Abstract
Background
The prevalence of CGG repeat expansion mutation in FMR1 gene varies among different populations. In this study, we investigated the prevalence of this mutation in women of reproductive age from northern China.
Methods
A total of 11,891 pre-conceptional or pregnant women, including 5037 pregnant women and 7357 women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos, were recruited. The number of CGG repeats in FMR1 was measured by the TRP-PCR method. We also offered genetic counseling and prenatal diagnosis to the women carrying pre-mutation or full mutation alleles.
Results
The prevalence of pre-mutation in reproductive women in northern China was 1/410, higher than that in southern China and Korea but lower than that in western countries. We also found that the prevalence of pre-mutation was relatively high (1/320) in women with abortion history.
Conclusion
Screening for CGG repeat expansion mutation in FMR1 should be recommended to the women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos.
Similar content being viewed by others
Background
Fragile X syndrome (FXS, OMIM 300624), one of the common forms of familial intellectual disability, is caused by CGG repeat expansion in the 5′-untranslated region of FMR1 gene on X chromosome. According to the standards and guidelines for fragile X testing from American College of Medical Genetics (ACMG), 5–44 CGG repeats can be defined as normal, 45–54 CGG repeats as intermediate or in a grey zone, 55–200 CGG repeats as pre-mutation, and > 200 CGG repeats as full mutation [1, 2].
Individuals with the full mutation are typically with FXS. Pre-mutation carriers are not associated with FXS, but have an increased risk for fragile X associated primary ovarian insufficiency (FXPOI) or fragile X associated tremor/ataxia syndrome (FXTAS). Female pre-mutation carriers have a higher chance to have FXS children because of the potential repeat instability of pre-mutation allele after transmission. Intermediate carriers are at a higher risk for expanding of CGG repeats to give pre-mutation offspring or full mutation patients in subsequent generations [3, 4]. Therefore, identification of pre-mutation in women of reproductive age is of clinically significance for providing information about the risk for FXPOI and the birth of FXS children [4,5,6].
The screening for CGG expansion mutation in FMR1 has been conducted in many countries [7,8,9,10,11]. The prevalence of CGG repeat pre-mutation varies in different populations but is unknown in northern China. In this study, we demonstrate the prevalence of CGG repeat mutation in FMR1 in a cohort of 11,891 women of reproductive age from northern China as well as pregnancy outcome in the pre-mutation and full mutation carriers in this cohort.
Methods
Subjects
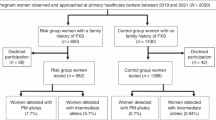
A total of 11,891 pre-conceptional or pregnant women from obstetrics department or family planning department were tested at the Central Laboratory of Peking University First Hospital during the period from Jan. 2015 to Sep. 2017. They asked for the test after the education and genetic counseling from doctors. A part of them had the history of spontaneous abortion or induced abortion due to delayed growth of the embryos. The family history of mental retardation was excluded by questionnaire. Informed consent was obtained from these women. This study was approved by the Medical Ethics Committee of Peking University First Hospital.
Their age ranged from 21 to 50 years (31.33 ± 5.87 years). Among these women, 5037 were pregnant (12–22 weeks), 7357 had the history of spontaneous abortion or induced abortion due to delayed growth of the embryos, and 4534 had no specific history. After the test, the women with pre-mutation or full mutation alleles were advised to take prenatal diagnosis for CGG repeats in FMR1 in amniotic fluid, chorionic villi or cord blood when they became pregnant.
Laboratory methods
The number of CGG repeats in FMR1 was measured by triplet repeat primed PCR (TRP-PCR) using the Amplide X FMR1 PCR Kit (Asuragen, Austin, TX, USA) following the manufacturer protocol. This method can detect the CGG repeats from 8 to > 200. Total DNA was extracted from peripheral leukocytes by routine method. PCR was performed in an ABI GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). Amplicon was sized on an ABI 3730xl Genetic Analyzer (Applied Biosystems) and analyzed using GeneMapper 4.0 software (Applied Biosystems). Number of CGG repeats in FMR1 was then categorized as normal (< 45 repeats), intermediate (45–54 repeats), pre-mutation (55–200 repeats) or full mutation (> 200 repeats).
For prenatal diagnoses, DNA were extracted from chorionic villi, amniotic fluid or cord blood using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and the same TRP-PCR method. Linkage analyses were also included using five STR markers nearby FMR1 to exclude false results due to maternal blood contamination.
