Abstract
Background
The purpose of this study was to detect the effects of vascular endothelial growth factor (VEGF) on systemic lupus erythematosus (SLE) risk.
Methods
Associated studies were extracted from the China Biological Medicine Database (CBM), and PubMed on June 10, 2018, and applicable investigations were pooled and analyzed by meta-analysis using RevMan 5.3.
Results
VEGF levels was associated with SLE risk (mean differences (MD) =196.02, 95% CI: 135.29–256.75, P < 0.00001), and VEGF levels was associated with active SLE risk (MD =77.51, 95% CI: 10.98–144.05, P = 0.02). We also found that VEGF levels was associated with SLE developing into lupus nephritis (LN) risk (MD =223.16, 95% CI: 144.38–301.93, P < 0.00001). However, VEGF -634G/C gene polymorphism (rs2010963) was not associated with SLE risk.
Conclusions
VEGF levels was associated with SLE risk, active SLE risk and SLE developing into LN risk. However, there was no an association between VEGF -634G/C gene polymorphism and SLE risk.
Similar content being viewed by others
Background
Vascular endothelial growth factor (VEGF), located on chromosome 6 (6p21.1) and a key regulator of vascular formation, is initially characterized by its actions on the endothelial fenestration and maintains permeability of capillary vessels, inducing vasculogenesis, angiogenesis [1, 2]. VEGF, an essential growth factor, is involved in the glomerular development and the postnatal homeostasis, and it is secreted by podocytes into the primary urine in high amounts, back-filtered across the glomerular capillary wall to act on the endothelial cells [3]. VEGF can repair interstitial tubule compartment in the cyclosporine nephrotoxicity, but the mRNA level of VEGF has been up-regulated in the tubules in hypoxic states [2]. VEGF gene polymorphism can affect the activation of VEGF, and there are some studies find that VEGF gene polymorphism is associated with SLE risk.
Systemic lupus erythematosus (SLE), characterized by the formation of immune complexes with nuclear antigens and the production of antibodies to components of the cell nucleus (antinuclear antibodies or ANAs) [4], is a prototypic and heterogeneous autoimmune disease with a wide clinical expression [5], and presents highly heterogeneous clinical manifestations and multi-systemic involvement [6]. Lupus nephritis (LN) is one of the most frequent and crucial complication of SLE, considered as the major predictor of poor prognosis [7]. Some factors were reported that gene expression, protein expression, and gene polymorphism were associated with the risk of some diseases [8,9,10,11,12]. Furthermore, results from meta-analysis are more robust when compared to individual study. There was no meta-analysis to assess the relationship between VEGF levels / VEGF gene polymorphism and SLE risk, and the association of VEGF with SLE developing into LN. In this study, we widely collected the related research on these relationships and used meta-analysis method to pool the results.
Methods
Search strategy
The retrieval strategy of vascular endothelial growth factor, VEGF, systemic lupus erythematosus, SLE, lupus nephritis and LN were entered into China Biological Medicine Database (CBM), and PubMed on June 10, 2018, without language limitations. We also checked the references cited in the recruited articles for additional reports.
Inclusion and exclusion criteria
Inclusion criteria
(1) The study should be a case-control study; (2) The outcome should be SLE or LN; (3) There were two groups (case group vs control group). (4) Levels of VEGF should be detected by enzyme-linked immunosorbent assay (ELISA).
Exclusion criteria
(1) Case reports, editorials and review articles; (2) Articles did not provide the VEGF levels or detail genotype data; (3) Association of VEGF genotype / VEGF level with other diseases which were not related to SLE or LN. (4) non-traditional ELISA method for the determination of VEGF levels, such as sandwich ELISA test.
Data extraction and synthesis
Data and information from each investigation was extracted independently by at least 2 investigators: the surname of first author, publication year, VEGF protein levels, and the sample number of case group and control group for VEGF genotypes. The frequencies of alleles were counted for the SLE group and the control group. The results were compared and disagreements were resolved by discussion.
The diagnosis of SLE was based on according to ACR (American College of Rheumatology) criteria [13]. Active SLE was defined as follows: disease activity score of SLE was evaluated by the systemic lupus erythematosus disease activity index (SLEDAI) score, and a patient was diagnosed as active if SLEDAI score was higher than or equals to 10 [14].
