Abstract
Background
Long QT syndrome (LQTS) is an inherited arrhythmic disorder characterized by prolongation of the QT interval, a risk of syncope, and sudden death. There are already a number of causal genes in LQTS, but not all LQTS patients have an identified mutation, which suggests LQTS unknown genes.
Methods
A cohort of 178 LQTS patients, with no mutations in the 3 major LQTS genes (KCNQ1, KCNH2, and SCN5A), was screened for mutations in the transient potential melastatin 4 gene (TRPM4).
Results
Four TRPM4 variants (2.2% of the cohort) were found to change highly conserved amino-acids and were either very rare or absent from control populations. Therefore, these four TRPM4 variants were predicted to be disease causing. Furthermore, no mutations were found in the DNA of these TRPM4 variant carriers in any of the 13 major long QT syndrome genes. Two of these variants were further studied by electrophysiology (p.Val441Met and p.Arg499Pro). Both variants showed a classical TRPM4 outward rectifying current, but the current was reduced by 61 and 90% respectively, compared to wild type TRPM4 current.
Conclusions
This study supports the view that TRPM4 could account for a small percentage of LQTS patients. TRPM4 contribution to the QT interval might be multifactorial by modulating whole cell current but also, as shown in Trpm4−/− mice, by modulating cardiomyocyte proliferation. TRPM4 enlarges the subgroup of LQT genes (KCNJ2 in Andersen syndrome and CACNA1C in Timothy syndrome) known to increase the QT interval through a more complex pleiotropic effect than merely action potential alteration.
Similar content being viewed by others
Background
Congenital long QT syndrome (LQTS) is characterized by a prolonged QT interval on the electrocardiogram (ECG), and can lead to cardiac events such as syncope or sudden cardiac death due to torsade de pointes or ventricular fibrillation. Prevalence in the population is about 1 over 2000 to 3000 [1, 2].
Similarly to other cardiomyopathies, a number of genes were described to be responsible for LQTS. To date, 15 genes have already been identified with more than 700 mutations in LQTS patients [3, 4]. Six of these genes encode for pore-forming ion channel subunits, while others encode for regulatory subunits or proteins. Among ion channel genes, mutations in KCNQ1 and KCNH2, which encode voltage-gated K+ channels involved in cardiac action potential (AP) repolarization, are responsible for about 70% of LQTS [3]. Mutations of the voltage-gated Na+ channel encoded by the SCN5A gene are responsible for 1.7 to 8% of LQTS while mutations in other genes are rare (below 1% of LQTS). Altogether, the quest to find mutations remains elusive in about 25% of LQTS patients [5], which leaves place for the discovery of additional causal genes for this syndrome.
At the mechanistic level, LQT is mainly explained by a delayed ventricular repolarization due to a prolongation of AP in ventricular cardiomyocytes. It explains why loss of function mutations in voltage-gated K+ channel encoding genes, such as KCNQ1 and KCNH2, which participate in cardiomyocyte repolarization (AP phases 2–3), result in LQTS. Similarly, any ion channel known to participate in the shape of AP may be involved in LQTS. Several clues pointed to the transient potential melastatin 4 gene (TRPM4) as a pertinent contender for LQTS: 1- TRPM4 encodes a non-selective cation channel and the mRNA is present in the heart [6]. 2- TRPM4 currents were recorded in cardiomyocytes of several mammals including humans [7]. 3- Trpm4 −/− mice have an increased QT interval [8]. 4- TRPM4 mutations were reported in patients with other cardiac electrical disturbances such as, cardiac conduction block [9, 10] and Brugada syndrome [11]. The involvement of TRPM4 in several pathologies is reminiscent to the voltage-gated Na+ channel encoded by SCN5A, which was associated with LQTS, Brugada syndrome, and conduction blocks [12].
According to this, we hypothesized that TRPM4 variants might be linked to LQTS. To address this hypothesis, we screened a cohort of 178 patients with LQTS and had no identified mutations in the major LQTS genes (KCNQ1, KCNH2 and SCN5A). TRPM4 variants were found in 4 patients (2.2% of the cohort). The electrophysiological properties of 2 of these mutants were further studied. Both mutations resulted in a decrease of function of the TRPM4 channel.
