Abstract
Background
In Malaysia, the prevalence of chronic kidney disease is high (9.1%). To date, no questionnaire that specifically assesses the health-related quality of life of patients with chronic kidney disease has been validated in Malaysia. Malay is the national language of Malaysia and spoken by the majority of its citizens. Therefore, the aim of our study was to cross-culturally adapt and validate the Malay Kidney Disease Quality of Life-36 (KDQOL-36) among patients with chronic kidney disease.
Methods
The English version of the KDQOL-36 was translated according to international guidelines to Malay. Content validity was verified by an expert panel and piloted in five patients. Our instrument was then administered to patients with chronic kidney disease stage 1-3A and patients on hemodialysis at baseline and 4 weeks later.
Results
A total of 181/232 patients agreed to participate (response rate = 78.0%). The majority were male (69.6%) with a median age of 51.0 years. Exploratory factor analysis found that the KDQOL-36 had three domains. All three domains showed low to moderate correlation (Spearman’s Rho = 0.297–0.610) with the Europe Quality of Life Five Dimension questionnaire. Patients on hemodialysis (physical component summary = 39.8; mental component summary = 53.1;burden of disease = 37.5; symptoms/burden list = 75.0; effects of kidney disease on daily life = 68.8) had significantly worse quality of life than patients with chronic kidney disease stage 1-3A (physical component summary = 49.9; mental component summary = 52.9; burden of disease = 75.0; symptoms/burden list = 85.4; effects of kidney disease on daily life = 93.8, p < 0.001) except for the mental component summary. This indicates that the Malay KDQOL-36 has achieved adequate known-groups validity. Cronbach alpha ranged from 0.872–0.901, indicating adequate internal consistency. At retest, intraclass correlation coefficient ranged from 0.584–0.902, indicating moderate to good correlation.
Conclusion
The Malay Kidney Disease Quality of Life-36 was found to be a valid and reliable tool to assess the quality of life in patients with chronic kidney disease. This tool can now be used to assess the health-related quality of life (HRQOL) in patients with chronic kidney disease, as HRQOL is an important independent predictor of patient outcome.
Similar content being viewed by others
Background
Globally, chronic kidney disease (CKD) is one of the important causes of mortality and morbidity [1]. More than ten million people worldwide have CKD which may progress to end stage renal disease (ESRD) [2]. In 2014, the prevalence of CKD in the United States was 13.6%. A 2016 review showed that the prevalence of CKD was higher in developed countries (such as the United States, Europe and Canada) than in economically developing countries (such as sub Saharan Africa and India) [3]. This may be due to the prevalence of higher dietary risks, body mass index (BMI), systolic blood pressure and co-morbid conditions in developing countries [3]. In Malaysia, the prevalence of CKD was 9.1% in 2011 [4]. In 2015, there were 37,183 patients receiving dialysis in Malaysia with 7597 new patients for dialysis [5].
Health-related quality of life (HRQOL) is a marker for burden of disease which can be used to assess the effectiveness of a treatment and predict the risk of adverse outcomes [1]. HRQOL is the patient’s subjective perception of their illness and treatment with regards to their physical, psychological and social-well-being [6]. Patients on dialysis have significant symptom burden and impaired quality of life, as they have a high number of comorbidities [7, 8]. In a study conducted in Hong Kong, patients on dialysis had a symptom burden of at least 9 symptoms (mean = 9.3 + 4.7) with fatigue (75.4%), cold aversion (68.7%), pruritus (65.7%), lower torso weakness (59.7%) and difficulty in sleeping (61.9%) as the most prevalent symptoms [8]. Dialysis patients with sleep disturbance was associated with lower HRQOL [9]. Therefore, assessing HRQOL of patients with ESRD is important as it is an independent predictor for patient outcomes.
