Abstract
Background
End stage renal disease (ESRD) is on the rise globally. Varicella infection among adult patients with ESRD has been reported to lead to multiple complications and even death. While varicella vaccination has been recommended in paediatric renal patients; recommendation on varicella vaccination among adult patients with ESRD remained sparse. This review is aimed at evaluating the impact of varicella infection among adult patients with ESRD and make a recommendation for vaccination.
Methods
Three databases (PubMed, Embase and Cumulative Index to Nursing and Allied Health Literature (CINAHL)) were searched in April 2018 with keywords ‘varicella, chronic kidney failure, chronic kidney disease, renal replacement therapy, kidney transplantation, end stage renal disease, end stage renal failure, chicken pox, vaccine, vaccination and complications’.
Results
29 articles were selected for review. The studies were mainly case reports, and they included measured outcomes: prevalence of seronegativity, impact (morbidity, length of stay, and mortality) of varicella among patients with ESRD, seroconversion rates and safety of varicella vaccination. The prevalence of seronegativity among varicella-infected ESRD adults was found to be at 42 to 100%. Nineteen deaths were reported. At least 54 patients have had complications from varicella infection. Seroconversion rate post vaccination was found to be around 64–94%.
Conclusion
Varicella is associated with significant morbidity and mortality rates in adult patients with ESRD. Varicella vaccination should be considered for the vulnerable, seronegative patients.
Similar content being viewed by others
Background
End stage renal disease (ESRD) is a prevalent chronic condition in many countries. ESRD incident rate in developed countries had largely stabilized in the past one decade, although incident rates rose for many developing countries during the same period [1]. The lifetime risk for an individual to develop chronic kidney disease (CKD) is high, with more than half the adults aged 30–64 years in the United States likely to develop CKD [2]. About 2.6 million people were on dialysis in 2010; 93% in high or upper-middle-income countries [3]. By 2030, worldwide use of renal replacement therapy (RRT) is projected to more than double, with a most projected increase in Asia [3].
Patients with ESRD have impaired immune system and therefore are susceptible to infections [4]. The disturbance to the immunity system is caused by uraemia, haemodialysis procedure, complications of CKD and therapeutic interventions for their treatment. Fehr et al.’s literature review on cases of disseminated varicella infection in adult renal allograft recipients, showed an overall mortality of 34% [5]. The mortality rate from pulmonary infections was 14 to 16-fold higher in dialysis patients and about two-fold higher in renal transplant recipients compared to general population [6]. One large cohort observational study showed hazard ratio of hospitalisation due to infection among patients with CKD or ESRD to be as high as 2.55 with a corresponding hazard ratio of 3.76 for infection-related deaths [7].
Varicella (chickenpox) is a primary infectious disease that is caused by varicella-zoster virus (VZV), an alpha herpes virus belonging to the Herpesviridae family. The secondary household attack rate of over 90% showed that varicella is highly contagious [8]. Transmissions are mostly airborne and by direct contact with vesicular fluids. The course of the disease is usually benign among paediatric patients; however, this is not so with adult patients. When it occurs in adult renal transplant recipients, it follows a virulent course and carries a very high risk of morbidity and mortality [9, 10]. Pneumonia, pneumonitis, acute obstructive respiratory disease, encephalitis, meningitis, neutropenia, thrombocytopenia, Henoch-Schonlein purpura, synovitis, Reye’s syndrome, secondary bacterial infections (sepsis, cellulitis, impetigo, abscesses, necrotizing fasciitis, and toxic skin syndrome) - the list of possible complications from varicella infection are numerous.
Since the advent of varicella vaccination, it had been proven to be effective in seroconverting paediatrics patients (including children with leukaemia), adolescents and adults, with a low occurrence of vaccine-associated rash among immunocompetent patients [11]. Similarly, seroconversion rates in adults have been encouraging, although adults respond less effectively than children group. In adults with ESRD, there are few studies on the efficacy of varicella vaccination in seroconverting this group of patients who are known to respond less efficiently to vaccinations. This is followed by lack of consensus and guidelines recommendation on vaccinating ESRD patients with VZV vaccines. This review is aimed at identifying the prevalence of seronegativity among patients with ESRD, evaluating the impact of varicella infection to adult patients with ESRD, and synthesizing current recommendations on VZV vaccination.
