Abstract
Background
A relationship exists between birth weight (BW) and glomerular filtration rate (GFR) in postnatal kidney. Willing to fill a gap of knowledge in sub-Saharan Africa, we assessed the effect of BW on blood pressure (BP), proteinuria and GFR among Cameroonians children.
Methods
This was a cross-sectional hospital-based study from January to April 2018 at the Yaounde Gynaeco-Obstetric and Paediatric Hospital (YGOPH). We recruited low BW (LBW) [< 2500 g], normal BW (NBW) [2500-3999 g] and high BW (HBW) [> 4000 g] children, aged 5–10 years, born and followed-up at YGOPH. We collected socio-demographic, clinical (weight, height, BP), laboratory (proteinuria, creatinine), maternal and birth data. The estimated GFR was calculated using the Schwartz equation.
Results
We included 80 children (61.2% boys) with 21 (26.2%) LBW, 45 (56.2%) NBW and 14 (15.5%) HBW; the median (interquartile range) age was 7.3 (6.3–8.1) years and 17 (21.2%) were overweight/obese. Two (2.5%) children, all with a NBW (4.4%), had an elevated BP whereas 2 (2.5%) other children, all with a LBW (9.5%), had hypertension (p = 0.233). Seven (8.7%) children had proteinuria with 19, 2.2 and 14.3% having LBW, NBW and HBW, respectively (p = 0.051). Equivalent figures were 18 (22.5%), 14.3, 24.2 and 28.6% for decreased GFR, respectively (p = 0.818). There was a trend towards an inverse relationship between BW and BP, proteinuria and GFR (p > 0.05).
Conclusion
Proteinuria is more pronounced in childhood with a history of LBW and HBW while LBW children are more prone to develop hypertension. Regular follow-up is needed to implement early nephroprotective measures among children with abnormal BW.
Similar content being viewed by others
Background
There is a correlation between birth weight (BW) and glomerular number, density, volume, size and filtration rate in postnatal kidney [1,2,3,4,5,6]. This is supported by Barker’s and Brenner’s hypothesis; the latter, which supports the intrauterine origin of later-life health, explains the relationship between BW and the number of nephrons as well as the risk of developing hypertension and chronic kidney disease (CKD). Consequently, long-term cardiovascular and renal health disorders are likely to occur [7,8,9].
It has been evidenced that low birth weight (LBW), prematurity and growth restriction are markers of an adverse intrauterine environment whereas high birth weight (HBW), exposure to maternal diabetes and rapid growth during early childhood emerge as developmental risk factors for chronic diseases [10]. Compared with normal birth weight (NBW), LBW is associated with reduced kidney volume, increased risk of perinatal morbi-mortality, underweight and short stature. It is also linked to elevated blood pressure, hypertension, proteinuria, natriuresis, podocytopenia and focal segmental glomerulosclerosis, and occurrence and rapid progression of CKD [4, 5, 9,10,11,12,13,14,15,16]. Moreover, studies revealed that LBW is associated with male sex predilection of CKD and early onset of end stage renal disease in patients with autosomal dominant polycystic kidney disease, without racial predominance [12, 17,18,19]. In contrast, HBW increases the risk of hypertension in children and decreases the risk in adults; furthermore, it increases proteinuria in children with maternal diabetes as well as the risk of diabetes-associated end stage renal disease [10].
In sub-Saharan Africa, the relationship between BW and renal markers has been scarcely explored, especially in school-aged children. Studies assessing the relationship between BW and blood pressure revealed a consistently positive association in neonates, an inverse association among children and inconsistent results in adolescents [20,21,22,23,24,25]. LBW was also identified as a non-traditional risk factor for CKD in this setting, and contributed to the burden of disease [26].
In Cameroon, CKD is highly prevalent in adult populations, ranging from 10 to 14.2% and driven by known modifiable risk factors for chronic nephropathy [27, 28]. In children, studies revealed no association between BW and blood pressure (BP) [29, 30]. However, no study had yet assessed the spectrum of renal markers according to BW among children. In this context, we undertook the present study aiming to evaluate the relationship between BW and blood pressure, proteinuria and glomerular filtration rate (GFR) in children aged 5–10 years and living in Yaoundé, Cameroon.