Statistical analysis
Available data was analyzed using Cochrane Review Manager Version 5 (Cochrane Library, UK). When the P value of heterogeneity test was more than 0.1, the pooled statistic was counted using the fixed effects model, otherwise, a random effects model was conducted. Odds ratios (OR) was used to express the results for dichotomous data and mean differences (MD) was used to express the results for continuous data, and 95% confidence intervals (CI) were also counted. A p-value of 5% or lower was considered to be statistically significant, and I2 was used to test the heterogeneity among recruited studies.
Results
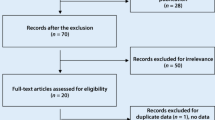
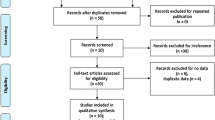
Search results
Fifteen articles [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] were related to VEGF levels in SLE vs control in this meta-analysis, including 573 SLE patients and 436 controls (Table 1). Six reports [14, 22, 23, 25, 26, 28] were included for the meta-analysis of Active SLE vs Inactive SLE. Two studies [16, 27] were recruited for the meta-analysis of LN vs SLE without LN. Three reports [29,30,31] were included for the analysis of the effect of VEGF -634G/C gene polymorphism (rs2010963) on SLE risk, including 523 SLE patients and 550 controls.
Association of VEGF with SLE risk
In this meta-analysis, we found that VEGF levels was associated with SLE risk (MD =196.02, 95% CI: 135.29–256.75, P < 0.00001; Fig. 1). The p-value of the heterogeneity test was < 0.00001. Thus, a random effects model was conducted.
Association of VEGF with active SLE risk
In this meta-analysis, we found that VEGF levels was associated with active SLE risk (MD =77.51, 95% CI: 10.98–144.05, P = 0.02; Fig. 2). The p-value of the heterogeneity test was < 0.00001. Thus, a random effects model was conducted.
Association of vascular endothelial growth factor protein levels with active SLE risk. SLE: systemic lupus erythematosus; SD: standard deviation; Total: the total number of SLE group or control group; CI: confidence intervals; I2: test the heterogeneity among recruited studies; df: degrees of freedom
Association of VEGF with SLE developing into LN
In this meta-analysis, we found that VEGF levels was associated with SLE developing into LN risk (MD =223.16, 95% CI: 144.38–301.93, P < 0.00001; Fig. 3). The p-value of the heterogeneity test was 0.25. Thus, a fixed effects model was conducted.
Association of vascular endothelial growth factor protein levels with SLE developing into LN. SLE: systemic lupus erythematosus; LN: Lupus nephritis; SD: standard deviation; Total: the total number of SLE group or control group; CI: confidence intervals; I2: test the heterogeneity among recruited studies; df: degrees of freedom
Association of the VEGF -634G/C gene polymorphism with SLE susceptibility
In this meta-analysis, we found that VEGF -634G/C gene polymorphism was not associated with SLE risk (C allele: OR = 0.96, 95% CI: 0.81–1.15, P = 0.66; CC genotype: OR = 0.91, 95% CI: 0.65–1.29, P = 0.61; GG genotype: OR = 1.03, 95% CI: 0.80–1.33, P = 0.80; Fig. 4).
Association between vascular endothelial growth factor -634G/C gene polymorphism (rs2010963) with SLE susceptibility. SLE: systemic lupus erythematosus; M-H: Mantel-Haenszel; Total: the total number of SLE group or control group; CI: confidence intervals; I2: test the heterogeneity among recruited studies; df: degrees of freedom
Discussion
In this study, we found that VEGF levels was associated with SLE risk, and VEGF levels was associated with active SLE risk. We also found that VEGF levels was associated with SLE developing into LN risk. It indicated that VEGF was associated with the SLE risk, the activation of SLE, and the SLE progression. The sample size for the meta-analysis on the relationship between VEGF levels and SLE risk was larger, and the results might be robust. SLE is an autoimmune disease, associated with the primary site represented by vascular endothelial cell injury, and VEGF has been regarded as a key mediator of modulator of neovascularization and endothelial dysfunction. We speculated that the increased VEGF protein levels was associated with the SLE vascular inflammation and associated with SLE risk. However, more studies should be performed to confirm it.