A generic quality of life (QOL) tool (e.g. SF-12) is designed to assess the function and well-being of individuals regardless of their specific condition [10]; whilst a “disease targeted” HRQOL instrument assesses QOL in specific disease conditions [10]. The most comprehensive method for assessing QOL would be to include both generic and disease targeted content in the instrument [10]. Several tools such as Kidney Disease Questionnaire (KDQ) [11], Kidney Transplant Questionnaire (KTQ) [12] and Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) [13] have been developed for assessing HRQOL in patients with CKD [14]. Among these tools, we selected the Kidney Disease Quality of Life (KDQOL) as it was developed for individuals with kidney disease who may or may not be on dialysis [15]. The KDQOL also has adequate to excellent internal consistency [16]. The Kidney Disease Quality of Life-36 (KDQOL-36) is an instrument that consists of both a generic core (SF12) and disease specific components [10]. However, the KDQOL-36 has not been validated in Malaysia. When adapting questionnaire, the cultural, idiomatic, linguistic and contextual aspects concerning its translation should be considered [17]. Therefore, the cross-cultural adaptation of a health status self-administered questionnaire in a different language requires a unique method to reach equivalence between the source and target version of the questionnaire through the process of translation, adaptation and assessment of validity and reliability of the targeted questionnaire [17]. It was important for us to validate the KDQOL-36 in Malay as Malay is the national language of Malaysia and spoken by the majority of its citizens. In addition, the prevalence of CKD in Malaysia was 9.1% [4].
Therefore, the aim of this study was to cross-culturally adapt and validate the Malay Kidney Disease Quality of Life among patients with chronic kidney disease.
Methods
Translation of the English kidney disease quality of life (KDQOL-36™) to Malay
Permission to use the KDQOL-36 was obtained from the original developer (via email on 25 June 2016). Translation of the English KDQOL-36 to Malay was performed according to international guidelines (Additional file 1) [18].
Face and content validity
Face validity is defined as “the degree to which (the items of) an health-related-patient-reported outcome (HR-PRO) instrument indeed looks as though they are an adequate reflection of the construct to be measured” [19]. Content validity is defined as “the degree to which content of an HR-PRO instrument is an adequate reflection of the construct to be measured” [19] Face and content validity of the Malay KDQOL-36 was assessed by an expert panel (consisting of a nephrologist, an academician experienced in the validation of instruments and a pharmacist). A pilot study was then conducted on five patients with CKD stage 1-3A. Two participants were confused with item no. 3 (“climbing several flights of stairs”) as they were unsure whether the item meant “climbing several flights of stairs” or just “several steps”. In Malay, “climbing several flights of stairs” or just “several steps” are expressed in the same way. Hence, for this item, the researcher had to explain to each participant that this item meant “climbing several flights of stairs”.
Validation of the Malay kidney disease quality of Life-36
This validation study was conducted at the Nephrology clinic in a tertiary hospital and its affiliated dialysis centers located in Kuala Lumpur from July 2016 to July 2017.
Participants
Known-groups validity is demonstrated when a test or questionnaire can discriminate between two groups known to differ on the variable of interest [20]. Previous studies showed that HRQOL progressively declined across the stages of CKD [21,22,23]. Hence, two groups of participants were recruited so that known-groups validity could be assessed. We hypothesized that the HRQOL of patients on hemodialysis would be worse than patients with CKD stage 1-3A.
Patients on hemodialysis (patient group)
Patients > 21 years of age, who could understand Malay and were on hemodialysis for at least 3 months were recruited. Patients with mental disabilities were excluded.
Patients with chronic kidney disease stage 1-3A (control group)
Patients > 21 years of age, who could understand Malay and with CKD stage 1-3A (defined as glomerular filtration rate (eGFR) value of 45 to > 90 mL/min/1.73 m2 with evidence of kidney damage) were recruited. Patients who were diagnosed with rheumatoid arthritis, active cancer and mental disabilities were excluded.
Sample size
Sample size was calculated based on the number of items to participant ratio of 1:5 to perform factor analysis [24]. There are 36 items in the KDQOL-36. Therefore, the minimum number of participants required was 36*5 = 180.
Baseline demographic form
A baseline demographic from was used to collect participants’ baseline demographic data and other relevant information.