Methods
Study design and setting
This was a cross-sectional hospital-based study carried out over a period of 4 months (January to April 2018) at the Yaounde Gynaeco-obstetric and Paediatric Hospital (YGOPH). The YGOPH is one of the tertiary health care facilities in Yaounde, the capital city of Cameroon. Inaugurated in 2002, the YGOPH has a capacity of 240 beds; it offers mainly gynaeco-obstetric and paediatric care to patients. The study was approved by the Institutional Review Board of the Faculty of Heath Sciences of the University of Buea and the Ethical Committee of the YGOPH.
Study participants
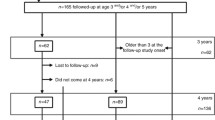
The study involved children between 5 and 10 years, born between 2007 and 2012 at YGOPH, who used to consult in this hospital for pediatric problems, and whose parents/guardians gave their consent. We excluded from the study children suffering from renal and urinary tract malformations, CKD, diabetes, HIV infection, hepatitis B and C infection, and sickle cell disease. Children born between 2007 and 2012 were identified from the maternity registers and recorded in a book. Based on the BW of the maternity register, children were divided into 3 groups: LBW (< 2500 g), NBW (2500-3999 g) and HBW (≥4000 g). We used the sample size calculation formula for cross-sectional studies and considered the clinical prevalence of hypertension in children of 3.5% to obtain a minimum sample size of 70 participants [31, 32]; we selected 1 HBW child for 2 LBW and 4 NBW children. The recruitment of LBW and HBW participants was consecutive on their appearance in the register while NBW children were randomly selected. In case of refusal to participate, the next name on the register was selected and the procedure repeated.
Data collection
For each eligible participant, the child’s parent/guardian was contacted through a phone call during which the study procedures were fully explained and an appointment fixed based on their availability. During the meeting with the parent/guardian and their child, the study was once again explained to them and an assent form signed. A self-designed and pre-tested questionnaire was used for data collection. Data collected included socio-demographic details (age, sex), clinical characteristics (weight, height, systolic and diastolic blood pressure), maternal history of pregnancy (age, type of pregnancy, maternal illness, smoking and alcohol consumption), birth characterisitics (weight, gestational term and age, and history of child reanimation and/or hospitalization) and laboratory parameters (proteinuria, and serum and urinary creatinine). We used an appropriate cuff size of 13.5 to 22 cm according to the American Academy of Pediatrics guidelines to measure BP [32]. After 5 min of rest with the participant in the sitting position, the back supported and feet uncrossed on the floor, we used an automated sphygmomanometer (OMRON HEM705CP, Omron Matsusaka Co, Matsusaka City, Mie-Ken, Japan) to measure BP on the right arm placed at the level of the heart, stretched out on the table with the palm facing up. The cuff was appropriately placed and then the machine was switched on. Three readings were taken consecutively and their mean calculated and recorded. Using BP table levels for sex, age and height percentiles, the BP percentile was recorded. When the BP was ≥90th percentile, it was repeated weekly up to two times; when it remained the same on the third measurement, it was measured using an aneroid sphygmomanometer twice consecutively; the mean of these measures were calculated and recorded as the final value.
Each participant provided 50 ml first morning mid-stream urine for an immediate semi-quantitative measurement of dipstick proteinuria using the CombiScreen 7SL PLUS 7 test strips (Analyticon Biotechnologies AG, D-35104 Lichentenfeis, Germany). Participants with at least traces on urine dipstick for proteinuria on the first sample were given an appointment 1 week later to repeat the urine dipstick. Those with a second urine sample still showing at least traces for proteinuria were seen 1 week later for another urine dipstick test. When the proteinuria persisted even just as traces after the second repeated urine dipstick, we proceeded to estimate the 24-h proteinuria from urine protein to creatinine ratio (PCR). We equally collected 3 ml of whole blood from an antecubital vein for serum creatinine and subsequent calculation of the GFR. Urine and blood samples were transported to the laboratory for processing. Serum and urinary creatinine were measured with a kinetic modification of the Jaffé reaction using a Human visual spectrophotometer (Human Gesellschaft, Biochemica und Diagnostica mbH, Wiesbaden, Germany) and Beckman creatinine analyzer (Beckman CX systems instruments, Anaheim, CA, USA) while urinary protein was measured using pyrogallol red-molybdate complex with Teco diagnostics tests (Teco Diagnostics, Anaheim, CA, USA).
