Abstract
Background
The silent progression of chronic kidney diseases (CKD) and its association with other chronic diseases, and high treatment costs make it a great public health concern worldwide. The population burden of CKD in Peru has yet to be fully described.
Methods
We completed a cross sectional study of CKD prevalence among 404 participants (total study population median age 54.8 years, 50.2 % male) from two sites, highly-urbanized Lima and less urbanized Tumbes, who were enrolled in the population-based CRONICAS Cohort Study of cardiopulmonary health in Peru. Factors potentially associated with the presence of CKD were explored using Poisson regression, a statistical methodology used to determine prevalence ratios.
Results
In total, 68 participants (16.8 %, 95 % CI 13.5–20.9 %) met criteria for CKD: 60 (14.9%) with proteinuria, four (1%) with eGFR <60mL/min/1.73m2 , and four (1%) with both. CKD prevalence was higher in Lima (20.7 %, 95 % CI 15.8–27.1) than Tumbes (12.9 %, 95 % CI 9.0–18.5). Among participants with CKD, the prevalence of diabetes and hypertension was 19.1 % and 42.7 %, respectively. After multivariable adjustment, CKD was associated with older age, female sex, greater wealth tertile (although all wealth strata were below the poverty line), residence in Lima, and presence of diabetes and hypertension.
Conclusions
The high prevalence rates of CKD identified in Lima and Tumbes are similar to estimates from high-income settings. These findings highlight the need to identify occult CKD and implement strategies to prevent disease progression and secondary morbidity.
Similar content being viewed by others
Background
Owing to the rising global epidemic of diabetes [1], hypertension [2], and obesity [3], chronic kidney disease (CKD) has become a worldwide public health problem with a substantial economic burden. For instance, the World Health Organization estimated a high cost-effectiveness ratio for dialysis, roughly $108,600 USD per disability-adjusted life-year [4, 5]. CKD further increases diabetes and hypertension-related complications, including cardiovascular risk and all-cause mortality [4, 6, 7]. In addition, due to major constraints on the availability of healthcare resources, CKD may impose a tougher challenge on low- and middle-income countries (LMIC). Understanding the epidemiology of CKD in LMIC is a fundamental step to addressing the burden of CKD and will guide disease surveillance, screening, prevention activities as well as healthcare resource allocation.
Given its diverse range of socio-economic trends and climatic and geographical zones, Peru provides a unique opportunity in which to assess CKD burden [8]. In 2013, approximately 65 % of the Peruvian population has some form of health insurance [9], but only an estimated 30 % of all Peruvians are able to access renal replacement therapy [10]. Another emerging challenge to current vulnerable health systems in Peru is its rapid population growth, which is reflected by changes in age structure and urbanization [11, 12]. However, as Peru is experiencing a marked demographic and epidemiologic transition with a rising prevalence of cardiovascular disease risk factors such as obesity, hypertension and type-2 diabetes, related illnesses such as CKD are also likely to be rising in prevalence [13, 14]. This scenario highlights the importance of detection of CKD and implementation of interventions that stem its progression toward end-stage disease.
In Peru, as in many other countries in Latin America, the information related to the epidemiology of CKD and end-stage renal disease is limited. Early hospital-derived data from 1990 suggested the prevalence of CKD in Lima to be only 12.2/100,000 people [15]. Since 1991, the Latin American Dialysis and Renal Transplant Registry has collected data from 20 countries and reported a high utilization of renal replacement therapy [16]. These data suggest that the burden of CKD has been under-appreciated and the need to design CKD surveillance programs as well as appropriate end-stage renal disease management [17, 18].
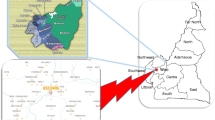
The CRONICAS cohort study is a general population-based longitudinal study in Peru assessing cardiopulmonary risk factors [19]. This study examined CRONICAS study participants identified at two of the three study sites, Lima and Tumbes (Fig. 1), to estimate the prevalence of CKD and its potential risk factors.
Methods
Study design and setting
This cross-sectional study collected baseline CKD marker data in a random subsample of the ongoing CRONICAS cohort study [19]. Data were collected from February to May 2011 in the highly-urbanized site of Pampas de San Juan de Miraflores, Lima, Peru with 60,000 inhabitants in 4 km2, and the semi-urban site of Tumbes with 20,000 inhabitants in 80 km2. Lima, the country’s capital, is located in the central west coast region of Peru and Tumbes is located along the northwest coastline. Both regions have undergone different degrees of urbanization, including urban-industrial development [20].
