Abstract
Background
Schizophrenia is a severe, heritable, and refractory psychiatric disorder. Several studies have shown that the disrupted in schizophrenia 1 (DISC1) gene is closely associated with schizophrenia by its role in neuronal morphology, synaptic function, brain development, and dopamine homeostasis etc. This study intended to investigate the expression levels of DISC1 gene in schizophrenia patients compared with healthy controls, and the expression variation of DISC1 gene before and after antipsychotic treatment in schizophrenia patients.
Methods
In this study, we compared DISC1 expression levels in blood of 48 healthy controls, and 32 schizophrenia patients before and after 12 weeks of antipsychotic treatment using real-time quantitative PCR (RT-qPCR) analysis.
Results
The expression levels of DISC1 gene in peripheral blood mononuclear cells of schizophrenia patients before antipsychotic treatment were higher than those in healthy controls (P < 0.01); whereas after antipsychotic treatment, the expression levels of DISC1 gene in peripheral blood mononuclear cells of schizophrenia patients still remained increased (P < 0.01).
Conclusions
Our study provided further support for the involvement of DISC1 in the development of schizophrenia.
Similar content being viewed by others
Background
Schizophrenia (SCZ) is a chronic, severe mental disorder, accompanied by positive symptoms such as hallucinations, delusions, and negative symptoms including decreased motivation, anhedonia, cognitive impairment and social dysfunction [1,2,3]. The etiology of SCZ remains unclear, with environmental and genetic factors thought to play an important role [4, 5].
Since the disrupted in schizophrenia 1 (DISC1) gene was first discovered in a Scottish family with an unusually high incidence of SCZ and other mental disorders [6,7,8], it has been identified as a candidate risk gene for SCZ in multiple genetic and clinical association studies [6, 9]. DISC1 is a regulator of glutamate function, whose transmission dysfunction is considered to be at the core of mental disorder pathology [10, 11]. Devine et al. proposed that DISC1 controls transport of a wide range of neuronal cargos, including neurotransmitter receptors, mRNAs, vesicles, and mitochondria and regulates neuronal morphology and synaptic function, making it a key factor in the regulation of neuronal intracellular trade [12]. Degradation of the DISC1 subtype has been shown to lead to neurodevelopmental abnormalities, suggesting that the breakdown of DISC1 disrupts the mitochondrial dynamics of axons and dendrites [13].
Prenatal brain development has been implicated in the risk of mental illness, while gray matter has been shown to be substantially decreased in the neonatal homozygous for the DISC1 rs821616 serine alleles [14]. DISC1 has also been found to regulate astrocytes via modulating RAS/MEK/ERK signaling mediated by RASSF7 in the embryonic brain, whose defects might contribute to SCZ [7]. In addition, DISC1 translocation has been associated with decreased white matter integrity in the frontal junction and associated fiber bundles in both animal models and patients with psychosis [14, 15]. This cortical thinning observed in individuals with DISC1 translocation was confirmed to be highly similar to SCZ [16]. The DISC1 and SLC12A2 genes have been identified as SCZ risk genes, and their role in GABA depolarization co-regulates the development of hippocampal neurons. Two SNPs (rs1000731 in DISC1 and rs10089 in SLC12A2) have been shown to increase the risk of SCZ interactively, with subjects carrying both SNPs displaying a significant reduction in hippocampal activation as well as reduced connectivity with the prefrontal cortex [17]. In a model organism study, DISC1 has been shown to play a role in sleep regulation, suggesting a possible association between DISC1 and SCZ in terms of sleep [18]. Abnormal DISC1 and NDEL expression is linked to impaired cognitive function, which is a major symptom of SCZ [19], furthermore, it has been hypothesized that a relationship exists between DISC1 and dopamine in SCZ [5], as dopamine homeostasis is closely related to the integrity and expression level of DISC1 [20].
The use of antipsychotics by patients with SCZ has been known to alter gene expression in some cases, metabolism-related genes are an example of aforementioned genes whose expression has been shown to be affected [21, 22]. Moreover, several studies have revealed that many genes were up or down regulated in SCZ patients, while the gene expression of some genes may be restored to normal levels after treatment with antipsychotics [21, 23].