Definitions and calculations
Delivery was categorized as preterm (< 37 weeks of gestation), normal (37 to 42 weeks) or post-term (> 42 weeks). Small for gestational age (SGA) was defined by BW < 10th centile for that gestational age (GA) whereas large for GA (LGA) was a BW > 90th centile for that GA and appropriate for GA (AGA) corresponded to BW between the 10th and 90th centile for that GA. We grouped children in percentiles using the World Health Organisation height and weight for age percentile reference charts release in 2007 [33]. According to weight, underweight (<5th percentile for age), normal weight (5- < 95th percentile for age) or overweight (≥95th percentile for age) were distinguished. For height, short stature (< 5th percentile for age), normal height (5- < 95th percentile for age) or tall stature (≥95th percentile for age) were considered. BMI was estimated as weight (kg)/square height (m2). It was stratified into underweight (<5th percentile for age and sex), normal weight (5-85th percentile for age and sex), overweight (85-95th percentile for age and sex) and obesity (≥95th percentile for age and sex). BP was either normal [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] <90th percentile for sex, age and height), elevated (SBP and/or DBP ≥90- < 95th percentile for sex, age and height) whereas hypertension was defined as SBP and/or DBP ≥ 95th percentile for sex, age and height after three occasions according to the American Academy of Pediatrics guidelines to define BP categories and stages [32]. Dipstick proteinuria was defined by a persistent proteinuria (at least traces) after three measurements. The 24 h proteinuria was estimated from PCR and proteinuria corresponded to a PCR ≥ 200 mg/g. The Schwartz equation was used for estimate glomerular filtration rate (eGFR) [34]; it was either increased (≥120 ml/min/1.73 m2), normal (90- < 120 ml/min/1.73 m2) or decreased (< 90 ml/min/1.73 m2).
Statistical analysis
Data were entered and coded using EPI info version 7.0 and analysed using Statistical Package for Social Science (SPSS) version 23.0. Considering the non-Gaussian distribution of continuous variables, medians and interquartile ranges (IQR) were computed for continuous variables. Frequencies and proportions were computed for categorical variables. Frequencies were compared using the Fisher exact test or the Chi-square test where appropriate. To compare continuous variables according to BW strata, we used the non-parametric U-test of Mann-Whitney or the H-test of Kruskal-Wallis, where indicated. The Spearman correlation was used to correlate the BW with other continuous variables. A p-value was considered statistically significant at < 0.05.
Results
Sociodemographic and anthropometric characteristics of the study population
We included 80 children among whom 49 (61.2%) boys divided into 21 (26.2%) LBW, 45 (56.2%) NBW and 14 (15.5%) HBW. The median (IQR) age was 7.3 (6.3–8.1) years with no significant difference according to BW groups (p = 0.32). The median (IQR) for weight, height and BMI were respectively 23.3 (21.0–27.8) kg, 124 (116–134) cm and 15.3 (14.6–16.8) kg/m2 with no significant difference with respect to BW strata (all p > 0.211). There were 17 (21.2%) overweight/obese children without any difference with BW groups (p = 0.665), Table 1.
Maternal and birth history of participants
As presented in Table 2, the median (IQR) maternal age was 28 (23.2–32.0) years with no significant difference in BW (p = 0.486). Amongst the 10 (12.5%) of multiple pregnancies, 9 (90%) led to LBW infants with a statistical significance (p < 0.001). We observed that 3 (3.7%) women had diabetes mellitus among whom 2 (66.7%) delivered HBW children with a statistical significance (p = 0.048). In Table 3, the median (IQR) BW was 3200 (2421.5–3678.8) g with 2150 (1885–2375) g for LBW, 3200 (2900–3500) g for NBW and 4350 (4165–4642) g for HBW children (p < 0.001). The median (IQR) gestational term was 39 (38–40) weeks, with 10 (47.6%) LBW children born before 37 weeks (p < 0.001). All LBW children born before 37 weeks had a BW SGA whereas 1 (2.2%) NBW was LGA.
Blood pressure characteristics
The median (IQR) SBP and DBP was respectively 91 (85.3–97.8) mmHg and 56 (52.0–58.8) mmHg, without any statistical significance according to BW groups (all p > 0.187). There were 2 (2.5%) children with an elevated BP; both had a NBW, giving a prevalence of 4.4% of NBW children with an elevated blood pressure. Hypertension was observed in 2 (2.5%) children who all had a LBW, giving a prevalence of 9.5% among LBW population (see Table 4). None of the HBW children had an elevated BP or hypertension. Hypertension and elevated BP were not significantly associated with BW (p = 0.233).