Participants, recruitment, and ethics
Using the most updated local census data, study participants were identified using a single-stage sampling method stratified by sex and age (35–44; 45–54; 55–64; ≥ 65 years old) [19]. Participants were included if they were full-time residents in the area, capable of understanding the study’s procedures, and able to provide informed consent. Patients were excluded if they were pregnant or unable to sit upright or lay down, precluding measurement acquisition. Participants were selected until each age and sex category was filled. Only one member per household was enrolled in the study.
Trained fieldworkers went door-to-door to contact selected participants. After discussing the parent study protocol and receiving informed consent, participants were scheduled to gather anthropometric measurements. At this appointment, a sub-sample of participants were approached and invited to participate in the CKD ancillary study and enrolled after obtaining verbal informed consent. The study’s protocol was reviewed and approved by the Institutional Review Boards of Universidad Peruana Cayetano Heredia and University of Pennsylvania.
Data collection
The protocol for the CRONICAS Cohort Study has been published elsewhere [19]. Briefly, each participant completed a fieldworker-administered questionnaire to provide information on socio-demographic and lifestyle data including age, sex, education level, work status, alcohol consumption, smoking habits, previous medical history, and medication use. The questionnaire was based on the STEPS approach for surveillance of non-communicable diseases [21]. Trained fieldworkers measured height and weight from all participants during the clinic visit [19]. Each measurement was taken three times, and the average of the three was used for analyses. In addition, three systolic (SBP) and diastolic (DBP) blood pressure measurements were acquired using an automated monitor (OMRON HEM-780) [19]. The second and third blood pressure measurements were averaged for the analysis.
A trained laboratory technician obtained fasting venous blood and urine samples. Whole blood and plasma were collected in ethylenediaminetetraacetic acid (EDTA) tubes and sodium fluoride/EDTA tubes, respectively. Urine was collected in 15 mL containers. Blood serum and urine specimens were maintained at 4–8 °C for two weeks and then moved to a storage facility where the urine was aliquoted into 4 vials (1.5 mL each). All samples were then stored at −20 °C until laboratory analyses were performed. All samples were analyzed in a single laboratory. Assay quality was checked against regular external standards and internal duplicate assays and monitored by BioRad (http://www.biorad.com).
Details pertaining to the measurement of plasma glucose, serum insulin, hemoglobin A1C, total cholesterol and high-density lipoprotein (HDL)-cholesterol are outlined in the parent study protocol. [18] High-sensitivity C reactive protein was measured using Latex (Tina-quant CRP-HS Roche/Hitachi analyzer, Indianapolis, IN, USA). Serum and urine creatinine were measured by the modified kinetic Jaffé method. The enzymatic method was standardized to the IFCC’s isotope dilution mass spec (IDMS) method. Urine protein was determined via turbidimetry as measured by the Sequoia-Turner digital fluorometer.
Study variables
The glomerular filtration rate, a measure of kidney function, was estimated using the CKD-Epidemiology Collaboration equation using serum creatinine as a filtration marker [22]. To ensure consistency across studies, CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or proteinuria (protein-creatinine ratio) ≥150 mg/g creatinine, or both, based on the latest Kidney Disease Improving Global Outcomes (KDIGO) guideline [4].
Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or self-reported physician diagnosis or use of anti-diabetic medications [23]. Hypertension was defined as a systolic blood pressure (SBP) of ≥140 mmHg, a diastolic blood pressure (DBP) of ≥90 mmHg, receipt of anti-hypertensive therapy at the time of enrollment, or self-report of a diagnosis by a physician [24].
Insulin resistance was assessed using the homeostasis model assessment (HOMA-IR) originally described by Mathew et al. [25] The Framingham risk score (FRS) was calculated from National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III algorithm and based on six cardiovascular risk factors: age, gender, total cholesterol, HDL-cholesterol, systolic BP and smoking status [26]. A 10-year risk of coronary events was divided into three levels of risk: low (<10 %), intermediate (10–20 %), and high (>20 %) [26].
Socioeconomic status was assessed using a wealth index based upon current occupation, household income, assets and household facilities [27]. Smoking was categorized as current, former, or never. Alcohol consumption was categorized into non-current and current drinkers. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Statistical analysis and model selection
Categorical variables were described as proportions, and continuous variables were described as median with interquartile range (IQR). Differences between CKD and non-CKD participants were examined using Chi-square tests for categorical variables or Student t-tests for continuous variables. Both eGFR and protein-creatinine ratio were right skewed and, thus, log-transformed for linear regression. The 2-sided statistical significance level was set at α = 0.05.