Methods
Data sources and search terms
The relevant papers published were collected through a computerised search on three databases (PubMed, Embase and Cumulative Index to Nursing and Allied Health Literature, CINAHL) using the keywords: chronic kidney failure, renal replacement therapy, kidney transplantation, end stage renal disease, end stage renal failure, chicken pox, varicella, vaccine, vaccination and complication. For PubMed search, the Boolean search of (Kidney Failure, Chronic [Medical Subject Heading (MeSH) Terms]) OR Renal Replacement Therapy [MeSH Terms]) OR kidney transplantation [MeSH Terms]) OR end stage renal disease) OR end stage renal failure)) AND (“Chickenpox”[MeSH Terms]) OR “Varicella”) AND (Complicat* OR vaccin*) was used. The same search terms were used for Embase and CINAHL database searches. For CINAHL only academic journals were included, periodics and bulletins were not included. The search was conducted in April 2018. There was no time frame limitation applied for the inclusion of the studies.
Study selection and eligibility criteria
Two reviewers, O.C.Y and L.S.G, independently evaluated the articles for eligibility through screening of the title and abstract first, followed by full text. Consensus on the eligibility of the articles was sought, and F.F.V was involved if there was disagreement and would act as an adjudicator.
A study is included if it is found to be relevant with regards to varicella infection in ESRD: the prevalence of seronegativity, the complications of the infection, or safety and efficacy of varicella vaccination to adult patients with ESRD or CKD. Case reports and cohort were included if measurable outcomes of death, complications, or length of stay were described. Records on herpes zoster, acyclovir, and non-renal solid organ transplants were excluded. Records on paediatric/ child populations were excluded.
Data analysis
Selected studies were summarised in Table 1. The data was grouped into themes of seroprevalance, impact of the disease, immunogenicity and safety of the varicella vaccination. Each article was graded for quality of study based on the Strength of Recommendation Taxonomy (SORT); which was introduced by the United States family medicine and primary care journals (i.e., American Family Physician, Family Medicine, The Journal of Family Practice, Journal of the American Board of Family Practice, and British Medical Journal-USA) and the Family Practice Inquiries Network (FPIN) [12]. The SORT was used because it can be applied to many sources of evidence and therefore suitable for our review which included studies with heterogeneous designs. Study quality was included in Tables 2, 3, 4, and 5. Risks of bias of each study were not accessed directly as most studies were of grade three in qualities based on the SORT. No statistical analysis was performed.
Results
610 studies were retrieved from the search strategy. After removal of duplications, 536 records remained. Screening of title and abstract narrowed down the number of records to 83 which were then assessed for eligibility. Twenty-nine studies were included in this review after study selection process (Fig. 1). More than half of the studies were case reports; the remaining studies comprised of retrospective data collection, prospective cohort, and cross-sectional studies (Table 1).
Prevalence of varicella seronegativity among patients with ESRD
Out of the seven studies on the prevalence of seronegative results; four studies were on the prevalence of seronegativity among ESRD patients upon presentation of the varicella disease [9, 13,14,15]. The results showed that 42 to 100% of the patients who contracted varicella had no prior immunity to varicella. Three studies examined the prevalence of seronegativity among ESRD patients before contraction of primary varicella. Of the three, the first studied on transplant recipients [16], the second on both transplant recipients and candidates on waitlist [17], and the third on haemodialysis patients [18]. The latter three studies, however, showed that prevalence of seronegativity was low (2.1 to 9.8%).
The prevalence of VZV seronegativity varies among renal transplant recipients, haemodialysis patients, and renal transplant candidates awaiting transplant (Table 2). There was no mention of whether the candidates waiting transplant was on renal replacement therapy or not. Among transplant patients (n = 935), there was a huge range of prevalence seronegativity from 2.1 to 100% [9, 13, 14, 17]. Among haemodialysis patients (n = 187), the prevalence of seronegativity was 2.1% [18]. As for candidates awaiting transplant (n = 622), 3.2 to 9.8% was seronegative to VZV [16, 17].