Proteinuria and glomerular filtration rate characteristics
In Table 5, dipstick positive proteinuria was observed in 15 (18.8%) children, with a significantly higher prevalence in LBW (28.6%) and HBW (35.7%) children compared with NBW (8.1%) ones (p = 0.033). For the 15 children who performed PCR, the median (IQR) PCR was 185 (130–373) mg/g without any significant difference according to BW classes (p = 0.228). A proteinuria was noticed in 7 (8.7%) children with a higher prevalence in LBW (19%) and HBW (14.3%) in comparison to NBW (2.2%) children, at the limit of statistical significance (p = 0.051). The median (IQR) eGFR was 105.5 (90–118) ml/min/1.73m2 without any significant difference with regards to BW groups (p = 0.330). There was a decreased eGFR in 18 (22.5%) children with an increased prevalence according to BW groups, ranging from 3 (14.3%) LBW, 11 (24.4%) NBW to 4 (28.6%) HBW children (p = 0.818).
Effect of prematurity, weight for gestational age and overweight/obesity on blood pressure, proteinuria and glomerular filtration rate
As presented in Table 6, GA did not significantly affect any of these parameters. When comparing with NBW, LBW had significantly higher SBP (p = 0.029); however, LBW and HBW children had a trend toward an increase DBP, PCR and eGFR with no statistical significance. We observed that overweight/obese children had significantly high SBP and DBP, and a reduced eGFR (all p < 0.037).
Correlations between birth weight and variables
There was an inverse relationship between BW and SBP, DBP, PCR and eGFR although with no significant difference (all p > 0.05). However, we observed a significant weak positive correlation between BW and weight (r = 0.231) as well as BMI (r = 0.269), and a negative correlation between BW and weight percentile (r = − 0.241) (all p < 0.039) (Table 7).
Discussion
This study revealed a high prevalence of elevated blood pressure and hypertension, observed respectively in NBW and LBW children only. Nearly one out of ten children had proteinuria which was significantly associated with LBW and HBW. More than one out of five children presented with a decreased eGFR associated with an increased prevalence with regards to BW. We noticed an inverse relationship between BW and renal markers (blood pressure, proteinuria and GFR), with no significant association.
The reported high prevalence of hypertension as well as an elevated blood pressure were previously observed in this setting [30]. We did not find any association between BW and BP overall as well as with SBP or DBP as reported earlier [29, 30]. However, we found that all children with hypertension were of the LBW group, which could be explained by the Brenner’s hypothesis. Indeed, it supports a relationship between BW and the number of nephrons as well as the risk of developing hypertension [8, 20, 21]. Regarding the presence of hypertension in only LBW children, there is need for regular BP follow-up in this group of children.
Further, we observed that LBW and HBW children had significant proteinuria compared to NBW children, as reported elsewhere [10, 12]. This could be related to hyperfiltration mechanisms related either to reduced glomerular number in LBW infants, the association between HBW and maternal diabetes or glomerular lesions such as podocytopenia and focal segmental glomerulosclerosis [10].
There was no significant association between eGFR and BW as reported elsewhere in children with similar age using creatinine [19, 35, 36]. However, the eGFR using cystatin C showed an inverse relationship with BW [36]. This could be explained by the fact that cystatin C reflects better the eGFR in comparison to creatinine and suggests its preferential use in LBW children. Nevertheless, higher GFRs turned to be more frequent in the LBW children compared to NBW and HBW. This could be explained by the fact that LBW is associated with a reduced number of nephrons and a low glomerular density at birth; glomerular enlargement volume, hyperfiltration, increased GFR and proteinuria occur during childhood and adolescence due compensatory maladaptive changes as consequences of compromised nephrogenesis; furthermore with advanced age favored by overweight/obesity, there will be a reduction in the GFR and worsening of proteinuria due to focal segemental glomerulosclerosis lesions [1,2,3, 37].
We observed an inverse relationship between BW and renal markers without any statistical significance as previously observed [21, 29]. However, studies in SSA reported a significant inverse relationship between BW and BP [20, 25]. Meanwhile, some other studies showed a positive correlation between BW and SBP at birth [24]. The difference of correlation between renal markers and BW observed in this study compared to others could be related to the difference in the age of participants and the method of renal markers measurement.