Methods
Study population
In this study, 178 patients from the University Hospitals of Nantes and Lyon were tested. This study was approved by the local ethics committees (comité de protection des personnes Ouest IV and Sud-Est II) and is in accordance with the last version of the Declaration of Helsinki [13]. In all probands, the clinical diagnosis of LQTS was made using the Schwartz criteria [14, 15]. The QT interval was corrected by the formula of Bazett (QTc). Each patient underwent full medical examination to rule out syndromic forms of LQT. Blood samples were collected after written informed consent. All study participants provided written informed consent to participate to this study. All these probands were negative after mutation screening by dHPLC or direct sequencing in the KCNQ1, KCNH2, and SCN5A genes. The LQTS cohort of the University Hospital of Nantes (90 patients) had also been tested negative for copy number variations with a Multiple Ligation-dependent Probe Amplification test of MRC Holland (SALSA P114 MLPA, MRC-Holland, Amsterdam, the Netherlands). This test contains 20 probes interrogating the KCNQ1 gene, 9 for the KCNH2 gene, and 3 for the SCN5A gene [15]. The LQTS cohort of the University Hospital of Lyon (88 patients) was also negative for mutation in the KCNE1 and KCNE2 genes.
Genetic analysis
DNA was extracted from blood samples according to standard protocols. DNA concentration and quality were assessed using NanoDrop (Thermo Fisher Scientific, Villebon sur Yvette, France) and Qubit (Life technologies, Villebon sur Yvette, France) fluorometers. A260/A280 ratios of 1.8 to 2.0 and A260/A230 ratios >1.5 were accepted. Mutation screening of TRPM4 (ENST00000252826, NM_017636.3, human genome version GRCh37/hg19) was carried out by High Resolution Melting (HRM) analysis (Rotor-Gene Q, Qiagen, Courtaboeuf, France), followed by bi-directional sequencing of abnormal profiles or by direct sequencing. The primers were already published [10]. Variants were confirmed on a second sample and PCR product. For miniexome sequencing, enrichment of DNA samples was performed with the Nextera Rapid Capture Exome v1.0 kit (Illumina, San Diego, CA, USA). Sequencing was carried out on a NextSeq500 instrument (Illumina, San Diego, CA, USA) with a high-output flow cell. TruSight One targets 4813 clinically relevant genes and covers 12 Mb of genomic content. In particular, the following long QT genes are included: ANK2, CACNA1C, CALM1, CAV3, KCNE1, KCNE2, KCNJ2, KCNJ5, KCNQ1, SCN4B, SCN5A and SNTA1. A minimal coverage of 10x was obtained on 98.2% of target sequences. Bam files were loaded on Alamut Visual 2.7.1 (Interactive Biosoftware, Rouen, France) and the 13 long QT genes were sequentially visualized along with the sequence reads of each patient. A threshold of 30% was selected to highlight sequence variants. The coverage of all coding exons of LQ long genes was at least 10x.
Preparation of TRPM4 mutants
The complete human wild-type TRPM4 cDNA was cloned in a pcDNA4/TO vector (Invitrogen, Cergy Pontoise, France) [10]. Mutants were obtained by in vitro mutagenesis using QuickChange II site-directed mutagenesis kit (Agilent Technologies, Massy, France). Mutant cDNA clones were then systematically resequenced before using them in further experiments.
Stable TRPM4 mutant expression
PcDNA4/TO plasmid containing the 2 selected TRPM4 mutants, were used to transfect T-REx™ 293 cell lines with Lipofectamine 2000 (Invitrogen, Cergy Pontoise, France) according to manufacturer specifications. The T-REx™ 293 cell line stably expresses the tetracycline repressor protein, enabling the silencing of the gene of interest unless tetracycline is added to the culture medium. T-REx™ 293 was obtained by stably transforming HEK 293 cells with a plasmid containing the Tet repressor cDNA under the control of the human CMV promoter. Several stable clones (3–4) of each TRPM4 mutants were obtained according to Invitrogen protocol, by selecting with blasticidin (Tet repressor) and zeocin (TRPM4). These stable clones were used for the electrophysiological study.