The prevalence of CGG repeat mutation was defined as the ratio of pre-mutation and full mutation alleles to total FMR1 alleles. The 95% confidence interval (CI) was calculated using Wilson score interval method. Chi-square test was used to compare the groups with and without abortion history. A P value < 0.05 was considered to be statistically significant.
Results
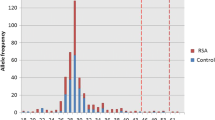
A total of 11,819 women of reproductive age from northern China were screened for CGG repeat expansion mutation in FMR1. The number of this CGG repeats was normally 29, encompassing 42.5% alleles of this cohort. Intermediate mutation carriers (45–54 CGG repeats), pre-mutation carriers (55–200 CGG repeats) and full mutation carriers (> 200 CGG repeats) were found in 76, 29 and 3 women, respectively, with the prevalence of 1/156 (CI 1:199~125), 1/410 (CI 1:588~286) and 1/3940 (CI 1:11,765~1351), respectively. Table 1 shows the distribution of CGG repeat number in the 32 pre-mutation and full mutation carriers.
The relationship between CGG repeat mutation carrier and abortion history is present in Table 2, which indicates that the prevalence of pre-mutation carriers was higher in women with abortion history (1/320) than in those without abortion history (1/756), but without statistically significance (P = 0.053).
In the 17 pregnant mothers of pre-mutation or full mutation carriers, 15 mothers agreed to perform prenatal diagnosis of the fetuses. Table 3 shows the prenatal diagnosis results. In the 13 pre-mutation mothers, 8 fetuses carried expanded CGG repeats from their mothers (CGG repeats = 78~115) and became full mutation carriers; 2 fetuses inherited the pre-mutation allele from their mothers with only minor expansion of the CGG repeats; and 3 fetuses inherited the normal FMR1 alleles from their mothers. In the 2 full mutation mothers, one fetus inherited the full-mutation allele (CGG > 200) from mother and was also a 21-trisomy (47, XY + 21) by karyotyping; the other fetus fortunately inherited the normal allele (CGG repeats = 36) from mother.
We also found a 32 years old woman carrying mosaic full mutation (CGG repeats = 200), pre-mutation (CGG repeats = 97) and normal CGG repeats (CGG repeats = 30). She had no neurological symptoms, menstrual irregularities or sex hormone problems but had the history of one abortion.
Discussion
To the best of our knowledge, this is the first study on the prevalence of CGG repeat expansion mutation in FMR1 in women of reproductive age in China. In this cohort, the prevalence of CGG repeat expansion mutation in FMR1 was 1/410 for pre-mutation and 1/3964 for full mutation. Totally, the prevalence of pre-mutation and full-mutation was 1/372, which is useful to estimate the risk for FXS transmitted from mothers.
Many researches have shown that the founder effect is partially responsible for the variation of carrier frequency in different populations. The prevalence of pre-mutation carrier in women of reproductive age without family history of intellectual disability or with unselected family history was 1/151–382 in USA [12,13,14,15], 1/259–549 in Canada [16], 1/83 in Finland [17] and 1/113–157 in Israel [18, 19]. However, the prevalence was lower in eastern countries. In a study of 5470 Korean women of reproductive age without family history, the prevalence of pre-mutation carrier was 1/781 [20, 21]. Two recent studies on the pregnant women from Hong Kong and Taiwan in southern China showed the prevalence of 1/1325 (total subjects = 2650) and 1/1955 (total subjects = 3911), respectively [22,23,24]. The prevalence of our cohort (1/410) is higher than that from Korea, Hong Kong and Taiwan, and is similar to that from Korea if we exclude the women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos from total subjects (1/756).
In this cohort, the prevalence of pre-mutation carriers was higher in women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos than in those without abortion history (1/320 vs. 1/756, P = 0.053), of which the information was not found in the literature. Totally, 61.2% of our subjects had abortion history. The prevalence of pre-mutation carrier of our subjects was 1/410, higher than that in other eastern countries. More samples should be tested to confirm the difference because of only 29 pre-mutation carriers we detected in this cohort.
The mechanism of abortion associated with pre-mutation of CGG repeat is not clear. In addition to FXPOI, pre-mutation carrier women are also at higher risks for the abnormalities involving neurology, reproductive, endocrinology, immunology and psychiatry systems [25]. Pre-mutation carrier women present a continuum of diminished ovarian follicular reserve, from which irregularity of menstrual cycle, decreased fertility, fluctuation of hormone levels, poor quality of oocytes in follicles and abortion occur [25,26,27].