There were some meta-analyses to detect the association between VEGF expression and diseases. Huang et al. [32] included nine articles met the inclusion criteria for our meta-analysis to examine the relationship between the protein expression of VEGF and lymph node metastasis (LNM) in papillary thyroid cancer, and reported that LNM occurred more frequently in papillary thyroid cancer patients with high VEGF expression than in those with low VEGF expression. Fafliora et al. [33] included 11 studies in the meta-analysis, and reported that VEGF levels in patients with malignant pleural effusion were increased as compared to the patients with benign pleural effusion. Lee et al. [34] conducted a meta-analysis of the VEGF levels in patients with rheumatoid arthritis and controls including 13 studies, and found that significantly higher circulating VEGF levels in patients with rheumatoid arthritis and a positive correlation between VEGF levels and disease activity in rheumatoid arthritis. VEGF levels might be a predicted factor for some diseases. In our meta-analysis, we also found that VEGF was associated with SLE risk and SLE progression.
In this study, we also conducted the meta-analysis for the association of VEGF gene polymorphism with SLE susceptibility, and we found that VEGF -634G/C gene polymorphism (rs2010963) was not associated with SLE risk. There was no other meta-analysis to assess this relationship. However, there was only three reports included for the analysis of the effect of VEGF -634G/C gene polymorphism on SLE risk. This result must be treated with caution. More studies should be conducted to confirm the results in the future. We speculated that the VEGF -634G/C gene polymorphism was not associated with the VEGF levels or the activity of VEGF, and it was not associated with SLE risk. However, more investigations should be conducted to confirm it.
In previous, there were some meta-analysis conducted to assess the relationship between VEGF -634G/C gene polymorphism and diseases. Zhuang et al. [35] included nine investigations with 2281 cases with gastric cancer and 2820 controls for meta-analysis, and reported that VEGF -634G/C G allele carrier may increase gastric cancer risk. Gong et al. [36] included six studies in their meta-analysis, and reported that the VEGF -634G/C gene polymorphism was not associated with an increased risk for renal cell carcinoma. Malik et al. [37] included six case-control studies for meta-analysis, and suggested that VEGF-634G/C gene polymorphism might not be associated with retinopathy of prematurity risk. In our meta-analysis, we found that VEGF -634G/C gene polymorphism was not associated with SLE risk.
Conclusions
In this study, we found that VEGF levels was associated with SLE risk, active SLE risk and SLE developing into LN risk. However, VEGF -634G/C gene polymorphism was not associated with SLE risk.
Abbreviations
- CBM:
-
China Biological Medicine Database
- CI:
-
confidence intervals
- LN:
-
Lupus nephritis
- LNM:
-
lymph node metastasis
- MD:
-
mean differences
- OR:
-
Odds ratios
- SLE:
-
systemic lupus erythematosus
- VEGF:
-
vascular endothelial growth factor
References
Acosta L, Morcuende S, Silva-Hucha S, Pastor AM, de la Cruz RR. Vascular endothelial growth factor (VEGF) prevents the downregulation of the cholinergic phenotype in Axotomized Motoneurons of the adult rat. Front Mol Neurosci. 2018;11:241.
Prakash S, Patel MR, Agrawal S, Jindal RM, Prasad N. Vascular endothelial growth factor gene polymorphism is associated with long-term kidney allograft outcomes. Kidney Int Rep. 2018;3(2):321–7.
Kuppe C, Rohlfs W, Grepl M, Schulte K, Veron D, Elger M, Sanden SK, Saritas T, Andrae J, Betsholtz C, et al. Inverse correlation between vascular endothelial growth factor back-filtration and capillary filtration pressures. Nephrol Dial Transplant. 2018;33(9):1514–25.
Mobarrez F, Svenungsson E, Pisetsky DS. Microparticles as autoantigens in systemic lupus erythematosus. Eur J Clin Investig. 2018;48:e13010.
Salman-Monte TC, Carrion-Barbera I, Garcia CP, Beltran JG, Monfort J: Inflammatory myopathy and autoimmune hepatitis in a patient with a flare of systemic lupus erythematosus: An exceptional association. Eur J Rheumatol 2018:1–4.
Sciascia S, Radin M, Roccatello D, Sanna G, Bertolaccini ML. Recent advances in the management of systemic lupus erythematosus. F1000Res. 2018;7.
Dumestre-Perard C, Clavarino G, Colliard S, Cesbron JY, Thielens NM. Antibodies targeting circulating protective molecules in lupus nephritis: interest as serological biomarkers. Autoimmun Rev. 2018.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006.