Strengths and limitations
The main limitations of this study were the estimation of GFR with creatinine instead of cystatin C which reflects better, the kidney function. Furthermore, the small sample size as well as the number of events might have an influence on possible lack of associations. Nevertheless, this study is the first in Central Africa, to the very best of our knowledge, to assess the relationship between BW and overall renal markers in childhood. We used up-to-date guidelines for blood pressure and proteinuria diagnosis in children [32, 38]. These findings contribute to enrich data on renal markers and BW in the sub-Saharan Africa setting and suggest further research using cystatin C-based equations for eGFR.
Conclusion
The present study shows that childhood life with history of abnormal birth weight can be impacted by foetal life; thus affecting BP and urine protein excretion rate. This study highlighted the importance for regular follow-up of children with LBW and HBW in order to implement early nephroprotective measures. Therefore, lifestyle education is a key; it can begin to be delivered to mothers of at risk babies identified in the delivery room and during the baby and maternal follow-up. We also suggest the implementation of annual screening of at risk children, overweight or obese, in order to identify hypertension, proteinuria and reduced eGFR.
Availability of data and materials
Data and materials are available with corresponding author which is the principal investigator. They can be consulted at anytime upon request. However, the ethical clearance and the inform consent form did mention that patient data could be share to a third party.
Abbreviations
- AGA:
-
Appropriate for gestational age
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- HBW:
-
High birth weight
- GA:
-
Gestational age
- IQR:
-
Interquartile range
- LGA:
-
Large for gestational age
- LBW:
-
Low birth weight
- NBW:
-
Normal birth weight
- PCR:
-
Proteinto creatinine ratio
- SBP:
-
Systolic blood pressure
- SCr:
-
Serum creatinine
- SGA:
-
Small for gestational age
References
Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63(6):2113–22.
Koike K, Ikezumi Y, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, et al. Glomerular density and volume in renal biopsy specimens of children with proteinuria relative to preterm birth and gestational age. Clin J Am Soc Nephrol. 2017;12(4):585–90.
Hoy WE, Hughson MD, Zimanyi M, Samuel T, Douglas-Denton R, Holden L, et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin Nephrol. 2010;74(Suppl 1):S105–12.
Starzec K, Klimek M, Grudzien A, Jagla M, Kwinta P. Longitudinal assessment of renal size and function in extremely low birth weight children at 7 and 11 years of age. Pediatr Nephrol. 2016;31(11):2119–26.
Iyengar A, Nesargi S, George A, Sinha N, Selvam S, Luyckx VA. Are low birth weight neonates at risk for suboptimal renal growth and function during infancy? BMC Nephrol. 2016;17(1):100.
Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58(2):770–3.
Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7.
Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–47.
Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21(6):898–910.
Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes--a global concern. Nat Rev Nephrol. 2015;11(3):135–49.
Reyes L, Manalich R. Long-term consequences of low birth weight. Kidney Int Suppl. 2005;97:S107–11.
Li S, Chen SC, Shlipak M, Bakris G, McCullough PA, Sowers J, et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 2008;73(5):637–42.
Schmidt IM, Chellakooty M, Boisen KA, Damgaard IN, Mau Kai C, Olgaard K, et al. Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int. 2005;68(2):731–40.
Zanardo V, Fanelli T, Weiner G, Fanos V, Zaninotto M, Visentin S, et al. Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int. 2011;80(1):119–23.
Ikezumi Y, Suzuki T, Karasawa T, Yamada T, Hasegawa H, Nishimura H, et al. Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol. 2013;38(2):149–57.
Vasarhelyi B, Dobos M, Reusz GS, Szabo A, Tulassay T. Normal kidney function and elevated natriuresis in young men born with low birth weight. Pediatr Nephrol. 2000;15(1–2):96–100.
Orskov B, Christensen KB, Feldt-Rasmussen B, Strandgaard S. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int. 2012;81(9):919–24.
Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69(4):671–8.
Cassidy-Bushrow AE, Wegienka G, Barone CJ 2nd, Valentini RP, Yee J, Havstad S, et al. Race-specific relationship of birth weight and renal function among healthy young children. Pediatr Nephrol. 2012;27(8):1317–23.
Woelk G, Emanuel I, Weiss NS, Psaty BM. Birthweight and blood pressure among children in Harare, Zimbabwe. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F119–22.
Chiolero A, Paradis G, Madeleine G, Hanley JA, Paccaud F, Bovet P. Birth weight, weight change, and blood pressure during childhood and adolescence: a school-based multiple cohort study. J Hypertens. 2011;29(10):1871–9.
