Abstract
Background
Central nervous system (CNS) hemangioblastomas are the most frequent cause of mortality in patients with Von Hippel–Lindau (VHL) disease, an autosomal dominant genetic disease resulting from germline mutations in the VHL tumor suppressor gene, with most mutations occurring in the exons. To date, there have been no reports of CNS hemangioblastoma cases related to pathogenic variants in intron 2 of VHL, which encodes a tumor suppressor protein (i.e., pVHL) that regulates hypoxia-inducible factor proteins.
Case presentation
We report the presence of a base substitution of c.464-1G > C and c.464-2A > G in the intron 2 of VHL causing CNS hemangioblastomas in six patients with VHL from two Chinese families. The clinical information about the two pathogentic variants has been submitted to ClinVar database. The ClinVar accession for NM_000551.3(VHL):c.464-1G > C was SCV001371687. This finding may provide a new approach for diagnosing and researching VHL-associated hemangioblastomas.
Conclusions
This is the first report of a pathogenic variant at intron 2 in VHL-associated hemangioblastomas. Gene sequencing showed that not only exonic but also intronic mutations can lead to the development of CNS hemangioblastomas.
Similar content being viewed by others
Background
Von Hippel–Lindau (VHL) disease is an autosomal dominant genetic disease resulting from germline mutations in the VHL tumor suppressor gene on chromosome 3 (3p25–26) within a 10 kb region. This gene consists of two introns and three exons and encodes a tumor suppressor protein (i.e., pVHL) that regulates hypoxia-inducible factor (HIF) proteins [1,2,3]. The main manifestation of VHL disease in the central nervous system (CNS) is hemangioblastoma (HGB). Some studies have shown that partial deletions are associated with a high risk of developing a large number of CNS HGBs. However, most mutations occur in the exons [4]. To date, no cases of intron 2 of VHL associated with CNS HGBs have been reported. Here, we report the cases of six patients with VHL-associated HGBs from two Chinese families with two types of intronic pathogenic variant in VHL.
Case presentation
The study was approved by the Ethics Committee of Peking University First Hospital and conformed to the Helsinki Declaration.
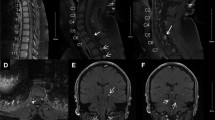
A 40-year-old male was admitted to Peking University First Hospital in 2018 with headache and dizziness that had been ongoing for 2 months. Notably, he had undergone surgical resection for cerebellar HGBs in 2015. When he was admitted in 2018, contrast-enhanced magnetic resonance imaging (MRI) of the cerebellum (3.0 T; Signa Excite HD 3.0 T; GE Healthcare, Chicago, IL, USA) showed multiple lesions in the cerebellum with obvious enhancement, highly suggestive of HGBs (Figs. 1a–c). In addition, abdominal computed tomography (CT) scanning with contrast revealed multiple cysts in the pancreas and kidneys (Fig. 1d). Because of the obvious occupy effect and symptoms, he underwent operation again. Postoperative pathology demonstrated that both lesions resected from the cerebellum were HGBs. The patient’s symptoms improved after surgery, and at two-year follow-up (FU), his condition was found to be stable with no recurrence. Five of his family members from three generations has also undergone surgical resections for CNS-related HGBs. The patient provided written informed consent to participate in this study and for publication of this case report.
A 58-year-old female was admitted to our hospital with symptoms similar to those of the male patient. Contrast-enhanced MRI showed multiple HGBs in the cerebellum with an obvious occupy effect (Fig. 2a–c). In addition, contrast-enhanced abdominal CT showed renal cell cancer and multiple renal and pancreatic cysts (Fig. 2d). She underwent surgical resection for HGBs in the bilateral cerebellum, and her symptoms improved. At two-year FU, her condition was stable with no recurrence.
Both patients and their family members who met the clinical diagnostic criteria underwent genetic testing. Clinical, genetic, and relevant imaging data were analyzed.