Impact of the disease (mortality and morbidity)
23 articles reported on the impact of the disease; including complications from varicella, length of stay, and mortality (Table 3). Collectively, there were nineteen deaths reported from the studies. Errasti, et al. reported four patients in which two died; both patients had significant complications (one with fulminant hepatitis, one had encephalitis) and multiorgan failure [19]. On the other hand, two other patients that had no complications survived the infection. Ishikawa, et al. reported two patients with disseminated intravascular coagulation [20]. Fehr et al. reported four cases in which all survived while their review of the literature revealed overall varicella mortality rates to be 34% [5]. Other deaths from varicella in ESRD were due to respiratory failures (one from pneumonia, one from pneumonitis), multiorgan failure (two cases), nervous system neuropathy (one case) and hepatitis (one case) [13, 21,22,23,24,25]. Length of stay has been reported to vary from 2 to 40 days. Other reported complications were pancreatitis, retinal necrosis, secondary bacterial infection, acute kidney injury, myocarditis, microangiopathy, Darrier’s disease, and even Guillain-Barre syndrome.
Most of the studies revealed that infected with primary varicella were treated with intravenous acyclovir. Standard dose of 10 mg/kg 8hourly (eight to fourteen days) were described in most cases (12 studies), renal adjusted dose were mentioned in seven reports, no dose of intravenous acyclovir was given in two reports, and in one study [9], all patients were given regimen of two weeks of intravenous acyclovir followed by three months of oral acyclovir was administered. One case was treated with three months of oral acyclovir. One case was treated with intravenous valaciclovir [26]. Intravenous ganciclovir was given in two cases [5, 9]. Cessation and reduction of immunosuppressant d rugs were described in four cases [5, 21, 25, 27, 28] and two studies [5, 9] respectively. Adjunctive antibiotics were initiated in five cases [5, 25, 27, 29, 30]. Foscarnet was given in one case following failure of initial treatment [23]. Immunoglobulins were administered in eight cases [13, 20, 31].
Immunogenicity and safety of varicella vaccination
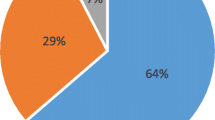
Three studies examined the seroconversion rate or post vaccination after administration of two doses of varicella vaccine. All three studies have limited number of patients. Crespo, et al. [16] reported a highly encouraging response rate of 94% while Geel, et al. [17] and Kho, et al. [32] found that the response rate to be around 64–77%. Table 4 summarises the seroconversion rates of selected studies.
As far as safety is concerned, Crespo, et al. and Geel, et al. found no secondary effect of vaccination [16, 32]. None of their vaccinated patients developed the varicella-zoster disease. Kho, et al. followed up 52 patients post-vaccination for complications and found one to have primary varicella and two to have herpes zoster [32]. Only one reported pain at injection site, no cellulitis or skin infection was reported. Interestingly, Scanlon-Kohlroser, et al. reported a case where transmission of varicella took place from two infants that were vaccinated to a post-renal transplant patient [28]. Table 5 summarises the complications of the vaccine.
Discussion
Summary of findings
In this review, the prevalence of seronegativity among varicella-infected ESRD adults was found to be significantly alarming at 42 to 100% [9, 13,14,15]. Nineteen deaths were reported in 23 studies that reported the varicella infections. At least 52 patients were reported to have complications from varicella infections. Efficacy of vaccination (measured by seroconversion rate after two doses of VZV vaccine) was found to be around 64–74%. Safety of vaccines showed that adverse effects or complications from vaccinations were zero in a cohort of fewer than twenty persons [16, 17]. Four adverse effects from vaccinations were reported in a study of 52 patients [32].