Multivariate Poisson regression was performed to calculate prevalence ratios [28] and to assess the relationship between CKD and associated potential risk factors. These models were initially adjusted for sociodemographic and lifestyle variables including age, education, smoking habits, alcohol consumption and BMI, followed by adjustments for comorbidities including diabetes mellitus, hypertension, and C-reactive protein. Exploratory analyses were conducted within subgroups of the following covariates: age, sex, obesity, smoking, alcohol, total cholesterol, hypertension, diabetes mellitus, and Framingham risk score. All statistical analyses were conducted using STATA version 11.0 statistical software (StataCorp LP, College Station, TX, USA). It should be noted that exact logistic regression was also used to for univariate analysis and yielded results consistent with those found with Poisson regression.
Results
Population characteristics
The overall participation rate in the CRONICAS parent study was 62.9 %. A total of 404 adults, mean age 54.9 years [SD 13.0], 50.2 % male, were invited and agreed to participate in this sub-study of CKD. All 404 participants, 203 from Lima and 201 from Tumbes, completed a questionnaire, a clinical examination and laboratory tests. Overall, the prevalence of diabetes and hypertension were 9.9 % (40/404, 95 % CI 7.4–13.3 %) and 29.2 % (118/404, 95 % CI 25.1–34.0 %), respectively.
Prevalence chronic kidney disease
The prevalence of CKD was 16.8 % (68/404, 95 % CI 13.5 %–20.9 %). Participants with CKD tended to be older, female (69.1 %), consumers of alcohol (98.5 %), less educated, and with more comorbid conditions, including diabetes and hypertension (Table 1).
Among the 68 participants meeting the definition of CKD, 60 had isolated proteinuria, 4 had isolated impaired estimated glomerular filtration rate (eGFR <60 ml/min), and 4 had both proteinuria and impaired eGFR. Among participants with reduced eGFR (n = 8), mean eGFR was 40.1 ml/min (range 15.0 – 57.7 ml/min). Among participants with proteinuria (n = 64), the mean protein/creatinine ratio was 345.71 (range 151.5 – 1840.9 mg/g creatinine).
Factors associated with CKD
In unadjusted Poisson analysis, older age, females, residents of Lima, and those with less education, greater levels of insulin resistance, diabetes, and hypertension were associated with higher probability of having CKD (Table 2).
In multivariable Poisson analysis, age, sex, higher wealth index, Lima residence, and diabetes were all independently associated with CKD (Table 2). Males had a 66 % lower prevalence of CKD than females and participants at the Tumbes site had a 46 % lower prevalence than among those recruited at the Lima site. Diabetes was associated with doubling prevalence of CKD. It is noted that HOMA, hs-CRP, and Framingham Risk Score were not significant in the final model.
Exploratory stratified analysis by sex and geographical site
A higher prevalence of CKD was observed among females overall as well as within each age stratum (Table 3, Fig. 2). Among men, the prevalence of CKD was similar between most subgroups evaluated. The only exception was a tendency towards a gradient of doubling prevalence estimates in the Framingham risk score groups (Fig. 3, see also Additional file 1). In women, both diabetes and hypertension were associated with double the prevalence of CKD contrasted to those without these conditions (Fig. 3, see also Additional file 1).
CKD prevalence was higher (p-value = 0.04) in Lima (21 %) than in Tumbes (13 %). Females, having diabetes, and hypertension were significantly associated with higher CKD prevalence in both sites (Fig. 4, see also Additional file 1).
Discussion
In this representative cross-sectional sample of Peruvian adults from two different geographical sites, the prevalence of CKD was 16.8 %. These estimates are similar to those from high-income countries. Proteinuria, rather than reduced eGFR, was the defining factor for CKD in the vast majority (88 %) of individuals detected to have disease. In our sample, individuals with CKD were more likely to be female, older, of a higher socioeconomic stratum, diabetic and hypertensive. Compared to Tumbes, the study population in Lima had a higher prevalence of CKD.