Lule SA, Elliott AM, Smeeth L, Webb EL. Is birth weight associated with blood pressure among African children and adolescents? A systematic review. J Dev Orig Health Dis. 2018;9(3):270–80.
Longo-Mbenza B, Ngiyulu R, Bayekula M, Vita EK, Nkiabungu FB, Seghers KV, et al. Low birth weight and risk of hypertension in African school children. J Cardiovasc Risk. 1999;6(5):311–4.
Nwokoye IC, Uleanya ND, Ibeziako NS, Ikefuna AN, Eze JC, Ibe JC. Blood pressure values in healthy term newborns at a tertiary health facility in Enugu, Nigeria. Niger J Clin Pract. 2015;18(5):584–8.
Levitt NS, Steyn K, De Wet T, Morrell C, Edwards R, Ellison GT, et al. An inverse relation between blood pressure and birth weight among 5 year old children from Soweto, South Africa. J Epidemiol Community Health. 1999;53(5):264–8.
Katz I. Kidney and kidney related chronic diseases in South Africa and chronic disease intervention program experiences. Adv Chronic Kidney Dis. 2005;12(1):14–21.
Kaze FF, Halle MP, Mopa HT, Ashuntantang G, Fouda H, Ngogang J, et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol. 2015;16:96.
Kaze FF, Meto DT, Halle MP, Ngogang J, Kengne AP. Prevalence and determinants of chronic kidney disease in rural and urban Cameroonians: a cross-sectional study. BMC Nephrol. 2015;16:117.
Youmbissi TJ, Oudou N, Mbede J, Nasah BT. Blood pressure profiles of a group of African children in the first year of life. J Trop Pediatr. 1989;35(5):245–6.
Chelo D, Mah EM, Chiabi EN, Chiabi A, Koki Ndombo PO, Kingue S, et al. Prevalence and factors associated with hypertension in primary school children, in the Centre region of Cameroon. Transl Pediatr. 2019;8(5):391–7.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–6.
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and Management of High Blood Pressure in children and adolescents. Pediatrics. 2017;140:3.
World Health Organization. Available from: https://www.who.int/growthref/who2007 (Accessed 7 Dec 2017).
Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am. 1987;34(3):571–90.
Vanpee M, Blennow M, Linne T, Herin P, Aperia A. Renal function in very low birth weight infants: normal maturity reached during early childhood. J Pediatr. 1992;121(5 Pt 1):784–8.
Franco MC, Nishida SK, Sesso R. GFR estimated from cystatin C versus creatinine in children born small for gestational age. Am J Kidney Dis. 2008;51(6):925–32.
Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–77.
K/DIGO Guidelines. Definition and classification. Kidney Int Suppl. 2013;3(1):19–62.
Acknowledgements
We thank the staff of paediatric service of the Yaounde Gynaeco-obstetric and Paediatric hospital. Gratitude goes to DR MANGA and technicians of «Laboratoire d’Analyses Medicales du Dr. MANGA».
Funding
The authors did not receive any fund for this study.
Author information
Authors and Affiliations
Contributions
FFK: Conception and design of the study, supervision of data collection, interpretation of data and drafting of the manuscript. SN: Conception and design of the study, supervision of data collection, interpretation of data and critical revision of the manuscript. CMA: Data collection and critical revision of the manuscript. JCAN:Conception and design of the study and critical revision of the manuscript. JRN: Data analysis and interpretation, and critical revision of the manuscript. MPK: Supervision of data collection, interpretation of data and critical revision of the manuscript. VN: Supervision of data collection, interpretation of data and critical revision of the manuscript. KGCM: Supervision of data collection, interpretation of data and critical revision of the manuscript. MPH: Conception and design of the study and critical revision of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Faculty of Heath Sciences of the University of Buea (Number: 2018/136/UB/SG/IRB/FHS) and the Yaounde Gynaeco-obstetric and Paediatric Hospital (Number: 646/CIERSH/DM/2017). All parents/guardians of the participants provided a written informed consent before enrolment.
Consent for publication
All authors gave their approval for publication.
Competing interests
The authors report no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kaze, F.F., Nguefack, S., Asong, C.M. et al. Birth weight and renal markers in children aged 5–10 years in Cameroon: a cross-sectional study. BMC Nephrol 21, 464 (2020). https://doi.org/10.1186/s12882-020-02133-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-02133-9