurements compared to 1 month prior to ICU admission in patients with diagnosed chronic kidney injury.
Exclusion criteria
Patients isolated with bacterial infections such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococcus (VRE), and Klebsiella; patients likely to be discharged or die within 24 h in the ICU; patients on dialysis; and pregnant women.
Patient characteristics
Patient information included age, weight, baseline serum creatinine and daily serum creatinine measurements, primary diagnosis, co-morbidities, the need for mechanical ventilation, and the use of vasoactive drugs were recorded for up to 7 days or until discharge from the ICU.
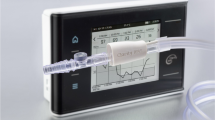
The RenalSense clarity RMS device
The RenalSense Clarity RMS device (Fig. 1) was used to electronically monitor UO every hour, and its technology is described elsewhere [15]. The data for the validation of the system was obtained from the first group of patients enrolled in this study. The electronic measurements were compared to manual UO measurements and the results were published previously. In brief, a total of 1046 h of electronic measurements recorded from 23 subjects measured with the RenalSense system were shown to be closer, with a better correlation, and narrower limits of agreement than the measurements obtained by the nurses, as compared to the scientific scale data [15].
The RenalSense Clarity RMS Sensor Kit incorporates a proprietary sensor in a standard urinary drainage assembly to monitor urine output as it exits the Foley catheter. The data is communicated to the Clarity RMS Console through a cable integrated within the custom-designed drainage tube to the bag and then to the Console, mounted on the footboard of the patient bed
For the purposes of this study the Clarity RMS Sensor Kit (a urine drainage apparatus incorporating a proprietary in-line sensor) was modified to incorporate a standard manual urinometer for the nursing staff to record UO as per their standard practice. Additionally, the sensor was connected by a cable to a RenalSense-designed data collection subsystem (DCS). Nursing staff was blinded to the Clarity RMS measurements console. No information recorded in the DCS had any bearing on the medical staff records, treatment interventions, or medical decisions for the patient. The drainage bag was placed in a container on a scientific scale (Precisa BJ 2200C) to be used as the reference for validation of the sensor measurements. The scale data were also recorded continuously by the same system.
AKI analysis
AKI was identified and scored according to the KDIGO criteria for both SCr and UO [2]. KDIGO Stage 1 is an increase in SCr by ≥0.3 mg/dl (≥26.5 mol/l) within 48 h or an increase in SCr to 1.5–1.9 times baseline or urine volume < 0.5 ml/kg/h for at least 6 h and up to 12 h. Stage 2 is an increase in SCr to 2.0–2.9 times baseline or urine volume < 0.5 ml/kg/h for ≥12 h. Stage 3 is SCr > 3.0 times baseline or initiation of renal replacement therapy (RRT) or urine volume < 0.3 ml/kg/h for ≥24 h or anuria for ≥12 h [1, 2].
AKI SCr was assessed for up to 7 days when measurements were available, following KDIGO guidelines that allow for use of a rolling baseline in the 7 days after admission for diagnosis of AKI by SCr [1, 2]. More than 50% of patients had no electronic record of SCr prior to hospital entry. Therefore, a uniform baseline SCr was defined as the first SCr drawn upon ICU admission.
Patients diagnosed in their medical records with chronic kidney injury as identified by their SCr were included if there was no evidence of “acute on chronic” kidney injury (identified by an increase in SCr upon initial ICU admission compared to previous measurement, or physician report referencing an acute increase).
SCr measurements were collected twice daily by the nurses according to department protocol and patients were treated accordingly as per standard of care. SCr measurements for the study included twice-daily measurements for up to 7 days following Clarity RMS removal if they remained in the ICU. Patients were connected to the Clarity RMS sensor kit upon admission to the ICU for a minimum of 24 h. AKI staging per KDIGO was applied to patient UO monitored consecutively and electronically up to 48 h after admission to the ICU.
Identification of AKI and time to identification of AKI
AKI was diagnosed in the study group by two different methods corresponding to the two sets of KDIGO criteria (UO and SCr).