Although the effects of antipsychotic drugs on gene expression have been well studied, reports focusing on DISC1 are scarce and often conflicting. A typical example of such contrasting reports is a study that found DISC1 expression to be increased and remain elevated in peripheral blood mononuclear cells (PBMCs) of SCZ patients of Sinhalese descent, despite the use of antipsychotics [23]. From the above studies, we know that antipsychotics can affect gene expression and treat SCZ on a molecular level [24], and additionally that DISC1 is strongly associated with SCZ. It is therefore necessary to investigate the impact of antipsychotic treatment on the expression of DISC1. As far as we know this is the first study of its kind in the Chinese Han population and aims to discover the therapeutic significance of DISC1 in SCZ.
Methods
Ethics statement
The clinical research procedures were confirmed by the ethics committee of the Wuxi Mental Health Center and followed the World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. All enrolled participants (or their legal guardian in cases where the patient lacked the capability to provide consent) were required to sign an informed consent form when the patient was assessed by a psychiatrist.
Subject recruitment
All participants were inducted from the Han Chinese population in Shanxi province of China, including 32 SCZ patients and 48 healthy controls (HCs). There was no significant statistical difference in gender, age and ethnicity between the SCZ group and the HC group. (Table 1).
The SCZ patients were recruited from the First Hospital of Shanxi Medical University and diagnosed by two experienced psychiatrists based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. All SCZ patients were antipsychotic-naïve and received a 12-week course of antipsychotic treatment after commencement of the study. The SCZ patients were treated with the oral second-generation antipsychotics which were comprised of olanzapine (n = 10), quetiapine (n = 6), aripiprazole (n = 6), risperidone (n = 5), amisulpride (n = 3) and ziprasidone (n = 2). All patients in the SCZ group showed improved clinical symptoms with a reduction rate of over 25% according to the evaluation of the Positive and Negative syndrome Scale. The following diseases such as serious organic brain injury, alcohol or substance abuse, epilepsy, intellectual disability and other mental disorders should be excluded. On the basis of a Structured Clinical Interview for DSM-IV and Non-patients edition, the HC participants were randomly enrolled from local communities of Shanxi Province and there were no mental or neurological disorders among them.
Analysis of gene expression by real-time quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was utilized to analyze the expression levels of DISC1 in PBMCs of 48 HCs and 32 SCZ patients before and after the 12-week antipsychotic treatment, as described previously [25]. AGGATGAGGAGGAGGAGAGC (forward) and TTTGGGCATTTTCCATTCAT (reverse) were the PCR primers for DISC1. Prior to this total RNA was extracted from PBMCs using TRIzol reagent (Invitrogen, USA) with on-column DNase I treatment as described by the manufacturer.
Statistical analysis
SPSS 20.0 was used for all statistical analysis. The comparative Ct (2−ΔΔCt) method was used to analyze the relative expression level of DISC1 of each individual after normalization to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene. The Mann-Whitney U test was used to compare the expression levels of DISC1 in SCZ patients before and after the 12-week antipsychotic treatment, as well as the HC subjects [26]. The threshold for statistical significance was set at P < 0.01 (two-tailed).
Results
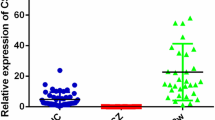
The expression levels of DISC1 gene in PBMCs of SCZ patients before antipsychotics were higher than those in HC subjects (Z = 5.34, P < 0.01). Nevertheless, the expression levels of DISC1 gene in PBMCs of SCZ patients were still elevated after 12-week antipsychotic therapy (Z = 3.59, P < 0.01) (Table 2 and Fig. 1).
Comparison of DISC1 expression levels in SZ, SZ_12w and with those in HCs. Notes: HCs: Healthy controls; SZ: schizophrenia patients before antipsychotics; SZ_12w: schizophrenia patients after 12-week antipsychotics; ① The expression levels of DISC1 gene in PBMCs of SCZ patients before antipsychotics were higher than those in HC subjects. P < 0.01 (Mann Whitney U test). ② The expression levels of DISC1 gene in PBMCs of SZ_12w patients were higher than those in baseline SZ patients. P < 0.01 (Mann Whitney U test)
Discussion
This study found that the expression levels of DISC1 gene in PBMCs of untreated SCZ patients were higher than those in HC subjects and continued to elevate despite 12 weeks of antipsychotic treatment. Our findings were consistent with previous research which reported that DISC1 expression increased in PBMCs of antipsychotic-naïve SCZ patients when compared to HCs and remained increased despite six to 8 weeks of antipsychotic treatment [23] (Table 3).