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). Three coding exons and their flanking intronic regions were amplified by polymerase chain reaction (PCR) using previously described primers [5]. Direct sequencing was performed to screen missense and splicing mutations. Large exon deletions were screened using a multiplex ligation-dependent probe amplification kit (P016-C2; MRC Holland, Amsterdam, the Netherlands) and confirmed by real-time quantitative PCR with primers described by Ebenazer et al. [6]. The reaction conditions and primers used for amplification were as previously described [7].
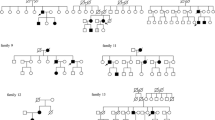
Gene sequencing revealed a heterozygous germline mutation (NM_000551.3(VHL):c.464-1G > C) in the splice site in intron 2 of VHL in the DNA from the peripheral blood of the male patient and nine of his family members (male 4, female 5) (Fig. 3a). In total, five of these nine patients from three generations underwent a total of nine surgical resections for CNS-related HGBs, with an average of 1.8 ± 1.3 surgeries (range: 1–4). All resected lesions were located in the cerebellum. Figure 4 shows the genetic investigation used in this study. In the first, second, and third generations, the ages at which the first operation for CNS HGBs was performed were 67, 32.3, and 17 years, respectively. Notably, the age at which the first operation for CNS HGBs was performed was younger in the children (younger generation) than in their parents (older generation). Additionally, only the mother of the male patient with the NM_000551.3(VHL):c.464-1G > C variant was diagnosed with renal cell carcinoma and underwent left nephrectomy. Another intronic mutation in VHL occurred in NM_000551.3(VHL):c.464-2A > G in the 58-year-old female patient (Fig. 3b), but none of her family members showed any abnormal changes in VHL. The clinical information about the two pathogentic variants has been submitted to ClinVar database. The ClinVar accession for NM_000551.3(VHL):c.464-1G > C was SCV001371687.
Family tree of the patients with c.464-1G > C point mutation. Three-generation pedigree of the patient family showing that the synonymous VHL variant c.464-1G > C segregates with affected family members. The number on the upper left corner of each patient with VHL-associated HGBs indicates the age of each operation for CNS HGBs
Discussion and conclusions
Some studies reported a germline mutation in intron 1 of VHL. This intronic mutation at the splice site occurs in various VHL-related diseases, such as erythrocytosis (NM_000551.4(VHL):c.340 + 770 T > C), renal cancer, or even tongue cancer (VHL c.340 + 5G > C) [8, 9]. However, the pathogenic mechanism and importance of this mutation remain unclear. The most likely cause of this condition is abnormal splicing. Lenglet et al. [8] also assumed that this intronic mutation (NM_000551.4(VHL):c.340 + 770 T > C) affects exonic splicing, resulting in the failure to produce a VHL protein. Genetic mutations also cause pVHL inactivation, resulting in dysfunction in the regulation of proteolytic degradation of HIF proteins [10]. In addition, uncontrolled HIF expression increases the expression of a wide range of target genes involved in angiogenesis, proliferation, and metabolism, including the vascular endothelial growth factor and C-X-C motif chemokine receptor 4. It is well-known that overexpression of VEGF is a leading factor in tumor angiogenesis, particularly for HGBs [11, 12]. In addition, several researchers have suggested that nearly one-third of HGBs are related to VHL disease [13]. Ong and Whaley reported the NM_000551.3(VHL):c.464-1G > C and NM_000551.3(VHL):c.464-2A > C mutations, respectively [14, 15]. However, both studies only described the intronic mutation in sporadic renal cell carcinoma. Neither study could determine whether the intronic variant was pathogenic. A query of the ClinVar database revealed that our research is the first report of a germline mutation at c.464-1G > C and c.464-2A > C in VHL-associated HGBs. This is also the first report of a pathogenic intron variant (NM_000551.3(VHL):c.464-1G > C) in a pedigree with VHL disease. Sequencing results showed that a base substitution of c.464-1G > C and c.464-2A > G in the intron 2 splice donor site of VHL could result in abnormal splicing. The family history of these patients with HGB was accordance with strong evidence