The first method to identify AKI was based on UO following 6 h of oliguria as defined by the first stage of the KDIGO criteria. Patients were divided into AKI UOelec versus non AKI UOelec.
The second method to identify AKI was an increase of SCr in patient records as defined by the first stage of the KDIGO criteria. Patients were divided into AKI SCr versus non AKI SCr. We compared the identification according to each of these methods separately.
Length of stay (LOS) (and related factors)
For this analysis, the population was divided differently than for AKI identification. This was done to isolate the AKI patients identified by the UOelec criteria alone (i.e., not by SCr criteria). The goal was to assess whether LOS or other related factors had a positive association with AKI UOelec in the absence of AKI SCr.
Thus, for the LOS analysis, the patients were divided into 3 mutually exclusive groups:
-
Group AKI-SCr for all of those patients classified as AKI by their SCr criteria and without regard to their UOelec measurements (i.e., this group includes both those identified as AKI SCr alone and those identified by ACI SCr and AKI UOelec).
-
Group AKI-UOelec′ for those patients classified as AKI using UOelec criteria alone, i.e., not including those already in group AKI-SCr. This group (with the prime symbol) is a subset of the previously described AKI UOelec group. This group represents those patients who would not have been identified as AKI using the standard SCr criteria.
-
Group non-AKI for those patients who were not classified as suffering from AKI, according to either SCr or UO criteria.
LOS in the ICU and hospital, hospital readmissions within 3 months of ICU discharge, RRT, and all-cause mortality were recorded during the entire length of the study and up to a year after the study was completed.
Statistical analysis
Baseline characteristics for the study population are presented. For continuous variables, mean, standard deviation (SD), median and IQR are presented. For dichotomous variables, count and proportion are presented. Rate of AKI is described according to various identification groups. A comparison between the rate of UOelec identification and SCr identification was performed using Wald test.
For all patients with AKI, time to identification is presented using Kaplan-Meier curves. Comparison between the different measures’ identification times was performed using the log-rank test. This analysis was repeated for the patients identified by both UOelec and SCr. Comparison between AKI identification groups was performed using the Wilcoxon rank sum test. LOS in the ICU and general hospitalization are presented by mean, SD, median, IQR, and range.
A comparison between all groups was performed using Kruskal-Wallis test, as well as specific post hoc between-group comparisons, using Wilcoxon rank sum test.
In order to account for mortality as censored data, sensitivity analyses were performed analyzing these parameters as time to discharge, using Kaplan-Meier curves and the log-rank test. These analyses were repeated for subpopulations by age. No adjustment for multiple comparisons was performed. All analyses were performed using R 3.6.2.
Results
Patient characteristics
The patient population was 67% male and 37% of the population was over 70 years of age with a median age of 63. As expected, in the GICU, the majority of the patients in our study group were surgical (67%). Patient characteristics including comorbidities and AKI risk factors are shown in Table 1.
Identification of AKI
A total of 60 out of the 95 (63.2%) patients in the study group were identified with AKI, applying the KDIGO criteria, using either SCr or UOelec or both criteria (Fig. 2). Twenty patients had AKI according to both UOelec and SCr criteria, while 5 were identified only by SCr criteria and 35 were identified only by UOelec measurements (Fig. 3a).
In total, AKI UOelec was identified in 57.9% (N = 55) of the patient population and AKI SCr was identified in 26.4% (N = 25) of the patient population. Out of the 60 AKI patients defined by the KDIGO classification, UOelec identified significantly more patients than SCr, 92% versus 42%, respectively, P < 0.0001 (Figs. 2, and 3b).
AKI severity
Out of the 55 AKI UOelec patients, 38% reached a maximum KDIGO score of stage 1, and 62% reached a maximum severity of stage 2 or 3. Out of the 25 patients with AKI SCr, 68% of the patients had a maximum severity score of stage 1 and 32% reached stages 2 or 3 as their maximum KDIGO severity score (Fig. 3a and b).