Previous studies in Iran and the United States have found CKD prevalence estimates ranging between 13–18 %, and in Asian countries, such as China, Japan and Korea, 14–22 % [29–33]. Although measures of CKD prevalence in Latin America are rare [34], reports from El Salvador and Nicaragua have estimated that 12.7 % of these populations are afflicted with CKD [35, 36]. In addition, a recent survey examining CKD prevalence in Latin America reported the prevalence of proteinuria in Mexico and Chile to be 9.2 % and 14.2 %, respectively [37]. Notably, the definition of CKD used in these studies has varied including differences in thresholds defining proteinuria and eGFR, in equations used to calculate eGFR, and in the reliance on eGFR and proteinuria to define CKD. We defined CKD in accordance with other epidemiological studies by considering functional abnormalities, such as proteinuria, with and without lowered eGFR and utilizing prototypical cutoff values for both variables. We were not, however, able to incorporate the requirement of persistence of abnormalities for greater than three months [38].
Also noteworthy is the variability in the predominating factor defining CKD across diverse national settings- proteinuria dominance versus eGFR dominance. For instance, in Nicaragua, proteinuria was the predominant factor seen among CKD patients; an uncommon trend given that eGFR is usually more prominent [36]. In our study, CKD was proteinuria driven.
Older age and female gender were independent predictors of CKD among our participants, consistent with reports from the United States and Iran [29, 30] but not from Nicaragua and Sri Lanka where males had a higher prevalence of disease. CKD prevalence was higher in patients with vascular disease risk factors like diabetes, particularly in female patients and older patients. Indeed, in our female sample, but not in males, CKD was more prevalent among those who had diabetes and hypertension. It is noted however that males had a higher percentage of diabetes and hypertension, compared to females. The mechanisms underlying the sex difference in CKD epidemiology and CKD progression remain unclear and may involve a differential impact of traditional risk factors and environmental influences [39].
Poverty and social deprivation are documented risk factors for the development and progression rate of CKD in high-income countries and LMIC [40]. The sites fall below average socioeconomic indicators for Peru and Lima. For example, access to health insurance in our Lima and Tumbes sites was 37 % and 50 %, respectively [41]. Of note, the type of insurance reported is likely government subsidized insurance schemes, which cover basic services such as immunizations, maternal health, and large infectious diseases programs such as tuberculosis and HIV [9]. This could infer that access to general medical care where CKD can be detected and managed is low. Within this socioeconomic setting, and compared to the group with lowest wealth index, we identified a higher prevalence of CKD among participants within the highest wealth index tertile (all wealth strata were below the poverty line). Following significant expansion of Seguro Integral de Salud in 2009, it was noted that among the Peruvian poor, the poorest of the poor were insured to a greater extent than the wealthier of the poor [Ref is same as the new ref inserted in Intro]. Perhaps this subgroup accessed care that lessened the extent of CKD risk factors or, perhaps survival bias contributed to the trend. This difference, however, was not observed by education level.
CKD prevalence was higher in Lima than in Tumbes by nearly two-fold. This difference could be partly related to the higher prevalence of diabetes and hypertension, among patients with CKD, in Lima compared to Tumbes. It has been suggested that certain environmental factors promote CKD, including pesticide exposure, and others slow progression of CKD, including high altitude [35, 42, 43]. We were not able to directly examine either of these attributes in this study. More specifically, high altitude has been associated with higher levels of eGFR [43]. Though Lima and Tumbes are both approximately at sea level, nearly 50–60 % of the population at the Lima site are within-country migrants originating largely from high altitude Andean locations [41]. This might partially explain the smaller number of individuals in Lima with low eGFR and their higher average eGFR, compared to Tumbes. However, the reasons for the two-fold greater number of participants with proteinuria in Lima compared to Tumbes are unclear.
Study strengths and limitations
The strengths of this study include high-quality data collection methods and surveillance of disease outcomes, all nested in a well-designed population-based study, a high response rate within the ancillary CKD component of the larger study, and standardized laboratory methods for measuring renal biomarkers. However, the study also had several limitations. First, while the cross-sectional design of this study provided the opportunity to estimate CKD prevalence in two Peruvian sites, causal relationships between CKD and measured risk factors cannot be determined. Further, not all potential risk factors were measured such as environmental exposures and health care accessibility. Second, misclassification of CKD status could not be excluded as we relied on a single measurement of kidney biomarkers rather than multiple measurements over time. Third, the sample size was moderate and may not have been sufficient for detection of small-to-moderate effects of hypothesized risk factors. Finally, some information biases related to the nature of the study cannot be discounted, such as temporal ambiguity and lead time biases [44].