Xu L, You X, Zheng P, Zhang BM, Gupta PK, Lavori P, Meyer E, Zehnder JL. Methodologic considerations in the application of next-generation sequencing of human TRB repertoires for clinical use. The Journal of molecular diagnostics : JMD. 2017;19(1):72–83.
Balasopoulou A, Stankovic B, Panagiotara A, Nikcevic G, Peters BA, John A, Mendrinou E, Stratopoulos A, Legaki AI, Stathakopoulou V, et al. Novel genetic risk variants for pediatric celiac disease. Human genomics. 2016;10(1):34.
An P, Xu J, Yu Y, Winkler CA. Host and viral genetic variation in HBV-related hepatocellular carcinoma. Front Genet. 2018;9:261.
An P, Penugonda S, Thorball CW, Bartha I, Goedert JJ, Donfield S, Buchbinder S, Binns-Roemer E, Kirk GD, Zhang W, et al. Role of APOBEC3F gene variation in HIV-1 disease progression and Pneumocystis pneumonia. PLoS Genet. 2016;12(3):e1005921.
Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & rheumatology (Hoboken, NJ). 1997;40(9):1725.
Zhou L, Lu G, Shen L, Wang L, Wang M. Serum levels of three angiogenic factors in systemic lupus erythematosus and their clinical significance. Biomed Res Int. 2014;2014:627126.
Navarro C, Candia-Zuniga L, Silveira LH, Ruiz V, Gaxiola M, Avila MC, Amigo MC. Vascular endothelial growth factor plasma levels in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Lupus. 2002;11(1):21–4.
Heshmat NM, El-Kerdany TH. Serum levels of vascular endothelial growth factor in children and adolescents with systemic lupus erythematosus. Pediatr Allergy Immunol. 2007;18(4):346–53.
Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S, Ciolkiewicz M. Vascular endothelial growth factor in systemic lupus erythematosus: relationship to disease activity, systemic organ manifestation, and nailfold capillaroscopic abnormalities. Arch Immunol Ther Exp. 2007;55(3):179–85.
Tanaseanu C, Tudor S, Tamsulea I, Marta D, Manea G, Moldoveanu E. Vascular endothelial growth factor, lipoporotein-associated phospholipase A2, sP-selectin and antiphospholipid antibodies, biological markers with prognostic value in pulmonary hypertension associated with chronic obstructive pulmonary disease and systemic lupus erithematosus. Eur J Med Res. 2007;12(4):145–51.
Ciprandi G, Murdaca G, Colombo BM, De Amici M, Marseglia GL. Serum vascular endothelial growth factor in allergic rhinitis and systemic lupus erythematosus. Hum Immunol. 2008;69(8):510–2.
Colombo BM, Cacciapaglia F, Puntoni M, Murdaca G, Rossi E, Rodriguez G, Nobili F, Pisciotta L, Bertolini S, Moccetti T, et al. Traditional and non traditional risk factors in accelerated atherosclerosis in systemic lupus erythematosus: role of vascular endothelial growth factor (VEGATS study). Autoimmun Rev. 2009;8(4):309–15.
Kuryliszyn-Moskal A, Ciolkiewicz M, Klimiuk PA, Sierakowski S. Clinical significance of nailfold capillaroscopy in systemic lupus erythematosus: correlation with endothelial cell activation markers and disease activity. Scand J Rheumatol. 2009;38(1):38–45.
Edelbauer M, Kshirsagar S, Riedl M, Billing H, Tonshoff B, Haffner D, Dotsch J, Wechselberger G, Weber LT, Steichen-Gersdorf E. Soluble VEGF receptor 1 promotes endothelial injury in children and adolescents with lupus nephritis. Pediatr Nephrol. 2012;27(5):793–800.
Robak E, Kulczycka-Siennicka L, Gerlicz Z, Kierstan M, Korycka-Wolowiec A, Sysa-Jedrzejowska A. Correlations between concentrations of interleukin (IL)-17A, IL-17B and IL-17F, and endothelial cells and proangiogenic cytokines in systemic lupus erythematosus patients. Eur Cytokine Netw. 2013;24(1):60–8.
Willis R, Seif AM, McGwin G Jr, Martinez-Martinez LA, Gonzalez EB, Doan E, Dang N, Papalardo E, Liu J, Vila LM, et al. Effects of statins on proinflammatory/prothrombotic biomarkers and on disease activity scores in SLE patients: data from LUMINA (LXXVI), a multi-ethnic US cohort. Clin Exp Rheumatol. 2014;32(2):162–7.
