Abstract
Background
Regional anticoagulation with citrate is the recommended first line treatment for patients receiving continuous renal replacement therapy (CRRT). There is wide variability in filter patency which may be due to differences in patient characteristics and local practice. It is also possible that citrate has effects on primary and secondary haemostasis, fibrinolysis and platelet function that are still unknown. The primary aim of the study is to describe the effect of citrate on coagulation and fibrinolysis pathways in both the patient and the haemodialysis circuit.
Methods
The study will recruit 12 adult patients admitted to the intensive care unit, requiring CRRT with regional citrate anticoagulation for acute kidney injury. Patients with pre-existing thrombotic or bleeding tendencies will be excluded. Thrombin generation, clot lysis and platelet function will be measured at baseline and at 12, 24, 36, 48 and 72 h after commencing CRRT (from the patient and from the circuit). We will describe the evolution of parameters over time as well as the differences in parameters between the patient and the circuit.
Discussion
The study will provide new data on the effects of citrate during continuous renal replacement therapy which is not currently available. We will minimise confounding factors through the use of tight exclusion criteria and accept that this will slow down recruitment. Depending on the results, we hope to incorporate the findings into existing clinical guidelines and clinical practice with the aim to prevent premature filter clotting and interruptions in treatment.
Trial registration
The study was registered with clinicaltrials.gov on 10th June 2015 (NCT02486614).
Similar content being viewed by others
Background
Citrate has emerged as the recommended first line anticoagulant for continuous renal replacement therapy (CRRT) in the Intensive Care Unit (ICU) based on its efficacy and safety profile [1]. It acts by chelating calcium and inhibiting the blood coagulation chemistry at several levels (Fig. 1). Several randomized controlled trials have shown significantly longer filter patency with citrate compared to anticoagulation with heparin [2]. However, the reported circuit life with citrate anticoagulation varies from a mean of 16 h to 125 h [2]. The exact reasons for this variation are not clear but are likely to be multifactorial.
Patient characteristics and vascular access problems are important risk factors for premature clotting. In fact, in patients with acute kidney injury (AKI) and sepsis, the coagulation/fibrinolysis balance is often deranged even prior to starting CRRT [3, 4]. A proportion of patients may also have an underlying medical condition that predisposes them to the formation of clots, such as Protein C deficiency or anti-phospholipid syndrome. In addition, no anticoagulant is effective at inhibiting all steps of the coagulation pathway completely. For instance, whilst citrate reduces thrombin generation by inhibiting reactions upstream, it does not impact all effects of thrombin (Fig. 1). It is also unknown whether citrate has any clinically important direct or indirect effects on fibrinolysis or platelet function [5,6,7]. Finally, not all events within the circuit are represented by systemic clotting results, and it has been argued that coagulation and fibrinolysis need to be studied separately in the patient and in the extracorporeal circuit [8]. We report our protocol to study the effects of citrate on coagulation and fibrinolysis pathways. (Version 1.1, 26 February 2014).
Methods/design
Aims
Our aim is to perform a detailed evaluation of the effects of citrate on the coagulation and fibrinolysis pathways during CRRT. The primary objective is to determine changes in primary haemostasis, coagulation and fibrinolysis parameters from baseline within the patient’s systemic circulation and the CRRT circuit over the course of 72 h of regional anticoagulation with citrate. The outcomes are parameters reflecting the coagulation and fibrinolysis pathways (see below).
Design
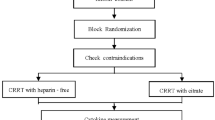
We plan to conduct a prospective observational cohort study.
Setting
The study is conducted in the critical care unit in a tertiary care centre in the United Kingdom.
Patient selection
Patients with AKI in whom the treating team intend to commence on CRRT with citrate anti-coagulation will be screened against the inclusion and exclusion criteria. Patients meeting all of the inclusion criteria and none of the exclusion criteria will be recruited.
The inclusion criteria are:
-
admitted to the critical care unit
-
treating clinician plans to initiate CRRT with regional citrate anticoagulation
-
age > 18 years
-
expected to require CRRT for >72 h
-
arterial or central venous catheter in situ to allow blood sampling
Exclusion criteria are:
-
known pre-existing thrombotic tendency
-
known pre-existing bleeding tendency
-
disseminated Intravascular Coagulation (DIC)
-
transfusion of any blood products in the 24 h before enrolment
-
active bleeding (ie. needing blood transfusion) at time of enrolment
-
haemoglobin at time of enrolment <75 g/L
-
haematocrit at time of enrolment >0.55 L/L
-
patient would refuse red blood cell transfusion (for example Jehova’s Witness)
-
platelet count at time of enrolment <100 × 103/μL
-
treatment with any anticoagulant or antiplatelet agent at enrolment or within 7 days of enrolment with the exception of heparin or low molecular weight heparin for deep venous thromboembolism (DVT) prophylaxis
-
intravenous heparin exposure within 4 h of commencing citrate anticoagulation
-
malnourishment, ie. body mass index (BMI) <18.5 kg/m2 or unplanned weight loss >10% actual body weight (ABW) in preceding 6 months or BMI <20 kg/m2 and unplanned weight loss >5% ABW in preceding 6 months.