PBMCs are routinely employed in investigating gene expression as a substitute to brain tissue [27] because of its similar gene expression profile to brain tissue and relative ease of access [23, 28]. DISC1 protein expression in hippocampal tissue has been reported to be elevated in the patients with SCZ [29] (Table 3). Our results indicated that DISC1 expression levels in PBMCs of SCZ patients may not respond to drug therapy, which supports DISC1 as a trait-related rather than a state-related biomarker for SCZ. There is also the possibility that the effects of drug therapy on DISC1 expression may be specific to specific tissues or organs [19], therefore the effects of antipsychotics on DISC1 expression in other tissues should be further studied.
An animal model of SCZ, Disc1-l100p mice, display several SCZ-like symptoms such as hyperactivity, abnormal pre-pulse inhibition, enlarged lateral ventricles, decreased social activity [4, 30] and tend to have a prolonged release of dopamine, which is consistent with clinical findings that increased release of synaptic dopamine in the striatum of SCZ patients can lead to the deterioration of psychiatric symptoms [5, 31]. Antipsychotics could improve the behavior abnormalities and break the psycho-stimulatory effect of amphetamine in Disc1-l100p mutants [3, 5]. Su et al. discovered that the levels of the D2R-DISC1 complex were elevated with reduced GSK-3α/β (Ser21/9) phosphorylation in post-mortem brain tissue of patients with SCZ and disc1-l100p mutant mice, while interfering peptides that disrupt the D2R-DISC1 complex and haloperidol can potentially reverse behaviors associated with SCZ [3]. They further hypothesized that DISC1 facilitated the D2-receptor-mediated transmission of GSK-3 signals, which could be responsible for SCZ’s psychotic symptoms [32] through D2R-DISC1 interaction. Hippocampal neurons of DISC1-deficient mice displayed exaggerated endoplasmic reticulum calcium responses that led to hyperactive dopamine function, while antipsychotic drugs such as clozapine and haloperidol, were found to be capable of reversing the abnormal endoplasmic reticulum calcium dynamics caused by DISC1 dysfunction [12].
These studies mentioned above have indicated that different types of DISC1 mutation or dysfunction are related to hyperactive dopamine function or the maturation of dopamine neurons, while some model organism studies showed that antipsychotics or antipsychotic substances could reverse dopamine-related dysfunction. The pathogenesis of SCZ is currently unknown but a popular hypothesis is that SCZ is caused by dopamine dysfunction, as most antipsychotics block dopamine receptors [33, 34]. Due to the relationship between DISC1 and dopamine we concluded that it is necessary to study the effects of antipsychotics on DISC1 expression in humans our study did not, for the time being, identify changes in DISC1 expression that were consistent with the effectiveness of antipsychotics and improvement in patient symptoms. However, the possibility that DISC1 expression response to drug therapy may be delayed does exist and needs to be verified by increasing the follow-up time in the future.
Several groups have investigated the expression levels of DISC1 in SCZ patients compared to HCs. Fazio et al. [19] found decreased expression levels of DISC1 in whole blood of SCZ patients, which may be due to different RNA sources used, since they extracted the RNA from whole blood instead PBMCs. There was also study that failed to find a difference in expression of DISC1 between SCZ patients and HCs in the dorsolateral prefrontal cortex from postmortem brain tissue [35] (Table 3). The aforementioned inconsistencies can be explained by the fact that DISC1 gene might express differently in diverse tissues or brain regions of the body; heterogeneity of study samples could lead to variation in DISC1 expression [19]; and/or insufficient statistical power due to the small sample size. In consequence, due to the small sample size of our current study, we still need to expand the sample size in the future to further verify our results and the expression levels of DISC1 in different tissues, organs or lineages warrant further study.
The main restriction of this study was relatively small sample size, which might influence the statistical effects for comparing DISC1 expression level between SCZ patients and HC subjects, a larger sample may be required to validate the present findings in the future. Secondly, for the qPCR experiments, we used one control gene (GAPDH) for normalization, and it is therefore possible that changes in GAPDH rather than DISC1 explain the results. Although GAPDH is a common control gene for normalization for qPCR analysis and was used alone as a control gene for normalization in many literatures, it still needs to additionally measure (and correct DISC1 expression by) at least one another control gene to show that this is not the case in the future. At last, the relationship between clinical symptoms and expression data was lacking.
Conclusions
Our results supported the involvement of the DISC1 gene in the development of SCZ.