Conclusions
In summary, this study has identified a high prevalence, most likely undiagnosed, of CKD in Lima and Tumbes. This trend may or may not be representative of CKD throughout Peru. CKD’s silent progression, its association with cardiovascular disease, and high treatment costs make this disease one of great public health concern. As such, every effort should be made to expand upon this study through larger nationwide surveillance efforts. This will inform policies aimed at preventing CKD from escalating in Peru, especially given that renal replacement therapy is out of financial reach for the majority of Peruvians under their current health care system. These actions would pave the way for interventions aimed at reducing CKD prevalence and effectively managing existing cases of disease.
Abbreviations
- CKD:
-
Chronic kidney disease
- CRONICAS:
-
CRONICAS Center of Excellence in Chronic Disease
- LMIC:
-
Low- and middle-income countries
- eGFR:
-
Estimated glomerular filtration rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- EDTA:
-
Ethylenediaminetetraacetic acid
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- FRS:
-
Framingham risk score
- NCEP:
-
National Cholesterol Education Program
- ATP:
-
Adult Treatment Panel
- HOMA-IR:
-
Homeostatic model assessment - Insulin Resistance
- HDL:
-
High-density lipoprotein cholesterol
- CRP:
-
C-reactive protein
- BMI:
-
Body mass index
- IQR:
-
Interquartile range
References
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14.
Andrassy KM. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013;84:622–3.
World Health Orgnization. Investing in health for economic developement. Commission on Macroeconomics and Health. Geneva: World Health Orgnization; 2001.
Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97.
Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–5.
Huynen MM, Vollebregt L, Martens P, Benavides BM. The epidemiologic transition in Peru. Revista Panamericana de Salud Pública. 2005;17:51–9.
Alcalde-Rabanal JE, Lazo-González O, Nigenda G. Sistema de salud de Perú. Salud Publica Mex. 2011;53:S243–54.
Dirks JH, Robinson S, Burdmann E, Correa-Rotter R, Mezzano S, Rodriguez-Iturbe B. Prevention strategies for chronic kidney disease in Latin America: a strategy for the next decade—a report on the Villarica Conference. Ren Fail. 2006;28:611–5.
Daar AS, Singer PA, Persad DL, Pramming SK, Matthews DR, Beaglehole R, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007;450:494–6.
Miranda JJ, Gilman RH, Smeeth L. Differences in cardiovascular risk factors in rural, urban and rural-to-urban migrants in Peru. Heart. 2011;97:787–96.
Jacoby E, Goldstein J, López A, Núñez E, López T. Social class, family, and life-style factors associated with overweight and obesity among adults in Peruvian cities. Prev Med. 2003;37:396–405.
McKeown RE. The epidemiologic transition: changing patterns of mortality and population dynamics. Am J Lifestyle Med. 2009;3:19S–26.
Cieza J, Huamán C, Alvarez C, Gómez J, Castillo W. Prevalencia de insuficiencia renal crónica en la ciudad de Lima-Perú, Enero 1990. Rev Peru Epidemiol. 1992;5:22–7.
Carlini R, Obrador G, Campistrús N, Andrade L, Chifflet L, Bregman R, et al. The first report of The Latin American Society of Nephrology and Hypertension (SLANH) anemia committee in chronic hemodialysis patients. Nefrología (Madrid). 2014;34:96–104.
Cusumano AM, Di Gioia C, Hermida O, Lavorato C; Latin American Registry of Dialysis and Renal Transplantation. The Latin American Dialysis and Renal Transplantation Registry Annual Report 2002. Kidney Int Suppl. 2005: S46-52.
Cusumano AM, Garcia-Garcia G, Gonzalez-Bedat MC, Marinovich S, Lugon J, Poblete-Badal H, et al. Latin American Dialysis and Renal Transplant Registry: 2008 report (data 2006). Clin Nephrol. 2010;74 Suppl 1:S3–8.
Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W; CRONICAS Cohort Study Group. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ open. 2012;2:e000610.
LIoyd P. The ‘young towns’ of Lima: Aspects of urbanization in Peru. Cambridge: Cambridge University Press; 1980.
World Health Orgnization. The WHO STEPwise approach to chronic disease risk factor surveillance (STEPS). Geneva: World Health Orgnization; 2009.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–20.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Howe LD1, Galobardes B, Matijasevich A, Gordon D, Johnston D, Onwujekwe O, et al. Measuring socio-economic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–86.
Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21.
Hosseinpanah F, Kasraei F, Nassiri AA, Azizi F. High prevalence of chronic kidney disease in Iran: a large population-based study. BMC Public Health. 2009;9:44.
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47.
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–22.
Nagata M, Ninomiya T, Doi Y, Yonemoto K, Kubo M, Hata J, et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant. 2010;25:2557–64.