showing that cosegregation with disease in multiple affected family members in a gene definitively known to cause the disease. According to the American College of Medical Genetics and Genomics standards and guidelines, we found that both variants were pathogenic [16]. According to previous research, there is increasing evidence that CNS HGBs are the most frequent cause of mortality in patients with VHL disease [17, 18]. However, some studies reported that approximately 20% of patients have VHL disease resulting from a de novo mutation and have no family history [19]. Thus, not all CNS HGB cases are screened for germline VHL mutations and the rate may be underestimated. Notably, both patients mentioned above, the female patient with c.464-2A > G and the male patient’s mother with c.464-1G > C, exhibited a nervous system manifestation as the initial symptom caused by CNS HGBs. Both patients underwent surgical treatment for renal cell carcinoma 2–3 years after CNS surgery. Hence, detecting molecular changes has several implications for the clinical management of these patients who are suspected to have VHL disease with CNS HGBs as the initial pathogenic manifestations. To our knowledge, this is the first report of a germline mutation in intron 2 in VHL-associated HGBs. The gene sequencing results showed that not only the exon region but also the intronic mutation could lead to the development of CNS HGBs. When no mutation can be detected in the exons, the possibility of intronic mutation cannot be ignored. In addition, pedigree analysis demonstrated that the age at which the first operation was performed was younger in the children than in their parents. Some researchers have suggested that the regularity with which this occurs is associated with genetic anticipation. The reasons for this phenomenon are considered to be related to telomere shortening in the offspring [20].
In this study, it was found that not only exonic mutations but also intronic mutations (c.464-2A > G and c.464-1G > C) in VHL play a pivotal role in the occurrence of CNS HGBs, which may provide a new approach for the diagnosis and research of VHL-associated HGBs.
Availability of data and materials
The raw datasets generated and analyzed during the current study are not publicly available because it is possible that individual privacy could be compromised but are available upon reasonable request with fulfillment of Materials Transfer Agreement. To request the datasets, please contact the corresponding authors (Liang Li doctzyf@126.com).
The dataset corresponding to the NM_000551.3(VHL):c.464-1G > C and NM_000551.3(VHL):c.464-2A > G gene can be found in NCBI under the accession number LOC107303340 (https://www.ncbi.nlm.nih.gov/gene/?term=LOC107303340). The c.464-1G > C variation was submitted to ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/) and the ClinVar accession number SCV001371687 assigned to it.
Abbreviations
- CNS:
-
Central nervous system
- HGBs:
-
Hemangioblastomas
- VHL:
-
Von Hippel–Lindau
- HIF:
-
Hypoxia-inducible factor
- VEGF:
-
Vascular endothelial growth factor
References
Maher E, Iselius L, Yates J, Littler M, Benjamin C, Harris R, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28(7):443–7. https://doi.org/10.1136/jmg.28.7.443.
Neumann H, Wiestler O. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337(8749):1052–4. https://doi.org/10.1016/0140-6736(91)91705-Y.
Kuzmin I, Duh FM, Latif F, Geil L, Zbar B, Lerman MI. Identification of the promoter of the human von Hippel-Lindau disease tumor suppressor gene. Oncogene. 1995;10(11):2185–94.
Lonser R, Butman J, Huntoon K, Asthagiri A, Wu T, Bakhtian K, et al. Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J Neurosurg. 2014;120(5):1055–62. https://doi.org/10.3171/2014.1.JNS131431.
Wu P, Zhang N, Wang X, Ning X, Li T, Bu D, et al. Family history of von Hippel–Lindau disease was uncommon in Chinese patients: suggesting the higher frequency of de novo mutations in VHL gene in these patients. J Hum Genet. 2012;57(4):238–43. https://doi.org/10.1038/jhg.2012.10.
Ebenazer A, Simon Rajaratnam S, Rekha Pai R. Detection of large deletions in the VHL gene using a real-time PCR with SYBR green. Familial Cancer. 2013;12(3):519–24.
Peng S, Shepard MJ, Wang J, Li T, Ning X, Cai L, et al. Genotype-phenotype correlations in Chinese von Hippel-Lindau disease patients. Oncotarget. 2017;8(24):38456–65.