Availability of data and materials
For access to the data in this paper, interested researchers may contact the corresponding author.
Abbreviations
- DISC1 :
-
Disrupted in schizophrenia 1
- DSM-IV:
-
Diagnostic and statistical manual of mental disorders, 4th edition
- GAPDH :
-
Glyceraldehyde-3-phosphate dehydrogenase
- HCs:
-
Healthy controls
- IQR:
-
Interquartile range
- PBMCs:
-
Peripheral blood mononuclear cells
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- SCZ:
-
Schizophrenia
- SZ:
-
Schizophrenia patients before antipsychotics
- SZ_12w:
-
Schizophrenia patients after 12-week antipsychotics
References
Ross CA, Margolis RL, Reading SAJ, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52(1):139–53.
Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12(9):1016–22.
Su P, Li S, Chen S, Lipina TV, Wang M, Lai TKY, Lee FHF, Zhang H, Zhai D, Ferguson SSG, et al. A dopamine D2 receptor-DISC1 protein complex may contribute to antipsychotic-like effects. Neuron. 2014;84(6):1302–16.
Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54(3):387–402.
Lipina TV, Niwa M, Jaaro-Peled H, Fletcher PJ, Seeman P, Sawa A, Roder JC. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav. 2010;9(7):777–89.
Srikanth P, Han K, Callahan DG, Makovkina E, Muratore CR, Lalli MA, Zhou H, Boyd JD, Kosik KS, Selkoe DJ, et al. Genomic DISC1 disruption in hiPSCs alters Wnt signaling and neural cell fate. Cell Rep. 2015;12(9):1414–29.
Wang S, Liang Q, Qiao H, Li H, Shen T, Ji F, Jiao J. DISC1 regulates astrogenesis in the embryonic brain via modulation of RAS/MEK/ERK signaling through RASSF7. DEVELOPMENT. 2016;143(15):2732–40.
Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St CD, Muir WJ, Blackwood DH, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9(9):1415–23.
Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13(1):36–64.
Wei J, Graziane NM, Wang H, Zhong P, Wang Q, Liu W, Hayashi-Takagi A, Korth C, Sawa A, Brandon NJ, et al. Regulation of N-methyl-D-aspartate receptors by disrupted-in-Schizophrenia-1. Biol Psychiat. 2014;75(5):414–24.
Kantrowitz J, Javitt DC. Glutamatergic transmission in schizophrenia: from basic research to clinical practice. Curr Opin Psychiatry. 2012;25(2):96–102.
Devine MJ, Norkett R, Kittler JT. DISC1 is a coordinator of intracellular trafficking to shape neuronal development and connectivity. J Physiol. 2016;594(19):5459–69.
Norkett R, Modi S, Birsa N, Atkin TA, Ivankovic D, Pathania M, Trossbach SV, Korth C, Hirst WD, Kittler JT. DISC1-dependent regulation of mitochondrial dynamics controls the morphogenesis of complex neuronal dendrites. J Biol Chem. 2016;291(2):613–29.
Knickmeyer RC, Wang J, Zhu H, Geng X, Woolson S, Hamer RM, Konneker T, Lin W, Styner M, Gilmore JH. Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex. 2014;24(5):1230–46.
Whalley HC, Dimitrova R, Sprooten E, Dauvermann MR, Romaniuk L, Duff B, Watson AR, Moorhead B, Bastin M, Semple SI, et al. Effects of a balanced translocation between chromosomes 1 and 11 disrupting the DISC1 locus on White matter integrity. PLoS One. 2015;10(6):e130900.
Doyle OM, Bois C, Thomson P, Romaniuk L, Whitcher B, Williams SCR, Turkheimer FE, Stefansson H, McIntosh AM, Mehta MA, et al. The cortical thickness phenotype of individuals with DISC1 translocation resembles schizophrenia. J Clin Invest. 2015;125(9):3714–22.
Callicott JH, Feighery EL, Mattay VS, White MG, Chen Q, Baranger DAA, Berman KF, Lu B, Song H, Ming G, et al. DISC1 and SLC12A2 interaction affects human hippocampal function and connectivity. J Clin Invest. 2013;123(7):2961–4.
Jaaro-Peled H, Altimus C, LeGates T, Cash-Padgett T, Zoubovsky S, Hikida T, Ishizuka K, Hattar S, Mongrain V, Sawa A. Abnormal wake/sleep pattern in a novel gain-of-function model of DISC1. Neurosci Res. 2016;112:63–9.