-
CRRT prescribed for an indication other than acute kidney injury
Sample size calculation
A formal sample size calculation was not possible due to lack of data in the literature. Following discussion with experts in the field, a sample size of 12 participants with complete data for 48–72 h was considered to be adequate to gain better understanding of coagulation and fibrinolysis during citrate based CRRT and to inform the design of future studies.
Blood sampling
Baseline
The following blood tests will be performed at baseline, i.e. immediately prior to commencing CRRT: Full Blood Count (FBC), prothrombin time (PT), activated partial thromboplastin time ratio (APTTr), fibrinogen, D-Dimers, antithrombin activity, protein C activity, free protein S antigen, resistance to activated protein C (APCR) screening, homocysteine, PT 20210 mutation test, factor VIII activity, von Willebrand factor antigen, Dilute Russell’s Viper Venom Time (DRVVT), Dilute Activated Partial Thromboplastin Time (DAPTT), anticardiolipin antibodies, anti-beta 2 glycoprotein I antibodies, thrombin generation assay, clot lysis, prothrombin fragment 1 + 2, thrombin-antithrombin complexes (TAT) enzyme linked immunosobent assays (ELISA) and platelet function screening.
During CRRT
At 12, 24, 36, 48 and 72 h after commencing CRRT, blood samples will be taken from the systemic circulation and from the CRRT circuit to measure the following parameters: FBC, PT, APTTr, fibrinogen, D-Dimers, thrombin generation assay, clot lysis and platelet function screening.
Laboratory tests
All laboratory tests except FBC will be conducted in the Haemostasis & Thrombosis Laboratories at Guy’s & St Thomas’ Hospital. Dade® Innovin® recombinant thromboplastin, Actin FS®, Thromboclotin®, Thrombin-Reagent®, Innovance D-dimer and HYPHEN BioMed Biophen FVIII:C chromogenic assay (Siemens Healthcare, Marburg, Germany) will be used on Sysmex CS2100i coagulation analysers (Sysmex UK, Milton Keynes, UK) for PT, APTT, Clauss fibrinogen, D-dimers and FVIII activity respectively. For lupus anticoagulant detection, DRVVT will employ Life Diagnostics LA Screen and LA Confirm reagents (Diagnostica Stago UK, Theale, UK), and DAPTT will use Stago PTT-LA (Diagnostica Stago) in the screen and addition of Bio/Data Corporation LA Confirmation Reagent (Alpha Labs, Eastleigh, UK) for the confirm. LA assays will be performed on a Sysmex 2000i analyser (Sysmex UK). Chromogenix Coamatic antithrombin, Coamatic protein C, Coatest APC Resistance, Coatest APCR resistance-V (Quadratech Diagnostic Ltd., Epsom, UK) and STA®-Liatest® Free protein S (Diagnostica Stago) will be used on Sysmex CS2000i analysers for antithrombin activity, protein C activity, phenotypic activated protein C resistance and free protein S antigen respectively. Inova Diagnostics QUANTA Lite® (ELISA) kits for IgG and IgM anticardiolipin and anti-β2 glycoprotein I antibodies (Werfen UK, Warrington, UK), Enzygnost® F1 + 2 and TAT will be performed on a Dynex DS2 ELISA analyser (Werfen UK) for anticardiolipin antibodies, anti-β2 glycoprotein I antibodies, prothrombin fragment 1 + 2 and thrombin-antithrombin complexes respectively. HemosIL VWF Antigen will be performed on an ACL TOP 500 coagulation analyser (Werfen UK) for von Willebrand factor antigen. Platelet function screening will be performed on a PFA-100 analyser (Sysmex UK) with collagen/ADP and collagen/epinephrine cartridges (Sysmex UK). Homocysteine will be assayed with Abbott ARCHITECT chemiluminescent microparticle assay (Abbott UK, Maidenhead, UK) on an Abbott ARCHITECT i2000SR immunoassay analyser (Abbott UK). The Thrombin Generation Assay will performed using a Technoclone kit (Technoclone, Vienna, Austria) on a Wallac Victor3 multilabel plate reader (Perkin Elmer, Turku, Finland). The Clot Lysis Assay will be performed as previously described [6] and read on a Wallac Victor3 multilabel plate reader (Perkin Elmer, Turku, Finland). The Prothrombin 20,210 polymorphism will be screened for using an in house developed allelic discrimination assay on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Baseline data collection
The following data will be collected: age, gender, ethnicity, admission diagnosis, comorbidities, biochemistry profile, C-reactive protein (CRP), Acute Physiology and Chronic Health Evaluation (APACHE) II score on admission and daily Sequential Organ Failure Assessment (SOFA) scores.