Varicella has been recognised as a potentially fatal disease among adults even though it has been largely regarded as a benign disease of childhood [33]. Although accounting for only 5% of reported cases of varicella, adults in general population contributed to 35% of all varicella deaths [34]. Furthermore, varicella is a more severe threat to adult patients with ESRD the myriad of organ and system-complications described. This dismisses the general perception of acute varicella being a self-limiting disease.
In the general population (adults and paediatrics), mortality rates were around 0.41 deaths per 1 million through 1990–1994. This decreased drastically to 0.14 deaths per 1 million during 1999 through 2001 [35, 36]. Compared to general population, mortality rates of varicella among adult patients with ESRD is much higher; suggesting the vulnerability of this group of patients to varicella infection.
Varicella-related complications derived from the review were no different from known complications of varicella infection [34]. Pneumonia, hepatitis, and encephalitis were found to be the leading complications. These complications may progress to multi-organ failure with high mortality.
Based on this review, seroconversion rates of 64–94% are encouraging and reflecting high immunogenicity when administered. This is in keeping with findings of live-attenuated varicella vaccinations being immunogenic, efficacious and safe in preventing varicella infections [35, 37]. Besides that, there are no major adverse effects in the cohort studies of vaccinated adult patients. This could suggest the positive role of vaccinating VZV seronegative patients with ESRD in preventing varicella infection.
In addition to the database search, we also searched specifically for guidelines on varicella vaccinations. As for recommendations for varicella vaccination in this group of patients; only a handful recommendations from published guidelines were found. The Advisory Committee on Immunization Practices (ACIP); Centres for Disease Control and Prevention (CDC) have recommended varicella vaccine for ESRD patients, who meet age criteria and who do not have contraindications to vaccine [38].
The American Society of Transplantation and the American Society of Transplant Surgeons recommended pre-transplantation VZV serology checking. Seronegative adults should receive one dose of varicella vaccine with serologic testing post vaccination. If seroconversion does not occur, the dose may be repeated once if time permits [39].
Similarly, the Korean Vaccination Society has recommended varicella vaccination for the seronegative adults; and this should be completed at least one month before transplantation [40]. The 2013 Infectious Disease Society of America (IDSA) Clinical Practice Guideline (CPG) for vaccination of the immunocompromised host advocated that varicella vaccine (VAR) should be given to immunocompetent patients without evidence of varicella immunity if it can be administered at least four weeks before initiating immunosuppressive therapy [41].
Both the US Department of Veterans Affairs and Department of Defence (2014) on their Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care (strong recommendation); and Public Health Agency of Canada (in their Canadian Immunisation Guide 2016) have extended the recommendation to include patients with chronic kidney disease or chronic renal disease [42, 43]. The Kidney Disease: Improving Global Outcomes (KDIGO) and the National Kidney Foundation’s Kidney Disease Outcome: Quality Improvement (KDOQI) have not specifically advocated for varicella vaccination post-transplant, the reason being varicella vaccine is a live-attenuated vaccine [44, 45]. At present, there is yet to be any recommendation by both KDIGO and KDOQI on pre-transplant vaccinations in general. While post-exposure prophylaxis with varicella immunoglobulin, and primary varicella treatment with acyclovir or valaciclovir has been recommended; they are still silent with regards to VZV immunisation as a preventive method [43, 45].
Clinical implications
There is a lack of guidelines in the Asia Pacific Region on varicella vaccination in patients with ESRD. Since most patients with ESRD or advanced CKD are managed by renal physicians and family physicians; it is critical to advocate, initiate planning, followed by implementing policies on varicella vaccination among these susceptible patients. This is of increasing importance considering the increasing number of patients developing ESRD in Asia.
Limitations and future research
The first limitation is the heterogeneity of the population in the studies that were included. The aim of this review is to review the available literature of adult populations with ESRD comprehensively. However, most studies included only subset populations of ESRD; namely renal transplant recipients or patients on haemodialysis and therefore findings may not be fully representative of the overall population of ESRD. Therefore, there is a real need for study varicella among patients with ESRD without renal transplantation. To date, guidelines by the US Veterans’ Affairs and Canadian Public Health Agency are the only two available ones to advocate vaccination even, among chronic kidney disease, while most of the published guidelines advocate vaccination among ESRD. Studies on varicella among CKD patients (before progressing into ESRD) may help to give insight whether vaccinating patients once they are diagnosed with CKD of certain stages (before their progression to ESRD) may prevent this vulnerable group of patients from contracting varicella.