The Malay kidney disease quality of life 36
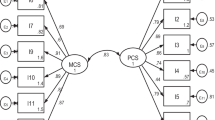
The KDQOL-36 is a self-administered tool which measures kidney disease-related HRQOL. The original tool consists of 134 items, and was too lengthy to administer in clinical practice [25]. Thus, a shorter version – the KDQOL-SF™ version 1.3 was developed. It consists of 36 items, and two cores: the SF-12 (i.e. generic QOL) and the disease-specific core. The generic core consists of two domains: the physical component summary (PCS) [6 items] and the Mental Component Summary (MCS) [6 items]. The disease-specific core consists of 24 items with 3 domains: symptoms and problems (12 items), burden of kidney disease (4 items) and effects of kidney disease (8 items). The raw scores were transformed according to the scoring manual [26] ranging from 0 to 100, where a higher score indicates better QOL. Patients that were not on hemodialysis were not required to answer item 28a “problems with your access point”.
The Malay EuroQol 5 dimensions questionnaire (EQ-5D-5L)
Convergent validity is the extent of different instruments to measure the same construct and that correlates with each other [27]. The Malay EQ-5D-5 L was used to assess the convergent validity of the KDQOL-36. The EQ-5D-5L consists of 5 items (which assesses five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a visual analog scale (EQ-VAS) [28]. The response for the five items was a 5-point Likert scale where 1 indicated better QOL, whilst 5 indicated poorer QOL. Scores were converted to 0 to 100%. The EQ-VAS requires patients to rate their own health using a scale which ranged from 0 (worst imaginable health) to 100 (best imaginable health).
Data collection
Convenience sampling was used to recruit participants. Potential participants were approached, and the purpose of the study was explained to them. For those who agreed to participate, written informed consent was obtained. At baseline, participants were asked to fill the baseline demographic form, the Malay KDQOL-36 and the EQ-5D-5L. One month later, participants were asked to answer the Malay KDQOL-36 again.
Data analysis
Data was analyzed using the Statistical Package for Social Science (SPSS) version 20.0 software (Chicago, Illinois, USA). Normality was assessed using the Kolmogorov Smirnov test. Non-parametric tests were used as data was not normally distributed. Descriptive statistics were used to describe the demographic data of participants. Categorical variables were presented using percentages and frequencies, whilst continuous variables were presented using median and interquartile ranges.
Validity
Validity is defined as “the degree to which HR-PRO instrument measures the construct(s) it purports to measure” [19].
Factor analysis
The dimensionality of the Malay KDQOL-36 was analyzed using exploratory factor analysis (EFA). Principal factor analysis and promax oblique rotation was used as the domains were correlated [29]. The cut-off point for the factor loadings was 0.4. [30].
Convergent validity
Convergent validity is defined as “the degree to which scores of a measure associate with scores on other measures that are intended to assess similar construct” [17]. The score for the three domains of the KDQOL-36 were compared with the scores of the EQ 5D5L and VAS. Correlations were calculated using Spearman’s rho coefficient: < 0.20 shows a very weak correlation, 0.20–0.40 shows weak correlation, 0.40–0.70 shows moderate correlation, 0.70–0.90 shows strong correlation and > 0.90 shows very strong correlation [31].
Known-groups validity
The Mann-Whitney-U-test was used to determine whether the Malay KDQOL-36 was able to discriminate between patients with stage 1-3A CKD (eGFR > 90–45 ml/min/1.73m2) and patients undergoing dialysis.
Reliability
Reliability is defined as “the degree to which the measurement is free from measurement error” [19] In the reliability section, we analyzed the data of both patients with stage 1-3A and patients on hemodialysis as a whole.
Internal consistency
Internal consistency is defined as “the degree of interrelatedness among the items” [19]. Internal consistency was assessed using Cronbach’s alpha coefficient to determine the extent that all items in a test measures the same concept [32]. This was done for the entire instrument, and for the different domains. Cronbach’s alpha < 0.70 have inadequate consistency; 0.70–0.90 suggests adequate internal consistency [32]. Corrected item-total correlation was also performed. Corrected item-total correlation > 0.4 is considered acceptable [33]. The effect of removing an item on Cronbach’s alpha was also determined.
Test-retest
The intra-class correlation coefficient (ICC) was used to analyze responses obtained at test and retest. Values > 0.9 indicate excellent reliability, 0.75–0.90 indicate good reliability; 0.5–0.75 indicate moderate reliability and < 0.5 indicate poor reliability [34].
Results
A total number of 181/232 agreed to participate (response rate = 78.0%) [Fig. 1]. The demographic characteristics of participants are shown in Table 1. The majority were male (69.6%) with median age of 51 years (Table 1).