Time to identification of AKI
Out of the 60 AKI patients identified by at least one of SCr or UOelec, the time to identify AKI using UOelec was significantly earlier than with SCr (P < 0.0001) (Fig. 4a). Moreover, in the 20 patients that had AKI according to both criteria, time to AKI identification was significantly earlier using UOelec as compared to SCr (P < 0.0001). Among this population, the median (IQR) identification time was 12.75 (8.75, 26.25) hours for AKI UOelec and 39.06 (25.8, 108.64) hours for AKI SCr (Figs. 4b, and 5).
Length of stay and related factors
LOS in the ICU
There was no significant difference in the LOS in the ICU for the AKI-UOelec′ group as compared to the non-AKI group (P = 0.3354). LOS in the ICU for the AKI-SCr group was significantly longer than the AKI-UOelec′ group (P = 0.0043) (Table 2).
LOS in the hospital
the AKI-SCr group had a median (IQR) LOS in the hospital of 20.39 (8.48, 39.46) days. The AKI-UOelec′ group had a similar LOS in the hospital with a median of 20.27 (10.08, 45.99) days. Although it did not reach significance, this was approximately 5 days longer than the non-AKI group, 14.92 (8.88, 30.46) days (P = 0.4210) (Table 2).
LOS in the hospital with a cut-off below and above 70 years of age
Patients less than 70 years old in the AKI-UOelec′ group had the longest median (IQR) hospital stay, 28.42 (8.46, 48.18) days, as compared to the AKI-SCr group, and the non-AKI group of 23.84 (13.82, 38.72), and 16.31(10.5, 31.25) days, respectively (P = 0.9403 and P = 0.655, respectively) (Table 2).
Sensitivity analyses for these above parameters as time to discharge (taking into account censored data due to mortality) yielded similar results.
Discussion
The RIFLE, AKIN, and KDIGO criteria for AKI have recognized the need to incorporate smaller time intervals for the assessment of kidney injury using UO [2, 16]. A recent study in ICU and cardiac surgery patients compared consecutive hourly measurements of UO to mean UO over the time measured. In the ICU patients it was shown that using a mean UO, rather than consecutively measured hours, led to misclassification of the KDIGO severity stage. For cardiac surgery patients the study found that averaging UO over time, versus consecutive hourly measurements, significantly overestimated the incidence of AKI [9].
Detection of oliguria is limited by the available tools, human error, and varying approaches to its definition. A reliable method for consecutive measurements of UO would provide a consistent application of the KDIGO UO criteria for diagnosis of AKI. In this study we demonstrated that consecutive electronically monitored UO identified significantly more cases of AKI according to the KDIGO criteria that were not identified by SCr alone. Additionally, in patients that fulfilled AKI definitions using both the SCr and UO criteria, UOelec measurement identified AKI significantly earlier than SCr.
The advantage of continuous measurements of UO was presented in a study showing oliguric periods as a predictor of higher mortality in critically ill patients. The authors discussed the need for a broader understanding of UO as a continuous physiological variable as opposed to one measured in set intervals [17].
It has also been argued that UO is too sensitive a biomarker for identifying AKI since oliguria may occur in response to normal physiological mechanisms [16, 18,19,20]. However, many studies confirm that oliguria, even in the absence of a rise in creatinine, identifies patients who have worse short-term and long-term outcomes, increased LOS, increased severity of AKI, and require more dialysis [1, 3, 4, 13, 17].
Our study was blinded, and UO for the patients was not documented in their records according to the electronic monitoring. The course of patient treatment was not based upon the consecutive electronic monitoring of UO, so the electronic monitoring did not impact ICU outcomes. This may explain why the patients with AKI as defined by UOelec had a similar LOS to that of the non-AKI population. However, the extended time in the hospital after ICU discharge for the patients with AKI as defined by UOelec (> 5 days in the general population and 8 days in the under-70 group Table 2) compared to the non-AKI patients, suggest there may be clinical implications for earlier identification of AKI using UOelec.