Barbulescu AL, Vreju AF, Buga AM, Sandu RE, Criveanu C, Tudorascu DR, Gheonea IA, Ciurea PL. Vascular endothelial growth factor in systemic lupus erythematosus - correlations with disease activity and nailfold capillaroscopy changes. Romanian J Morphol Embryol. 2015;56(3):1011–6.
Liu J, Wang X, Yang X, Yan Q, Wang S, Han W. Investigating the role of angiogenesis in systemic lupus erythematosus. Lupus. 2015;24(6):621–7.
Ghazali WSW, Iberahim R, Ashari NSM. Serum vascular endothelial growth factor (VEGF) as a biomarker for disease activity in lupus nephritis. Malays J Med Sci. 2017;24(5):62–72.
Merayo-Chalico J, Barrera-Vargas A, Juarez-Vega G, Alcocer-Varela J, Arauz A, Gomez-Martin D. Differential serum cytokine profile in patients with systemic lupus erythematosus and posterior reversible encephalopathy syndrome. Clin Exp Immunol. 2018;192(2):165–70.
Lv HZ, Lin T, Zhu XY, Zhang JT, Lu J. A study on relationship between single nucleotide polymorphisms of vascular endothelial growth factor gene and susceptibility to systemic lupus erythematosus in China North Han population. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26(12):1189–92.
Ramirez-Bello J, Cadena-Sandoval D, Fragoso JM, Barbosa-Cobos RE, Moreno-Eutimio MA, Saavedra-Salinas MA, Valencia-Pacheco G, Lopez-Villanueva RF, Jimenez-Morales S. The VEGFA -1154G/a polymorphism is associated with reduced risk of rheumatoid arthritis but not with systemic lupus erythematosus in Mexican women. J Gene Med. 2018;20(6):e3024.
Wongpiyabovorn J, Hirankarn N, Ruchusatsawat K, Yooyongsatit S, Benjachat T, Avihingsanon Y. The association of single nucleotide polymorphism within vascular endothelial growth factor gene with systemic lupus erythematosus and lupus nephritis. Int J Immunogenet. 2011;38(1):63–7.
Huang XQ, He WS, Zhang HQ, Yang R, Huang T. Relationship between expression of vascular endothelial growth factor and cervical lymph node metastasis in papillary thyroid cancer: a meta-analysis. J Huazhong Univ Sci Technolog Med Sci. 2017;37(5):661–6.
Fafliora E, Hatzoglou C, Gourgoulianis KI, Zarogiannis SG. Systematic review and meta-analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol Rep. 2016;4:24.
Lee YH, Bae SC. Correlation between circulating VEGF levels and disease activity in rheumatoid arthritis: a meta-analysis. Z Rheumatol. 2018;77(3):240–8.
Zhuang M, Peng Z, Wang J, Su X. Vascular endothelial growth factor gene polymorphisms and gastric cancer risk: a meta-analysis. J BUON. 2017;22(3):714–24.
Gong M, Dong W, Shi Z, Qiu S, Yuan R. Vascular endothelial growth factor gene polymorphisms and the risk of renal cell carcinoma: evidence from eight case-control studies. Oncotarget. 2017;8(5):8447–58.
Malik MA, Shukla S, Azad SV, Kaur J. Vascular endothelial growth factor (VEGF-634G/C) polymorphism and retinopathy of prematurity: a meta-analysis. Saudi J Ophthalmol. 2014;28(4):299–303.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangzhou Medical Key Discipline Construction Project, Natural Science Foundation of the Guangdong Province (no. 2015A030310386) and Guangdong Medical Science and Technology Research Fund Project (no. A2018336). The role of the funding body supported the publication fee for this manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
TBZ was in charge of conceived and designed the study. WZT and TBZ were responsible for collection of data and performing the statistical analysis and manuscript preparation. ZQZ and HZZ were responsible for checking the data. All authors were responsible for drafting the manuscript, read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tang, W., Zhou, T., Zhong, Z. et al. Meta-analysis of associations of vascular endothelial growth factor protein levels and -634G/C polymorphism with systemic lupus erythematosus susceptibility. BMC Med Genet 20, 46 (2019). https://doi.org/10.1186/s12881-019-0783-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-019-0783-1