Construct validity
Factor analysis
EFA found that the Malay KDQOL-36 was a 3-factor model (Table 2). The scree plot for each domain is provided in Additional file 2.
Convergent validity
The scores for the three domains in the KDQOL-36 were found to be significantly correlated to the EQ-5D-5L. However, the association between the KDQOL-36 and EQ-5D-5L was weak to moderate. The association between the domains “burden of disease”, “signs/symptoms list”, “effects of kidney disease on daily life” with the EQ-5D-5L was − 0.456, − 0.610 and − 0.588 while the association these domains with the EQ VAS was 0.297, 0.434 and 0.361, respectively.
Known-groups validity
Patients on hemodialysis (physical component summary = 39.8; mental component summary = 53.1; burden of disease = 37.5; symptoms/burden list = 75.0; effects of kidney disease on daily life = 68.8) had significantly worse quality of life than patients with chronic kidney disease stage 1-3A (physical component summary = 49.9; mental component summary = 52.9; burden of disease = 75.0; symptoms/burden list = 85.4; effects of kidney disease on daily life = 93.8, p < 0.001) except for the mental component summary, indicating that the Malay KDQOL-36 has achieved adequate known-groups validity (Table 3).
Reliability
The overall Cronbach alpha of the Malay KDQOL-36 was 0.715. Cronbach’s alpha values for the domain ranged from 0.872–0.901. At test-retest, the ICC of the KDQOL-36 showed moderate to good correlation (ICC = 0.584–0.902) (Table 4).
Discussion
The Malay KDQOL-36 was found to be a valid and reliable tool to assess the HRQOL of patients with CKD in Malaysia.
EFA found that the Malay KDQOL-36 was a 3-factor model: “burden of kidney disease”, “symptoms/burden list” and “effects of kidney disease on daily life”. Our findings were similar to a Singaporean study which reported that the KDQOL-36 was a three-factor model [35]. However, the authors of this study used confirmatory factor analysis to confirm the number of factors, whereas we used EFA. We were not able to analyze our data using CFA, as the minimum sample size required to conduct CFA was 315 [36].
The Malay KDQOL-36 was able to discriminate between patients who were on hemodialysis and early stage (CKD stage 1-3A) in all domains except for the mental component summary. In a previous study, the HRQOL of patients were discriminated based on subgroups of demographic data of patients of the study [37]. The study showed that being female, unemployed, having history of hospitalization during the past 6 months, and being on a longer duration of hemodialysis had worse HRQOL [37]. At present, no other study has assessed the discriminative validity of the KDQOL-36 using patients at different stages of CKD. [23].
The scores from the three domains of the Malay KDQOL-36 were significantly correlated to the EQ-5D-5L and EQ VAS score, which was similar to a previous study [38]. The correlation in our study was negative because for the KDQOL-36, as a higher KDQOL-36 score indicates a better QOL, whilst a higher score in EQ5D5L indicates a worse QOL.
The overall Cronbach alpha of the Malay KDQOL-36 was 0.715, whilst the Cronbach alpha of the individual domains ranged from 0.872–0.901, which was similar to a previous study [38]. At test-retest, ICC values ranged from 0.584–0.902, which was lower compared to previous studies [37, 38]. This was due to item no. 18 (“in the past 4 weeks, to what extend were you bothered by chest pain?”) where two participants selected the answer “not at all bothered” at test (Likert scale = 1), whilst at retest they answered, “extremely bothered” (Likert scale = 5). These patients may have experienced chest pain during the period between test and retest.
One of the limitations of our study was that we were unable to perform CFA as the minimum sample size required to perform this analysis was 315. Another limitation was that our patients were recruited using convenience sampling, and may not be representative of the general population [39].