There is some heterogeneity in the reports of prevalence of varicella immunity among patients in ESRD. Three described the prevalence among ESRD patients who yet to contract varicella [16,17,18]; while four described the prevalence in already infected ESRD patients [9, 13,14,15]. Despite the comprehensive search, the number of available studies in the literature is low, they were summarised together in Table 2.
Another limitation is the design of the selected articles. As varicella in adult patients with ESRD has not been widely studied, there are no large-scale observational studies to date to give an impactful insight on the burden of the disease in this group of population. Most available studies are case reports and retrospective data collection and therefore are prone to selective bias (reporting bias).
Finally, future research on the cost-effectiveness on vaccinating all patients with ESRD compared to screening patients with ESRD for seronegativity before vaccinating them and monitoring will be helpful to guide national guidelines on varicella vaccination in adult patients with ESRD. This can be challenging and varies between countries depending on the robustness of national healthcare surveillance data on patients with ESRD and cost of delivering and administrating vaccines and serological tests.
Conclusion
Varicella is a disease with great morbidity and mortality in adult patients with ESRD. Preventing varicella infection in ESRD patients is critical, and has been proven safe and reasonably efficacious in ESRD and chronic kidney disease patients.
Abbreviations
- Ab:
-
Antibody
- AUC:
-
Area under curve
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- CKD:
-
Chronic kidney disease
- CMV:
-
Cytomegalovirus
- DIC:
-
Disseminated intravascular coagulation
- ESRD:
-
End stage renal disease
- Ig G:
-
Immunoglobulin G
- Ig M:
-
Immunoglobulin M
- LOS:
-
Length of stay
- MeSH:
-
Medical subject heading
- NA:
-
not applicable
- RRT:
-
Renal replacement therapy
- SORT:
-
Strength of recommendation taxonomy
- VZV:
-
Varicella zoster virus
- y.o.:
-
years old
References
Wetmore JB, Collins AJ. Global challenges posed by the growth of end-stage renal disease. Ren Replace Ther. 2016;2(1):15.
Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Burrows NR, Saydah SH, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD initiative. Am J Kidney Dis. 2015;65(3):403–11.
Garcia-Garcia G, Jha V, Tao Li PK, Garcia-Garcia G, Couser WG, Erk T, et al. Chronic kidney disease (CKD) in disadvantaged populations. Clin Kidney J. 2014;8(1):3–6.
Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Basic science and dialysis: disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20(5):440–51.
Fehr T, Bossart W, Wahl C, Binswanger U. Disseminated varicella infection in adult renal allograft recipients: four cases and a review of the literature. Transplant. 2002;73(4):608–11.
Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58(4):1758–64.
Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K. CKD and risk for hospitalization with infection: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2017;69(6):752–61.
Ong A, Goh KT, editors. A guide on infectious diseases of public health importance in Singapore. 7th ed. Singapore: Communicable Diseases Division, Ministry of Health [and] Communicable Disease Centre, Tan Tock Seng Hospital; 2011.
Kaul A, Sharma RK, Bhadhuria D, Gupta A, Prasad N. Chickenpox infection after renal transplantation. Clin Kid J. 2012;5(3):203–6.
Ross LF, Lantos JD. Immunisation against chickenpox. BMJ. 310(6971):2.
Gershon AA. Live attenuated varicella vaccine. Int J Infect Dis. 1997;1:130–4.
Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centred approach to grading evidence in the medical literature. J Am Board Fam Pract. 2004;17(1):59–67.
Rodríguez-Moreno A, Sanchez-Fructuoso AI, Calvo N, Ridao N, Conesa J, Marques M, et al. Varicella infection in adult renal allograft recipients: experience at one center. Transplant Proc. 2006;38(8):2416–8.