Electrophysiological recordings
Patch-clamp experiments on T-REx™ 293 transfected cells were performed at room temperature. An Axopatch 200B (Axon Instruments, Sunnyvale, CA, USA) amplifier was used and controlled by a Pentium PC connected by a Digidata 1322A A/D converter (Axon Instruments). It was also used for data acquisition and analysis using Pclamp software (Axon Instruments). Signals were filtered at 1 kHz using an 8-pole Bessel low pass filter before digitization at 10 kHz and storage. Patch pipettes resistance was typically 2 MΩ when filled with intracellular solution (below).
TRPM4 currents were recorded in the whole-cell configuration using a ramp protocol. The holding potential was −60 mV. The 400 ms increasing ramp from −100 to +100 mV ends with a 20 ms step at +100 mV to unmask a typical TRPM4 current [16]. The measured current was then reported to cell size estimated by capacitance measurement. A new ramp was performed every 5 s. TRPM4 current develops with time after the membrane break to stabilize within 10–20 min as previously shown [16]. Biophysical properties were estimated after current stabilization. Pipette solutions contained (in mM) 156 CsCl, 1 MgCl2 and 10 HEPES (pH adjusted to 7.2 with CsOH and [Ca2+] 10−4 M). Bath and perfused solutions contained (in mM) 156 NaCl, 5 CaCl2, 10 glucose and 10 HEPES (pH adjusted to 7.4 with NaOH).
Data analysis
Mutant electrophysiological parameters were compared to wild type using an unpaired Student t test, with a probability value below 0.05 considered as significant. A Fisher exact test was performed to compare the prevalence of TRPM4 variants in the ethnically matched control population, and in the LQTS cohort both of European origin.
Results
Study subjects and TRPM4 screening
The screening of TRPM4 in the cohort of 178 Long QT cases with no mutations in the 3 major LQT genes (KCNH2, KCNQ1, and SCN5A) evidenced 4 variants of interest (Table 1): p.V441M, p.R499W, p.R499P, and p.G844D. The putative pathogenicity of these variants was estimated on their prevalence in ethnically matched control populations, the interspecies conservation of reference amino acid, the physico-chemical gap between referenced and new residues, and prediction software (snp&go [17], PhD_SNP [18], SNAP [19] and PredictSNP1.0 [20]). The data is summarized in Table 1. All variants were new [9–11, 21] except p.G844D, which was already found in cases of conduction blocks [10, 21] and of Brugada syndrome [11]. All variants were heterozygous. To exclude that any of these 4 Patients had a variant in an already known long QT genes, the sequencing of a panel of 4813 clinically relevant genes was obtained from each patient. A careful reading with Alamut Visual 2.7.1 of sequence reads of each TRPM4 variant carriers did not detect any suspicious variant in coding exons of any of the 13 long QT genes (ANK2, CACNA1C, CALM1, CAV3, KCNE1, KCNE2, KCNH2, KCNJ2, KCNJ5, KCNQ1, SCN4B, SCN5A and SNTA1) tested with TruSight One gene panel. Interestingly, this gene panel includes the TRPM4 gene. It was thus possible to confirm the reported TRPM4 variant of each of the 4 Patients.
The clinical details of the 4 variant carriers are presented in Table 2. The range of age of discovery was wide from 3 months to 69 years of age. Circumstances of discovery were essentially related to disturbance in consciousness.