Kim S, Lim CS, Han DC, Kim GS, Chin HJ, Kim SJ, et al. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009;24(Suppl):S11–21.
Ordunez P, Saenz C, Martinez R, Chapman E, Reveiz L, Becerra F2. The epidemic of chronic kidney disease in Central America. Lancet Glob Health. 2014;2:e440–1.
Gracia-Trabanino R, Domínguez J, Jansà JM, Oliver A. Proteinuria and chronic renal failure in the coast of El Salvador: detection with low cost methods and associated factors. Nefrologia. 2005;25:31–8.
O'Donnell JK, Tobey M, Weiner DE, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–805.
Cusumano AM, Gonzalez Bedat MC. Chronic kidney disease in Latin America: time to improve screening and detection. Clin J Am Soc Nephrol. 2008;3:594–600.
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–100.
Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33:383–92.
Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–74.
Instituto Nacional de Estadística e Informática. Sistema de Difusión de los Censos Nacionales. Censos de Población y Vivienda 2007, vol. 2014. Lima: INEI; 2007.
Weiner DE, McClean MD, Kaufman JS, Brooks DR. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2013;8:504–11.
Ghahramani N, Ahmed F, Al-Laham A, Lengerich EJ. The epidemiological association of altitude with chronic kidney disease: Evidence of protective effect. Nephrology. 2011;16:219–24.
Delgado-Rodriguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58:635–41.
Acknowledgements
The CKD ancillary study was supported by a grant from the University of Pennsylvania’s Perelman School of Medicine. The CRONICAS Cohort Study was supported by the National Heart, Lung, and Blood Institute Global Health Initiative under the contract Global Health Activities in Developing Countries to Combat Non-Communicable Chronic Diseases (contract number 268200900033C-1-0-1). ERF was supported by funds from Weill Cornell Medical College (Global Health Fellowship) and the Johns Hopkins Bloomberg School of Public Health (Global Health Scholar, Center for Global Health).
We are indebted to the CRONICAS-UPCH and PRISMA teams who diligently recruited and cared for research participants. In addition, we are grateful for the faculty of the Johns Hopkins Bloomberg School of Public Health, including Dr. Marie Diener-West, Dr. James Tonascia, and Dr. Ebony Boulware, for their guidance in completing the data analysis as well the faculty at Weill Cornell Medical College, Dr. Oliver Fein and Dr. Madelon Finkel, for their support of this project. The authors would also like to thank Mrs. Susan Shultz for her help in editing the manuscript and Mr. Miguel Moscoso for helping format the manuscript.
CRONICAS Cohort Study Group
Cardiovascular Disease: Antonio Bernabé-Ortiz, Juan P. Casas, George Davey Smith, Shah Ebrahim, Héctor H. García, Robert H. Gilman, Luis Huicho, Germán Málaga, J. Jaime Miranda, Víctor M. Montori, Liam Smeeth; Chronic Obstructive Pulmonary Disease: William Checkley, Gregory B. Diette, Robert H. Gilman, Luis Huicho, Fabiola León-Velarde, María Rivera, Robert A. Wise; Training and Capacity Building: William Checkley, Héctor H. García, Robert H. Gilman, J. Jaime Miranda, Katherine Sacksteder.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
All the authors declared they have no conflicts of interest.
Authors’ contributions
All the efforts for this paper correspond to its authors and the contribution of each is shown below: Conception of the study: ABO, JJM, LN, HIF. Study design CKD ancillary study: ERF, ABO, LN, JJM, HIF. Study design CRONICAS Cohort Study: ABO, RHG, WC, JJM. Data collection: ERF, ABO, JJM. Data analysis: ERF, C-CK, ABO. Drafting the article: ERF, C-CK, ABO, JJM, HIF. Critical revisions to article: LN, RHG, WC. Provision of intellectual content of critical importance to the work described: All authors. Final approval of the version submitted: All authors.
J. Jaime Miranda and Harold I. Feldman are joint senior authors.
J. Jaime Miranda and Harold I. Feldman contributed equally to this work.
Additional file
Additional file 1:
Estimated prevalence of CKD and 95 % confidence interval by participants’ comorbidities stratified by sex and by area.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Francis, E.R., Kuo, CC., Bernabe-Ortiz, A. et al. Burden of chronic kidney disease in resource-limited settings from Peru: a population-based study. BMC Nephrol 16, 114 (2015). https://doi.org/10.1186/s12882-015-0104-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-015-0104-7