Due to the low incidence of CGG repeat expansion mutation in FMR1, it is not recommended to widely screen for this mutation in population in China. The guidelines for fragile-X test proposed by American Congress of Obstetricians and Gynecologists (ACOG) and American College of Medical Genetics (ACMG) [1, 3] recommend that this test should be performed in individuals with a personal or family history of fragile-X, fragile-X-related disorders, unexplained mental retardation or developmental delay, autism, ovarian insufficiency or elevated follicle-stimulating hormone before 40 years old of unknown cause. Because the prevalence of pre-mutation carriers is relatively high in women with abortion history in this cohort, the indications for screening CGG repeat mutation should include women having the history of spontaneous abortion or induced abortion due to delayed growth of the embryos.
In this study, 15 of the 17 pregnant women accepted prenatal diagnosis for the fetuses. All of the 9 mothers having full mutation fetuses agreed to terminate the pregnancy. In the 13 pregnant women with pre-mutation, the pre-mutation alleles were transmitted to 10 fetuses, in which 8 pre-mutation alleles with the CGG repeats of 78–115 expanded greatly to full mutation alleles. Our results are consistent with the previous notion that 55 (or 60) CGG repeats can be used as the cutoff value. Prenatal diagnosis is essential if the pregnant woman brings CGG repeats in FMR1 more than the cutoff value. Two of the 17 pregnant women with pre-mutation (60 and 69 CGG repeats, respectively) refused to perform prenatal diagnosis and had normal babies by follow-up study. But there was a limitation for this study that the methylation of status for the full mutation was not detected.
Some women with the indications were not willing to do CGG repeat expansion test due to medical expense problems. In addition, women with abortion were more likely to try this test. The sample bias impacts on the prevalence of pre-mutation carrier in this cohort.
Conclusions
The prevalence of pre-mutation in reproductive women from northern China was 1/410, higher than that in southern China and Korea but lower than that in western countries. The prevalence of pre-mutation was higher (1/320) in the reproductive women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos. Therefore, screening for CGG repeat expansion mutation in FMR1 should be recommended to the women with the history of spontaneous abortion or induced abortion due to delayed growth of the embryos.
Abbreviations
- ACMG:
-
American College of Medical Genetics
- FMR1 :
-
Fragile X mental retardation 1 gene
- FXPOI:
-
Fragile X associated primary ovarian insufficiency
- FXS:
-
Fragile X syndrome
- FXTAS:
-
Fragile X associated tremor/ataxia syndrome
- TRP-PCR:
-
Triplet repeat primed PCR
References
Monaghan KG, Lyon E, Spector EB. ACMG standards and guidelines for fragile X testing: a revision to the disease-specific supplements to the standards and guidelines for clinical genetics Laboratories of the American College of medical genetics and genomics. Genet Med. 2013;15:575–86.
Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–98.
Finucane B, Abrams L, Cronister A, Archibald AD, Bennett RL, McConkie-Rosell A. Genetic counseling and testing for FMR1 gene mutations: practice guidelines of the national society of genetic counselors. J Genet Couns. 2012;21:752–60.
Wheeler AC, Bailey DB Jr, Berry-Kravis E, Greenberg J, Losh M, Mailick M, et al. Associated features in females with an FMR1 premutation. J Neurodev Disord. 2014;6:30.
Gutiérrez JF, Bajaj K, Klugman SD. Prenatal screening for fragile x: carriers, controversies, and counseling. Rev Obstet Gynecol. 2013;6(1):e1–7.
Alfaro Arenas R, Rosell Andreo J, Heine Suñer D. Group for the study of FXS in the Balearic Islands. Fragile X syndrome screening in pregnant women and women planning pregnancy shows a remarkably high FMR1 premutation prevalence in the Balearic Islands. Am J Med Genet B Neuropsychiatr Genet. 2016;171:1023–31.
Grigsby J. The fragile X mental retardation 1 gene (FMR1): historical perspective, phenotypes, mechanism, pathology, and epidemiology. Clin Neuropsychol. 2016;30:815–33.
Hantash FM, Goos DM, Crossley B, Anderson B, Zhang K, Sun W, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13:39–45.
Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med. 2014;16:19–24.
Gabis LV, Gruber N, Berkenstadt M, Shefer S, Attia OL, Mula D, et al. Fragile X Premutation carrier epidemiology and symptomatology in Israel-results from a tertiary child developmental center. Cerebellum. 2016;15:595–8.