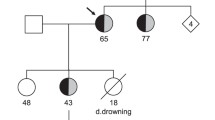
Patient 1 had hypertension, type II diabetes and was over-weighted. He was first seen at the cardiology clinics because he was complaining about a shortness of breath during exercise. He had a normal echocardiography and normal coronarography but a prolonged QTc interval was discovered. He was given 20 mg daily of Netaxolol. Four years later, he was 68 years old. He lost consciousness for several minutes while at rest. His wife noticed that he had neither seizures nor urine loss. He recovered consciousness spontaneously. Upon arrival of the emergency unit, his heart rate was low (30/min) with numerous premature ventricular beats occurring at the down-sloping part of the T wave and a QTc at 500 ms. However, no torsade-de-pointes were recorded during his monitoring. He had hypokaliemia (2.8 mEq/L) although he did not take any diuretic. He received potassium and the dosage of betablocker was lowered. Two years later, he started developing a mild cardiac failure and his ECG showed, in addition to the long QT, a left anterior hemiblock (Fig. 1). His echocardiogram showed a mild concentric hypertrophy (10 mm), a normal ejection fraction and a diastolic dysfunction. We consider a Schwartz score of 4 for this patient (QTc ≥ 480 ms and syncope at rest).
Patient 2 was a three-year-old baby when he had his first loss of consciousness. As he was sleeping, he had a cry. The parents who rushed to his bed noticed that he was unreactive and atonic for several minutes. Then, he recovered progressively. He was pale with circumoral cyanosis and he vomited. At hospital, he had an echocardiography which disclosed a patent foramen ovale, a prolonged QTc interval. A pH-metry showed no evidence for a gastro-oesophageal reflux. Blood analysis was in normal range. He had an ambulatory ECG which showed no arrhythmia. Subsequently, he had 3 losses of consciousness in the following year with no detection of any other anomalies except mild hypothermia (35.6 °C). Figure 2 presents one representative ECG. We gave a Schwartz score of 3.5 to this patient (QTc between 460 and 479 ms, syncope at rest and a slow heart rate for his age). His asymptomatic older sister and parents had a normal QTc interval.
Patient 3 is a 69-year-old female who lost consciousness while gardening. She recovered consciousness after an unknown lap of time and felt several episodes of dizziness during the following hours. Her ECG showed a prolonged QT interval (Fig. 3) and Holter recording discovered short stretches of ventricular beats (Fig. 4). Her echocardiogram was normal and her blood tests were normal. She was given a regular regimen of betablocker and did not experience any other episodes of loss of consciousness. In our opinion, her Schwartz score is 3.5 (QTc in the range of 460–479 ms or torsade-like arrhythmia and effort-associated syncope).
Patient 4, a fifteen-year-old boy, had a syncope while he was at rest. The next day at the hospital, his ECG showed a prolonged QTc interval but no arrhythmia (Fig. 5). He had a rSr’ QRS complex in V2 but the QRS duration was normal (90 ms). His echocardiography was normal. His Schwartz score is 4.5 (QTc ≥ 480 ms, syncope and a familial case of unexplained sudden death).
It was not possible to obtain blood samples from relatives of these patients.
TRPM4 currents in the whole-cell configuration
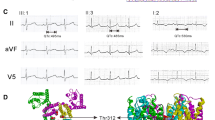
HEK-293 TRPM4 transfected cells were searched for ion currents using patch-clamp recordings in the whole-cell configuration. Wild type (WT) TRPM4 transfected cells exhibited a characteristic outward rectifying current with a density measured at +100 mV of 191 ± 25 pA/pF (n = 18), significantly higher than non-transfected HEK-293 cells (3 ± 1 pA/pF; n = 11, p < 0.001), which is in accordance with previous reports [11, 16].
Both, Val441Met and Arg499Trp, produced an outward rectifying current of 74.6 ± 33 pA/pF (n = 7) and 20 ± 7 pA/pF (n = 9) respectively, which is significantly higher than the untransfected cells (p = 0.014 and p = 0.020, respectively) but smaller than WT transfected cells (p = 0.016 and p < 0.001, respectively) (Fig. 6a and b). To characterize the current rectification, the current at +100 mV was expressed as a ratio to the current at −60 mV (Fig. 6c). No significant variation was induced by mutations when compared to WT, indicating that this parameter was not affected.