Abad CL, Razonable RR. α Herpes virus infections among renal transplant recipients. Sem Nephrol. 2016;36(5):344–50.
Ong CY, Low SG, Vasanwala FF, Stephanie MC, Kaushik M, Low LL. Incidence and mortality rates of varicella among end stage renal disease (ESRD) patients in Singapore General Hospital, a 12-year review. BMC Infect Dis. 2018;18(1):118.
Crespo JF, Gorriz JL, Avila A, Sancho A, Gavela E, Cano A, et al. Prevalence of past varicella zoster virus infection in candidates for kidney transplantation: vaccination in seronegative patients. Transplant Proc. 2002;34(1):77.
Geel AL, Landman TS, Kal JA, Van Doomum GJ, Weimar W. Varicella zoster virus serostatus before and after kidney transplantation, and vaccination of adult kidney transplant candidates. Transplant Proc. 2006;38(10):3418–9.
Talebi-Taher M, Hassanzadeh T, Ossareh S. Seroprevalence of antibodies against varicella-zoster virus among prevalent hemodialysis patients. Iranian J Kidney Dis. 2013;7(6):475.
Errasti P, Alvarex ML, Gomex G, Lavilla FJ, Garcia N, Ballester B, et al. Chickenpox in four adult transplant recipients. Transplant Proc. 1999;31(6):2341–2.
Ishikawa N, Tanabe K, Shimmura H, Tokumoto T, Toma H. Primary varicella virus in adult renal transplant recipients: case reports. Transplant Proc. 2000;32(7):1952–3.
Lauzurica R, Bayes B, Frias C, Fontseré N, Hernandez A, Matas L, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35(5):1758–9.
Robertson S, Newbigging K, Carman W, Jones G, Isles C. Fulminating varicella despite prophylactic immune globulin and intravenous acyclovir in a renal transplant recipient: should renal patients be vaccinated against VZV before transplantation? Clin Transpl. 2006;20(1):136–8.
Inokuchi R, Nakamura K, Sato H, Shinohara K, Aoki Y, Doi K, et al. Bronchial ulceration as a prognostic indicator for varicella pneumonia: case report and systematic literature review. J Clin Virol. 2013;56(4):360–4.
Sampathkumar K, Prabhakar A, Vijay Anand C. Quiz page April 2015: fever and encephalopathy in a kidney transplant recipient. Am J Kidney Dis. 2015;65(4):A18–21.
Depledge DP, Brown J, Macanovic J, Underhill G, Breuer J. Viral genome sequencing proves nosocomial transmission of fatal varicella. J Infect Dis. 2016;214(9):1399–402.
Nabi S, Kahlon P, Goggins M, Patel A. VZV encephalitis following successful treatment of CMV infection in a patient with a kidney transplant. BMJ Case Rep. 2014;2014 https://doi.org/10.1136/bcr-2014-206655.
Sato A, Amada N, Kikuchi H, Fukumori T, Haga I, Takahashi Y. Pneumonia due to varicella-zoster virus reinfection in a renal transplant recipient. Transplant Proc. 2009;41(9):3959–61.
Scanlon-Kohlroser CA, Fan PY, Primack W, Stoff JS. Primary varicella after transplantation. Am J Kidney Dis. 2002;39(6):1310–2.
Assi M, Abou Antoun S. Unusual neurologic manifestations of varicella zoster virus infection with the absence of rash in a kidney transplant recipient. Transpl Infect Dis. 2011;13(5):545–7.
Low LL, Vasanwala FF, Suhail SM. Varicella encephalitis and pneumonia in a patient with end stage renal failure. Asia Pac Fam Med. 2014;13(1):4.
Crowther N, Murugasu A, Sabto J, McLean CA. Late acute antibody-mediated rejection 16 years post cadaveric renal transplantation precipitated by recurrent varicella infection. Nephrol (Carlton). 2009;14(5):533–4.