Santa María L, Aliaga S, Faundes V, Morales P, Pugin Á, Curotto B, et al. FMR1 gene mutations in patients with fragile X syndrome and obligate carriers: 30 years of experience in Chile. Genet Res (Camb). 2016;98:e11.
Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:589–97.
Cronister A, Teicher J, Rohlfs EM, Donnenfeld A, Hallam S. Prevalence and instability of fragile X alleles: implications for offering fragile X prenatal diagnosis. Obstet Gynecol. 2008;111:596–601.
Cronister A, DiMaio M, Mahoney MJ, Donnenfeld AE, Hallam S. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet Med. 2005;7:246–50.
Strom CM, Crossley B, Redman JB, Buller A, Quan F, Peng M, et al. Molecular testing for fragile X syndrome: lessons learned from 119,232 tests performed in a clinical laboratory. Genet Med. 2007;9:46–51.
Lévesque S, Dombrowski C, Morel ML, Rehel R, Côté JS, Bussières J, et al. Screening and instability of FMR1 alleles in a prospective sample of 24,449 mother-newborn pairs from the general population. Clin Genet. 2009;76:511–23.
Ryynänen M, Heinonen S, Makkonen M, Kajanoja E, Mannermaa A, Pertti K. Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet. 1999;7:212–6.
Berkenstadt M, Ries-Levavi L, Cuckle H, Peleg L, Barkai G. Preconceptional and prenatal screening for fragile X syndrome: experience with 40,000 tests. Prenat Diagn. 2007;27:991–4.
Toledano-Alhadef H, Basel-Vanagaite L, Magal N, Davidov B, Ehrlich S, Drasinover V, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351–60.
Jang JH, Lee K, Cho EH, Lee EH, Kim JW, Ki CS. Frequency of FMR1 premutation carriers and rate of expansion to full mutation in a retrospective diagnostic FMR1 Korean sample. Clin Genet. 2014;85:441–5.
Kim MJ, Kim DJ, Kim SY, Yang JH, Kim MH, Lee SW, et al. Fragile X carrier screening in Korean women of reproductive age. J Med Screen. 2013;20:15–20.
Cheng YK, Lin CS, Kwok YK, Chan YM, Lau TK, Leung TY, et al. Identification of fragile X pre-mutation carriers in the Chinese obstetric population using a robust FMR1 polymerase chain reaction assay: implications for screening and prenatal diagnosis. Hong Kong Med J. 2017;23(2):110–6.
Tzeng CC, Tsai LP, Chang YK, Hung YJ, Chang YY, Su YP, et al. A 15-year-long southern blotting analysis of FMR1 to detect female carriers and for prenatal diagnosis of fragile X syndrome in Taiwan. Clin Genet. 2017;92:217–20.
Huang KF, Chen WY, Tsai YC, Lin CC, Chen SH, Tseng CY, et al. Original article pilot screening for fragile X carrier in pregnant women of southern Taiwan. J Chin Med Assoc. 2003;66(4):204–9.
Noto V, Harrity C, Walsh D, Marron K. The impact of FMR1 gene mutations on human reproduction and development: a systematic review. J Assist Reprod Genet. 2016;33:1135–47.
Elizur SE, Lebovitz O, Derech-Haim S, Dratviman-Storobinsky O, Feldman B, Dor J, et al. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One. 2014;9(8):e105121.
Hoyos LR, Thakur M. Fragile X premutation in women: recognizing the health challenges beyond primary ovarian insufficiency. J Assist Reprod Genet. 2017;34:315–23.
Acknowledgments
We thank the women who participated in the study and our colleagues in Beijing Huanuo Aomei Gene Biotech Co. Ltd. and Peking University First Hospital.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
YM designed the study and drafted the manuscript; XW, HP, SW, XW and participated in the study design; LZ, XW, XW, HY, FW, KW, LS, XQ, YY, XM, DL, GD, JM and XY participated in collecting samples and experiments; SZ did the statistics work; CY and YQ participated in the study coordination and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Peking University First Hospital. Written informed consent to participate was obtained from all patients for being included in the study on behalf of the patients.
Consent for publication
Not application.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ma, Y., Wei, X., Pan, H. et al. The prevalence of CGG repeat expansion mutation in FMR1 gene in the northern Chinese women of reproductive age. BMC Med Genet 20, 81 (2019). https://doi.org/10.1186/s12881-019-0805-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-019-0805-z