Lenglet M, Robriquet F, Schwarz K, Camps C, Couturier A, Hoogewijs D, et al. Identification of a new VHL exon and complex splicing alterations in familial erythrocytosis or von Hippel-Lindau disease. Blood. 2018;132(5):469–83. https://doi.org/10.1182/blood-2018-03-838235.
Asakawa T, Esumi M, Endo S, Kida A, Ikeda M. A mutation at IVS1 + 5 of the von Hippel-Lindau gene resulting in intron retention in transcripts is not pathogenic in a patient with a tongue cancer?: case report. BMC Med Genet. 2012;13:23. https://doi.org/10.1186/1471-2350-13-23.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72.
Pierscianek D, Wolf S, Keyvani K, El Hindy N, Stein KP, Sandalcioglu IE, et al. Study of angiogenic signaling pathways in hemangioblastoma. Neuropathology. 2017;37(1):3–11. https://doi.org/10.1111/neup.12316.
Kruizinga RC, van Marion DM, den Dunnen WF, de Groot JC, Hoving EW, Oosting SF, et al. Difference in CXCR4 expression between sporadic and VHL-related hemangioblastoma. Fam Cancer. 2016;15(4):607–16. https://doi.org/10.1007/s10689-016-9879-3.
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19(6):617–23. https://doi.org/10.1038/ejhg.2010.175.
Ong KR, Woodward ER, Killick P, Lim C, Macdonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007;28:143–9.
Whaley JM, Naglich J, Gelbert L, et al. Germ-line mutations in the von Hippel-Lindau tumor-suppressor gene are similar to somatic von Hippel-Lindau aberrations in sporadic renal cell carcinoma. Am J Hum Genet. 1994;55:1092–102.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Zhou B, Wang J, Liu S, Peng X, Hong B, Zhou J, et al. Hemangioblastoma instead of renal cell carcinoma plays a major role in the unfavorable overall survival of Von Hippel-Lindau disease patients. Front Oncol. 2019;9:1037. https://doi.org/10.3389/fonc.2019.01037.
Dornbos D, Kim H, Butman J, Lonser R. Review of the neurological implications of von Hippel-Lindau disease. JAMA Neurol. 2018;75(5):620–7. https://doi.org/10.1001/jamaneurol.2017.4469.
Richards FM, Payne SJ, Zbar B, Affara NA, Ferguson-Smith MA, Maher ER. Molecular analysis of de novo germline mutations in the von Hippel-Lindau disease gene. Hum Mol Genet. 1995;4(11):2139–43. https://doi.org/10.1093/hmg/4.11.2139.
Aronoff L, Malkin D, van Engelen K, Gallinger B, Wasserman J, Kim RH, et al. Evidence for genetic anticipation in vonHippel-Lindau syndrome. J Med Genet. 2018;55(6):395–402.
Acknowledgements
Not Applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZL, MD: Design and conceptualized study, analyzed the data, drafted the manuscript for intellectual content. JZ, MD: Performed the experiments and conducted the genetic sequencing. LL, MD: Data collection and analysis, drafting and revision of manuscript, drafted the manuscript for intellectual content. ZY, MD: Data collection and statistical analysis, drafting and revision of manuscript. RL, MD: Helped perform the analysis through constructive discussions. CL, MD: Helped perform the analysis through constructive discussions. KG, MD: Drafted the manuscript for intellectual content. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Peking University First Hospital, conforming to the Helsinki Declaration, and all patients provided written informed consent.. The patient and legal guardians of any participant under the age of 16 years gave written informed consent to participate in this study and for publication of this case report.
Consent for publication
All participants have provided written informed consents to publish all identifying images, personal details and clinical details anonymously. Moreover, written informed consents for publication of identifying images or other personal or clinical details were obtained from the parents or legal guardians of any participant under the age of 16.
Competing interests
The authors certify that there is no conflict of interest with any individual/organization for the present work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Z., Zhou, J., Li, L. et al. Intronic mutation of the VHL gene associated with central nervous system hemangioblastomas in two Chinese families with Von Hippel–Lindau disease: case report. BMC Med Genet 21, 191 (2020). https://doi.org/10.1186/s12881-020-01126-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-020-01126-7