Kho MM, Zuijderwijk JM, van der Eijk AA, de Kuiper R, Boer-Verschragen MJ, Weimar W, et al. Humoral and cellular response after varicella vaccination in VZV IgG seronegative kidney transplant candidates. Vaccine. 2017;35(1):71–6.
Meyer PA, Seward JF, Jumaan AO, Wharton M. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. J Infect Dis. 2000;182(2):383–90.
Centers of Disease Control and Prevention. Varicella In: Pinkbook, Epidemiology of vaccine preventable diseases. Centers of Disease Control and Prevention; 2015. https://www.cdc.gov/chickenpox/about/index.html. Accessed 3 July 2018.
Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. New Engl J Med. 2005;352(5):450–8.
Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287(5):606–11.
Carrillo-Santisteve P, Lopalco PL. Varicella vaccination: a laboured take-off. Clin Microb Infect. 2014;20(s5:86–91.
Marin M, Guris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1–40.
Danziger-Isakov L, Kumar D. Vaccination in solid organ transplantation. Am J Transplant. 2013;13(s4):311–7.
Kim YJ, Kim SI. Vaccination strategies in patients with solid organ transplant: evidences and future perspectives. Clin Exp Vacinne Res. 2016;5(2):125–31.
Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et aI. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infectious Dis 2013; 58(3):e44–100.
The Management of Chronic Kidney Disease Working Group. Va/DoD Clinical Practice Guideline for the Management of Chronic Kidney Disease in Primary Care. US Department of Veterans Affairs and Department of Defense, 2014. https://www.healthquality.va.gov/guidelines/CD/ckd/VADoDCKDCPG2014.pdf. Accessed 3 July 2018.
National Advisory Committee on Immunization (NACI), "Active Vaccines: Varicella Vaccine,” from Public Health Agency of Canada, Ottawa, ONT: Minister of Public Works and Government Services Canada. Available at http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-vari-eng.php; last accessed 20 July 2017.
Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kid Int. 2010;77(4):299–311.
Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kid Dis. 2010;56(2):189–218.
Sinha S, Jha R, Lakhtakia S, Narayan G. Acute pancreatitis following kidney transplantation–role of viral infections. Clin Transpl. 2003;17(1):32–6.
Shahbazian H, Ehsanpour A. An outbreak of chickenpox in adult renal transplant recipients. Exp Clin Transplant. 2007;5(1):604–6.
Kandasamy R, Hecker M, Choi M, Pile J. Darier disease complicated by disseminated zoster. Dermatol Online J. 2009;15(2):6.
Mustapic Z, Basic-Jukic N, Kes P, Lovcic V, Bubic-Filipi L, Mokos I, et al. Varicella zoster infection in renal transplant recipients: prevalence, complications and outcome. Kidney Blood Press Res. 2011;34(6):382–6.
Chiang E, Pyatetsky D. Acute retinal necrosis secondary to varicella zoster virus in an immunosuppressed post-kidney transplant patient. Clin Med Res. 2012;10(4):240–1.
Chhabra P, Ranjan P, Bhasin DK. Simultaneous occurrence of varicella zoster virus-induced pancreatitis and hepatitis in a renal transplant recipient: a case report and review of literature. Perm J. 2017;21:16–083.
Momani H, Algeizawi S, Shamoun B, Taha AA, Alshakhatreh H, Breizat AH. Preliminary results of a newly established organ transplantation program in a teaching hospital. Exp Clin Transplant. 2017;15(Suppl 1):110–2.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request but restrictions apply to the availability of these data.
Author information
Authors and Affiliations
Contributions
OCY and LLL formulated the search strategy. OCY and LSG, independently evaluated the articles for eligibility through screening of the title and abstract first, followed by full text. FFV adjudicated the evaluation of articles. OCY wrote the draft. All authors participated in the editing of the manuscript. All authors read and confirmed the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval or consent is needed based on institution’s guidelines.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ong, C.Y., Low, S.G., Vasanwala, F.F. et al. Varicella infections in patients with end stage renal disease: a systematic review. BMC Nephrol 19, 185 (2018). https://doi.org/10.1186/s12882-018-0976-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-018-0976-4