Conclusion
The Malay KDQOL-36 was found to be a valid and reliable tool to assess the HRQOL in patients with CKD. This tool can now be used to assess the HRQOL in patients with chronic kidney disease, as HRQOL is an important independent predictor of patient outcome.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CFA:
-
Confirmatory factor analysis
- CKD:
-
Chronic kidney disease
- EFA:
-
Exploratory factor analysis
- eGFR:
-
Glomerular filtration rate
- EQ-5D-5L:
-
Europe quality of life-5-dimension-5-level
- EQ-VAS:
-
Europe quality of life-visual analog scale
- ESRD:
-
End stage renal disease
- HR-PRO:
-
Health-related-patient-reported outcome
- HRQOL:
-
Health-related quality of life
- MCS:
-
Mental component summary
- PCS:
-
Physical component summary
- QOL:
-
Quality of life
References
Periman RL, Finkelstein FO, Liu L, Roys E, Kiser M, Eisele G, et al. Quality of life in Chronic Kidney Disease ( CKD ): A cross-sectional analysis in the Renal Research Institute-CKD study. 2005(APRIL).
Tajima R, Kondo M, Kai H. Measurement of health-related quality of life in patients with chronic kidney disease in Japan with EuroQol ( EQ-5D ). Clin Exp Neprol. 2010:340–8.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Hooi LS, Ong LM, Ahmad G, Bavanandan S, Ahmad NA, Naidu BM, et al. A population-based study measuring the prevalence of chronic kidney disease among adults in West Malaysia. Kidney Int. 2013;84(5):1034–40.
Ahmad G, Leong GB, Ngo LY, Meng OL, Guat LD. 24th Report of the Malaysian Dialysis and Transplant Registry 2016. National Renal Registry Malaysia; 2016.
Juczynski Z. Health-related quality of life: theory and measurement. Acta Universitatis Lodziensis folia Psychologica; 2006.
Y-w C, Teitelbaum I, Misra M, Leon EMD, Adzize T. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance Dialysis patients. Clin J Am Soc Nephrol. 2009:1089–96.
Yong D, Kwok A, Wong D, Suen M, Chen W, Tse D. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23(2):111–9.
Shayamsunder AK, Patel SS, Jain V, Peterson RA, Kimmel PL. PSYCHOSOCIAL FACTORS IN PATIENTS WITH CHRONIC KIDNEY DISEASE: sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Semin Dial. 2005;18(2):109–18.
Kalantar-zadeh K, Unruh M. Health related quality of life in patients with chronic kidney disease; 2005. p. 367–78.
Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron. 1992;60(3):302–6.
Laupacis A, Pus N, Muirhead N, Wong C, Ferguson B, Keown P. Disease-specific questionnaire for patients with a renal transplant. Nephron. 1993;64(2):226–31.
Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT. Physical symptoms and quality of life in patients on chronic dialysis: results of the Netherlands cooperative study on adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant. 1999;14(5):1163–70.
Mosconi P, Apolone G, Mingardi G. Quality of life assessment and instruments in end-stage renal disease. J Nephrol. 2008;21(SUPPL. 13:107–12.
Korevaar JC, Merkus, Mp, Fau - Jansen MAM, Jansen Ma Fau - Dekker FW, Dekker Fw Fau - Boeschoten EW, Boeschoten Ew Fau - Krediet RT, Krediet RT. Validation of the KDQOL-SF: a dialysis-targeted health measure. Quality of Life Research. 2002(0962–9343 (Print)).
Peipert JD, Bentler PM, Klicko K, Hays RD. Psychometric properties of the kidney disease quality of life 36-item short-form survey (KDQOL-36) in the United States. Am J Kidney Dis. 2018;71(4):461–8.
Arafat SM, Chowdhury HR, Qusar MS, Hafez MA. Cross Cultural Adaptation and psychometric validation of research instruments: a methodological review. J Behav Health. 2016;5:129–36.
Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-lorenz A, et al. Principles of good practice for the translation and Cultural adaptation process for patient-reported outcomes ( PRO ) measures : report of the ISPOR task force for translation and Cultural adaptation. Value Health. 2005;8(2):94–104.
Mokkink LB, Prinsen CAC, Bouter LM, Vet HCW, Terwee CB. The COnsensus-based standards for the selection of health measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz J Phys Ther. 2016;20(2):105–13.
Davidson M. Known-groups validity. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer Netherlands; 2014. p. 3481–2.
Manavalan M, Majumdar A, Harichandra Kumar K, Priyamvada P. Assessment of health-related quality of life and its determinants in patients with chronic kidney disease. Indian J Nephrol. 2017;27(1):37–43.
Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–301.
Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10:71.
Jason WO, Anna BC. Sample size and subject to item ratio in principal components analysis. Practical Assessment. Res Eval. 2004;9(11):1–9.