Withdrawal criteria
Patients will be excluded from the study at their own request or the request of their legal representative. They will be excluded from further blood sampling if any of the following events occur during the 72 h sampling period:
-
Development of Disseminated Intravascular Coagulation (DIC).
-
Need for treatment with any systemic anticoagulant or antiplatelet agent with the exception of heparin or low molecular weight heparin for DVT prophylaxis
-
Need for treatment with platelets or clotting factors
Statistical analysis
The baseline characteristics will be summarised using appropriate descriptive statistics [i.e. frequency (percentage) for categorical data and mean (standard deviation) or median (interquartile range) for continuous data]. All coagulation and fibrinolysis parameters at baseline will be summarised as means (standard deviation) unless the distribution is highlight skewed, in which case median values (interquartile range) will be used.
Average coagulation and fibrinolysis parameters at each time point, and the mean (standard deviation) of the difference relative to baseline will be presented. The evolution of parameters will also be illustrated graphically by plotting the mean (with 95% confidence interval) against time points. Trajectories of individual patients over time will be shown graphically. Separate figures will be used to summarise data obtained from bloods taken from the patient and that from the CRRT circuit.
Repeated measures analysis of variance (ANOVA) (one for each of the parameters obtained from patient’s bloods and separately for those from the circuit), will be used to further explore changes in parameters over time. Where CRRT ends before 72 h patients will not have measurements taken at 72 h. The ANOVA will therefore include all measurements up to 48 h (unless all patients have complete data to 72 h, in which case the final time point will also be included). If a main effect of time is identified, post-hoc comparisons will be carried out to determine where differences lie.
Coagulation and fibrinolysis parameters from the patients’ blood will be plotted against the corresponding value obtained from blood drawn from the CRRT circuit. The mean (standard deviation) of the differences will be calculated overall and at each time point and Bland-Altman limits of agreement calculated.
Discussion
To our best knowledge, this is the first study that will provide a detailed description of clotting and fibrinolysis processes before CRRT and the effects of citrate on coagulation pathways, fibrinolysis and platelet function during CRRT.
The strengths of this project are:
-
i)
The project will provide data that are currently not available.
-
ii)
By using very tight inclusion and exclusion criteria we are minimising confounding influences as much as possible.
-
iii)
Serial blood samples will be taken from the systemic circulation and the CRRT circuit separately to differentiate between events in the patient and in the extracorporeal circuit.
-
iv)
The results have potential to inform the design of future exploratory studies and intervention trials aimed a prolonging circuit life during CRRT and preventing unplanned interruptions in treatment.
We are aware of potential limitations and risks of the project which we will eliminate as much as possible.
-
i)
As a result of very strict criteria for inclusion, exclusion and withdrawal and a relatively long sampling period, recruitment is likely to be slow.
It is possible that we identify medical problems through this research that may have implications for patients and their close relatives, for instance a genetic predisposition to clotting which was previously not known about. With approval by the Research Ethics Committee, we have put measures in place to make sure that affected patients and relatives will receive the necessary medical attention.
Depending on the findings of the study, we plan to incorporate the results into current guidelines and clinical practice and hope that premature filter clotting can be prevented and the delivery of CRRT improves.
Abbreviations
- ABW:
-
actual body weight
- AKI:
-
Acute kidney injury
- ANOVA:
-
Repeated measures analysis of variance
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- APCR:
-
Resistance to activated protein C
- APTTr:
-
Activated partial thromboplastin time ratio
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- CRRT:
-
Continuous renal replacement therapy
- DAPTT:
-
Dilute Activated Partial Thromboplastin Time (DAPTT)
- DIC:
-
Disseminated Intravascular Coagulation
- DRVVT:
-
Dilute Russell’s Viper Venom Time
- DVT:
-
Deep vein thrombosis
- ELISA:
-
Enzyme linked immunosobent assays
- FBC:
-
Full blood count
- ICU:
-
Intensive care unit
- PT:
-
Prothrombin time
- SOFA:
-
Sequential Organ Failure Assessment
- TAT:
-
Thrombin-antithrombin complexes
- VWF:
-
Von Willebrand factor
References
Disease K, Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138.
Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20:144.
Stefanidis I, Hägel J, Frank D, Maurin N. Hemostatic alterations during continuous venovenous haemofiltration in acute renal failure. Clin Nephrol. 1996;46(3):199–205.
Cardigan R, McGloin H, Mackie I, Machin S, Singer M. Endothelial dysfunction in critically ill patients: the effect of haemofiltration. Intensive Care Med. 1998;24:1264–71.
Etulain J, Negrotto S, Carestia A, Pozner RG, Romaniuk MA, D'Atri LP, Klement GL, Schattner M. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb Haemost. 2012;107:99–110.
Lamberth EL Jr, Warriner RA III, Batchelor ED, Des Prez RM. Effect of metabolic acidosis and alkalosis on human platelet aggregation induced by epinephrine and ADP. Proc Soc Exp Biol Med. 1974;145:743–6.
Dai L, Mitchell M, Savidge G, Alhaq A. The profibrinolytic effect of plasma thrombomodulin in factor XI deficiency and its implications in hemostasis. J Thromb Haemost. 2004;2(12):2200–4.
Schilder L, Nurmohamed SA, ter Wee PM, Paauw NJ, Girbes ARJ, Beishuizen A, Beelen RHJ, Groeneveld ABJ. Coagulation, fibrinolysis and inhibitors in failing filters during continuous venovenous haemofiltration in critically ill patients with acute kidney injury: effect of anticoagulation modalities. Blood Purif. 2015;39:297–305.
Acknowledgements
The authors wish to thank the research nurses for their help recruiting patients.
Funding
This work was supported by research funding from Fresenius Medical Care (UK) Ltd. following review by an independent “Investigator Driven Study Evaluation Committee (IDSEC)”. Fresenius Medical Care (UK) Ltd. was not involved in the design of the protocol and has no involvement in the conduct of the study. The results will remain intellectual property of the research team. Fresenius Medical Care (UK) Ltd. will not be involved in the data analysis and writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study will be available from the corresponding author following completion of the study on reasonable request.
Sponsor
The study was sponsored by Guy’s & St Thomas’ Hospital London.
Author information
Authors and Affiliations
Contributions
RF, MM, GM and MO conceptualised the study and designed the protocol. HD and LT reviewed the protocol and helped with patient recruitment. RF, KL, MM, GM and MO authored the study protocol. SC authored the statistical analysis plan and will perform the statistical analysis. The laboratory analyses will be conducted under the leadership of GM and MM. RF, MO, GM and MM will interpret the laboratory analyses. All co-authors will contribute to the interpretation of the results. The final draft of this manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Authors’ information
MO is a Consultant in Nephrology and Critical Care and an international expert in the field of acute kidney injury and critical care nephrology. GM is a Consultant Biomedical Scientist and Head of the Diagnostic Haemostasis and Thrombosis Laboratory at Guy’s & St Thomas Hospital London.
Ethics approval and consent to participate
Full ethical approval was granted by the London – Harrow National Research Ethics Committee (14/LO/0138). Participants will be asked to give their consent to participate in the study. In the event that they do not have capacity to consent, a Personal Consultee will be consulted in accordance with the Mental Capacity Act 2005. In case a Personal Consultee is not available, a Nominated Consultee (a clinician who is not connected to the research study) will be consulted. When the patient regains capacity, they will be informed about the study and asked to give consent to remain in the study. The Research Ethics Committee agreed that the study had the potential to benefit participants lacking capacity without imposing a disproportionate burden on them. Upon completion, the study team will submit the study report for publication in a peer reviewed scientific journal.
Consent for publication
All participating individuals consented to the publication of the study protocol and results in fully anonymised format.
Competing interests
The authors declare that they have no conflict of interest. The study was supported by funding from Fresenius Medical Care (UK) Ltd. following review by an independent “Investigator Driven Study Evaluation Committee (IDSEC)”. The authors are not affiliated with Fresenius Medical Care (UK) Ltd.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fisher, R., Lei, K., Mitchell, M.J. et al. The effect of regional citrate anti-coagulation on the coagulation system in critically ill patients receiving continuous renal replacement therapy for acute kidney injury - an observational cohort study. BMC Nephrol 18, 304 (2017). https://doi.org/10.1186/s12882-017-0718-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-017-0718-z