Biophysical properties of currents from HEK-293 cells transfected with WT and TRPM4 variants. a Representative current tracings recorded in the whole-cell configuration (ramp protocol under the traces) (b) Mean current densities for WT and variants estimated using the maximal current recorded during the ending step of 20 ms at Vm = +100 mV (see a). c Rectification factor estimated by the current amplitude at +100 mV divided by the current amplitude at −60 mV . *p < 0.05, Error bar: standard error of the mean. Number of experiments is indicated in bars. Abbreviations: NT: Non-Transfected; WT: Wild-type
Discussion
Four coding variants, which occurred in less than 0.3% of more than 18,000 control chromosomes (and less than 0.02% of more than 78,000 control chromosomes, if G844D is excepted), were identified in 178 LQT cases with no mutations in the 13 major LQTS genes. Among the genes associated with long QT syndrome, only AKAP9 and CALM2 are not included in the clinical exome of Illumina. AKAP9 was associated with a single case of long QT syndrome [22] and might rather be a QT interval modifier than a long QT causal gene [23]. CALM2 mutations may result in neonatal bradycardia, cardiac arrest at a young age, a markedly prolonged QTc interval and eventually mild neurodevelopmental delay [24, 25]. Only two patients of this TRPM4 study were within the age range of CALM2 mutations (Patients 2 and 4) but their QTc interval was never markedly prolonged as in CALM2 mutations.
The TRPM4 variants of this study are all heterozygous missense variants. Interestingly, one patients had in addition to LQTS, a conduction block (patient 1 had a Left Anterior Hemiblock) but he was 70 years old when the heart block appeared. This is consistent with Trpm4−/− mice, which also present multilevel conduction blocks [8] in addition to increased QT interval. The variant of patient 4 (p.G844D) was found in other cases with conduction blocks [10, 21] or Brugada syndrome [11]. Among these latter cases, one also had a prolonged QTc interval of 458 ms (patient 13 of reference 11).
In this study, comparing whole-cell currents recorded from WT and Val441Met and Arg499Trp TRPM4 transfected HEK293 cells, showed densities significantly lower in both variants. The present study does not discriminate between a decrease in current density due to a modification of biophysical/regulatory properties of the ion channel, or a decreased protein expression as previously reported for other TRPM4 mutants [11]. However, there is a clear channel loss of function at the level of the membrane.
Until now, TRPM4 impact on cardiac activity was investigated only with regards to its contribution to the action potential (AP). According to its non-selective cation permeability (Na+ and K+), its opening is suspected to induce an outward repolarizing current in positive voltages but an inward depolarizing current in negative voltages. Since channel activity increases with membrane depolarization, it is suspected to be largely open at the upstroke and during the plateau of the AP. This is potentiated by the fact that TRPM4 is activated by internal Ca2+, which increases during the AP. Altogether, one can predict that TRPM4 activation reduces phase 2 duration of AP and prolongs phase 3 counteracting repolarization. It seems that the effect on phase 2 is predominant, at least in mouse atria, since TRPM4 pharmacological inhibition by 9-phenanthrol reduces AP duration [26]. Consistent with this result, Trpm4−/− mice exhibit a shortened AP in atria, but also in ventricle [26, 27]. Surprisingly, ECGs from Trpm4−/− mice showed a prolonged QT interval, despite the reduction of ventricular AP duration [8, 27]. The rationale for this counter-intuitive change might be that Trpm4 −/− mice also show an increased heart/body weight ratio, which is not due to cardiomyocyte hypertrophy but rather neonatal hyperplasia [8]. QT duration depends on both ventricular AP duration and AP propagation within the ventricle. Thus, hyperplasia could increase the time for the excitation to reach the entire ventricles, and thus prolong the QT interval. Even if AP parameters are different between mice and human, the model of Trpm4 −/− mice might be representative of heart disease related to TRPM4 mutations in human. Indeed, mutations which induce loss of function may impact cardiac development as does Trpm4 disruption in mice. TRPM4 is not the first gene encoding a channel whose mutations result in LQT and developmental anomalies as it was already found in Andersen [28] and Timothy [29] syndromes. Unfortunately, contribution of the TRPM4 current to these phases of the AP was not evaluated yet in human. It was also not possible to estimate a putative cardiomyocyte hyperplasia in patients of this study. At least, it can be stated that none of the five TRPM4 variant carriers fulfilled the echographic criteria for hypertrophic cardiomyopathy.