Ricardo AC, Hacker E, Lora CM, Ackerson L, DeSalvo KB, Go A, et al. Validation of the kidney disease quality of life short form 36 (KDQOL-36™) US Spanish and English versions in a cohort of Hispanics with chronic kidney disease. Ethn Dis. 2013;23(2):202–9.
Hays RD, Kallich JD, Mapen DL, Coons SJ, Amin N, Carter WB, et al. Kidney disease quality of life short form (KDQOL-SFTM), version 1.3: a manual for use and scoring. 1997.
Cunningham WA, Preacher KJ, Banaji MR. Implicit attitude measures: consistency, stability, and convergent validity. Psychol Sci. 2001;12(2):163–70.
Fermont JM, Blazeby JM, Rogers CA, Wordsworth S. On behalf of the by-band-sleeve study management G. the EQ-5D-5L is a valid approach to measure health related quality of life in patients undergoing bariatric surgery. PLoS One. 2017;12(12):e0189190.
Costello A, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7).
Jr JFH, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. 7th ed. United Kingdom: Pearson education limited; 2014.
Overholser BR, Sowinski KM. Biostatistics primer: part 2. Nutr Clin Pract. 2008;23(1):76–84.
Jensen MP. Questionnaire validation: a brief guide for readers of the research literature. Clin J Pain. 2003;19(6):345–52.
Ware JE, Gandek B. Methods for testing data quality, scaling assumptions, and reliability: the IQOLA project approach. J Clin Epidemiol. 1998;51(11):945–52.
Koo TK, Li MY. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63.
Yang F, Wang VW, Joshi VD, Lau TWL, Luo N. Validation of the English version of the kidney disease quality of life questionnaire (KDQOL-36) in Haemodialysis patients in Singapore. Patient. 2013;6(2):135–41.
Chao S, Yen M, Lin T-C, Sung J-M, Wang M-C, Hung S-Y. Psychometric properties of the kidney disease quality of life–36 questionnaire (KDQOL-36™). West J Nurs Res. 2016;38(8):1067–82.
Tao X, Ka S, Chow Y, Kam F, Wong Y. Determining the validity and reliability of the Chinese version of the kidney disease quality of life questionnaire ( KDQOL-36 ™ ), vol. 2014. p. 1–9.
Thaweethamcharoen T, Srimongkol W, Noparatayaporn P, Jariyayothin P, Sukthinthai N, Aiyasanon N, et al. Validity and reliability of KDQOL-36 in Thai kidney disease patient. Value Health Reg Issues. 2013;2(1):98–102.
Etikan I. Comparison of convenience sampling and purposive sampling. Am J Theor Appl Stat. 2016;5(1).
Acknowledgements
We would like to thank Associate Professor Dr. Karuthan Chinna, Department of Social and Preventive Medicine, University of Malaya, for his assistance in our data analysis. We also would like to all the patients who participated in our study.
Consent of publication
Not applicable
Funding
This research was funded by the University Malaya Research Grant (UMRG) (RP048B-17HTM). Funding was used to pay the forward and backwards translators of the Malay KDQOL-36, and to pay participants an honorarium for their participation.
Author information
Authors and Affiliations
Contributions
KGKK was involved in the conception of the study design, collected and analyzed the data and wrote the manuscript. PLSM was involved in the conception of the study design, analyzed the data and wrote the manuscript. LSK was involved in the conception of the study design, assisted in the recruitment of participants, and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Research Ethics Committee of University Malaya Medical Centre prior to the commencement of the study (approval number: MECID No. 20165–2493). Written informed consent was obtained from all participants who agreed to participate.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Translation of the English Kidney Disease Quality of life (KDQOL-36) to Malay. (DOCX 39 kb)
Additional file 2:
Scree plot for exploratory factor analysis of burden of disease, symptoms/burden list and effects of kidney disease on daily life for the Malay KDQOL-36. (DOCX 42 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Goh, K.K.K., Lai, P.S.M. & Lim, S.K. Cross cultural adaptation and validation of the Malay Kidney Disease Quality of Life (KDQOL-36™). BMC Nephrol 20, 226 (2019). https://doi.org/10.1186/s12882-019-1397-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-019-1397-8