Retrospective studies have shown a prolonged LOS and higher mortality in the ICU and cardiac surgery patients that had AKI according to both the UO and SCr criteria [8, 21]. Physicians in our study were not blinded to SCr measurements (as they were with the UOelec measurements), which may have contributed to the LOS in the ICU observed in the AKI-SCr group in our study. This may be further highlighted with the similar overall LOS in the hospital in the AKI-SCr (20.39 days) and the AKI-UOelec′ (20.27 day) groups, despite the significant difference between these groups for their median LOS while in the ICU.
A retrospective study in over 15,000 adults compared intensive vs non-intensive monitoring of UO and intensive vs non-intensive SCr monitoring. Intensive UO monitoring was shown to be independently associated with improved survival to 30 days among patients developing AKI. Intensive monitoring of SCr had no effect on 30-day mortality associated with AKI. With or without AKI, patients who had intensive UO monitoring had significantly less cumulative fluid volume and fluid overload. They were also significantly less likely to receive vasopressors over the first 72 h of their ICU stay [22]. Intensive monitoring in that study was considered a measurement recorded at least once every 3 h and was exclusively manual monitoring of UO. Our study shows that automated, continuous, consecutive hourly monitoring of UO can provide a uniform application of KDIGO criteria to identify AKI early and in real-time. This may provide support to improve outcomes similar to that found in intensive manual monitoring, and merits further study. Furthermore, a uniform and consistent application of UO data for early identification of AKI may enable a consensus definition for, and earlier intervention to prevent progression of the disease [17].
Study limitations
In our study, UOelec measurements were recorded every hour and SCr measurements were taken twice a day (which is considered very frequent for standard of care [22]). This gives UOelec a certain advantage in identifying AKI by assessing the previous 6 hours once an hour, while SCR can only identify AKI approximately once in 12 h. This inherent limitation also highlights an inherent advantage of the use of UOelec criteria for the early identification of AKI. This is unlikely to change due to the labor-intensive and invasive nature of SCr tests.
Due to the nature of the pilot study, our patient population was limited in size. Follow-up for readmissions within 3 months after hospital discharge and 1 year mortality, as well as time to RRT were too small for analysis. Further prospective studies using real-time continuous UOelec monitoring on larger patient cohorts that includes tracking fluid balance, and fluid response to diuretics should be designed to assess the advantage of early detection of AKI and these additional criteria.
Conclusion
Electronic UO monitoring provides a uniform and consistent definition for identifying AKI patients in the ICU according to the KDIGO UO criteria. Application of KDIGO criteria for AKI using continuous monitoring of UO identifies more AKI patients, and identifies them earlier, than using the SCr criteria alone. This tool can enable the clinician to set protocol goals for earlier intervention for the prevention or treatment of AKI.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum Creatinine level. J Am Soc Nephrol. 2015;26(9):2231–8. https://doi.org/10.1681/ASN.2014070724.
Section 2: AKI Definition. Kidney Int Suppl (2011). 2012 Mar;2(1):19–36. doi: https://doi.org/10.1038/kisup.2011.32.
Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012 May;81(9):819–25. https://doi.org/10.1038/ki.2011.339.
Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X. Beijing Acute Kidney Injury Trial (BAKIT) workgroup. a comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18(4):R144. https://doi.org/10.1186/cc13977.
Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care. 2007;13(6):638–44. https://doi.org/10.1097/MCC.0b013e3282f07570.
Galley HF. Can acute renal failure be prevented? J R Coll Surg Edinb. 2000;45(1):44–50 PMID: 10815380.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–53. PMID: 1394976. https://doi.org/10.1093/clinchem/38.10.1933.
Allen JC, Gardner DS, Skinner H, Harvey D, Sharman A, Devonald MAJ. Definition of hourly urine output influences reported incidence and staging of acute kidney injury. BMC Nephrol. 2020;21(1):19. https://doi.org/10.1186/s12882-019-1678-2.
Koeze J, Keus F, Dieperink W, van der Horst IC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18(1):70. https://doi.org/10.1186/s12882-017-0487-8.
Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26(2):509–15. https://doi.org/10.1093/ndt/gfq332.
Fujii T, Uchino S, Takinami M, Bellomo R. Validation of the kidney disease improving global outcomes criteria for AKI and comparison of three criteria in hospitalized patients. Clin J Am Soc Nephrol. 2014;9(5):848–54. https://doi.org/10.2215/CJN.09530913.
Cruz DN, Ricci Z, Ronco C. Clinical review: RIFLE and AKIN-time for reappraisal. Crit Care. 2009;13(3):211. https://doi.org/10.1186/cc7759.
Bedford M, Stevens PE, Wheeler TW, Farmer CK. What is the real impact of acute kidney injury? BMC Nephrol. 2014;15(1):95. https://doi.org/10.1186/1471-2369-15-95.
Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J. Traumabase® Group. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care. 2018;22(1):344. https://doi.org/10.1186/s13054-018-2265-9.
Goldman A, Azran H, Stern T, et al. A novel electronic device for measuring urine flow rate: a clinical investigation. Clin Med Insights. 2017;8:1–6. https://doi.org/10.1177/117956031730032.
Lehner GF, Forni LG, Joannidis M. Oliguria and biomarkers of acute kidney injury: star struck lovers or strangers in the night? Nephron. 2016;134(3):183–90. https://doi.org/10.1159/000447979.
Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011;80(7):760–7. https://doi.org/10.1038/ki.2011.150.
Prowle JR, Liu YL, Licari E, Bagshaw SM, Egi M, Haase M, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care, 2011. 15(4):R172. https://doi.org/10.1186/cc10318.
Klein SJ, Lehner GF, Forni LG, Joannidis M. Oliguria in critically ill patients: a narrative review. J Nephrol. 2018;31(6):855–62. https://doi.org/10.1007/s40620-018-0539-6.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network. acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. https://doi.org/10.1186/cc5713.
Howitt SH, Grant SW, Caiado C, Carlson E, Kwon D, Dimarakis I, et al. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: a validation study. BMC Nephrol. 2018;19(1):149. https://doi.org/10.1186/s12882-018-0946-x.
Jin K, Murugan R, Sileanu FE, Foldes E, Priyanka P, Clermont G, et al. Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest. 2017;152(5):972–9. https://doi.org/10.1016/j.chest.2017.05.011.
Acknowledgements
We would like to acknowledge Aliza Rozenberg and Ilan Yanuv from Global Statistics for their contribution to the statistical analysis of the data in this study. Thank you to the dedicated GICU nursing staff at Hadassah Medical Center for their cooperation and support of this study.
Funding
RenalSense provided all equipment, supplies and funding for this study.
Author information
Authors and Affiliations
Contributions
DW- Study conception, study design, data interpretation, and project manager. AG- review of data integrity, drafting of manuscript and preparation of Figs. HA- IRB submission, data collection setup and design. TS- Data collection, review of data integrity and support for statistical review by the outsourced biostatistics analysis company. DK- Study design, patient selection. GR- Study design, study PI, manuscript review and edit. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations.
Local IRB approval was obtained from the Hadassah Medical Center Helsinki Ethics Committee and because this was a non-invasive observational trial and standard of care was not altered in any way, informed consent was waived.
Consent for publication
Not applicable.
Competing interests
Goldman A, Azran H, and Stern T are employees of RenalSense. Kirshenbom D is the head nurse of the GICU at Hadassah Medical Center and has disclosed no competing interests. Willner D was affiliated with Hadassah Medical Center in the Anaesthesiology Department at the time of the study and has disclosed no competing interests. Rosenthal G is affiliated with Hadassah Medical Center as the head of the Neurological ICU and has disclosed no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Willner, D., Goldman, A., Azran, H. et al. Early identification of acute kidney injury in the ICU with real-time urine output monitoring: a clinical investigation. BMC Nephrol 22, 293 (2021). https://doi.org/10.1186/s12882-021-02485-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-021-02485-w