Rampino A, Walker RM, Torrance HS, Anderson SM, Fazio L, Di Giorgio A, Taurisano P, Gelao B, Romano R, Masellis R, et al. Expression of DISC1-interactome members correlates with cognitive phenotypes related to schizophrenia. PLoS One. 2014;9(6):e99892.
Trossbach SV, Bader V, Hecher L, Pum ME, Masoud ST, Prikulis I, Schable S, de Souza SM, Su P, Boulat B, et al. Misassembly of full-length disrupted-in-schizophrenia 1 protein is linked to altered dopamine homeostasis and behavioral deficits. Mol Psychiatry. 2016;21(11):1561–72.
Crespo-Facorro B, Prieto C, Sainz J. Schizophrenia gene expression profile reverted to normal levels by antipsychotics. Int J Neuropsychoph. 2015;18(4):1–7.
Frank A. Middleton,1 Karoly Mirnics, Joseph N. Pierri, David a. Lewis, pat Levitt. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718–29.
Kumarasinghe N, Beveridge NJ, Gardiner E, Scott RJ, Yasawardene S, Perera A, Mendis J, Suriyakumara K, Schall U, Tooney PA. Gene expression profiling in treatment-naive schizophrenia patients identifies abnormalities in biological pathways involving AKT1 that are corrected by antipsychotic medication. Int J Neuropsychopharmacol. 2013;16(07):1483–503.
Chana G, Bousman CA, Money TT, Gibbons A, Gillett P, Dean B, Everall IP. Biomarker investigations related to pathophysiological pathways in schizophrenia and psychosis. Front Cell Neurosci. 2013;7:95.
Xu Y, Yao SY, Wang G, Cheng Z, Jin C, Zhang K, Wang J, Yu H, Yue W, Zhang F, et al. Altered expression of mRNA profiles in blood of early-onset schizophrenia. Sci Rep. 2016;6:16767.
Liu Y, Fu X, Tang Z, Li C, Xu Y, Zhang F, Zhou D, Zhu C. Altered expression of the CSMD1 gene in the peripheral blood of schizophrenia patients. BMC Psychiatry. 2019;19(1):113.
Kumarasinghe N, Tooney PA, Schall U. Finding the needle in the haystack: a review of microarray gene expression research into schizophrenia. Aust N Z J Psychiatry. 2012;46(7):598–610.
Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006; 141 B (3):261–268.
Lipska BK, Peters T, Hyde TM, Halim N, Horowitz C, Mitkus S, Weickert CS, Matsumoto M, Sawa A, Straub RE, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum Mol Genet. 2006;15(8):1245–58.
Lee FHF, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, Roder JC, Wong AHC. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci. 2011;31(9):3197–206.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300.
Beaulieu J. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatr Neurosci. 2012;37(1):7–16.
Agarwal V. Urinary incontinence with risperidone. J Clin Psychiatry. 2000;61(3):219.
Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient's clinical profile. CNS Spectr. 2004;9(10 Suppl 11):6–14.
Rastogi A, Zai C, Likhodi O, Kennedy JL, Wong AH. Genetic association and post-mortem brain mRNA analysis of DISC1 and related genes in schizophrenia. Schizophr Res. 2009;114(1–3):39–49.
Acknowledgments
The authors thank all participants for their cooperation in our study.
Funding
This study was funded by grants from Primary Research & Development Plan of Jiangsu Province (BE2016630), Suzhou Municipal Bureau of Science and Technology Program (SYSD2017136, SYSD2017140), The key program of intergovernmental international science and technology innovation cooperation (2017YFE0103700), The program of the Jiangsu Commission of health (LGY2019013), Key technology project of Suzhou Science and Technology Bureau (SS201882) and Suzhou municipal health and Family Planning Commission (LCZX201719). The funding body had no role in the study design; in collection, analysis, and interpretation of data; and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
FZ designed the study and performed data analyses. XF, CZ, GZ, YL, and LZ were responsible for manuscript writing. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committees of the Wuxi Mental Health Center. All participants signed a written informed consent prior to participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, X., Zhang, G., Liu, Y. et al. Altered expression of the DISC1 gene in peripheral blood of patients with schizophrenia. BMC Med Genet 21, 194 (2020). https://doi.org/10.1186/s12881-020-01132-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-020-01132-9