Conclusion
This study supports the view that TRPM4 variants could be responsible for about 2% of LQT syndrome cases. The impact of these variants might result not only in electrophysical disturbances, but putatively also in other anomalies including cell proliferation.
Abbreviations
- AP:
-
Action potential
- cDNA:
-
copy Deoxyribonucleic acid
- CMV:
-
CytoMegalovirus
- dHPLC:
-
denaturating High Performance Liquid Chromatography
- ECG:
-
Electrocardiogram
- Euro-Am EVS:
-
European-American Exome Variant Server
- ExAC:
-
Exome Aggregation Consortium
- HEK:
-
Human Embryonic Kidney
- HRM:
-
High Resolution Melting
- LAHB:
-
Left anterior hemiblock
- LQTS:
-
Long QT syndrome
- MLPA:
-
Multiple Ligation-dependent Probe Amplification
- mRNA:
-
messenger Ribonucleic acid
- PCR:
-
Polymerase Chain Reaction
- QTc:
-
Corrected QT interval (Bazett formula)
- SCD:
-
Resuscitated sudden cardiac death
- TRPM4:
-
Transient potential melastatin 4 gene
- WT:
-
Wild type
- Y:
-
Year
References
Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7.
Zumhagen S, Stallmeyer B, Friedrich C, Eckardt L, Seebohm G, Schulze-Bahr E. Inherited long QT syndrome: clinical manifestation, genetic diagnostics, and therapy. Herzschrittmacherther Elektrophysiol. 2012;23:211–9.
Amin AS, Pinto YM, Wilde AAM. Long QT syndrome: beyond the causal mutation. J Physiol. 2013;591:4125–39.
Nakano Y, Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2016;61:51–5.
Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–39.
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2 + −activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407.
Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, et al. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83.
Demion M, Thireau J, Gueffier M, Finan A, Khoueiry Z, Cassan C, et al. Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS One. 2014;9:e115256.
Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119:2737–44.
Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, et al. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2010;3:374–85.
Liu H, Chatel S, Simard C, Syam N, Salle L, Probst V, et al. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS One. 2013;8:e54131.
Tfelt-Hansen J, Winkel BG, Grunnet M, Jespersen T. Inherited cardiac diseases caused by mutations in the Nav1.5 sodium channel. J Cardiovasc Electrophysiol. 2010;21:107–15.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124:2181–4.
Barc J, Briec F, Schmitt S, Kyndt F, Le Cunff M, Baron E, et al. Screening for copy number variation in genes associated with the long QT syndrome: clinical relevance. J Am Coll Cardiol. 2011;57:40–7.
Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, et al. 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol. 2008;153:1697–705.
Calabrese R, Capriotti E, Fariselli P, Martelli PL, Casadio R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum Mutat. 2009;30:1237–44.
Capriotti E, Calabrese R, Casadio R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics Oxf Engl. 2006;22:2729–34.
Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35:3823–35.
Bendl J, Stourac J, Salanda O, Pavelka A, Wieben ED, Zendulka J, et al. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput Biol. 2014;10:e1003440.
Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hébert J-L, Ferrer X, et al. Mutational spectrum in the Ca(2+)--activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat. 2012;33:109–17.
Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104:20990–5.
de Villiers CP, van der Merwe L, Crotti L, Goosen A, George AL, Schwartz PJ, et al. AKAP9 is a genetic modifier of congenital long-QT syndrome type 1. Circ Cardiovasc Genet. 2014;7:599–606.
Crotti L, Johnson CN, Graf E, De Ferrari GM, Cuneo BF, Ovadia M, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–17.
Makita N, Yagihara N, Crotti L, Johnson CN, Beckmann B-M, Roh MS, et al. Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circ Cardiovasc Genet. 2014;7:466–74.
Simard C, Hof T, Keddache Z, Launay P, Guinamard R. The TRPM4 non-selective cation channel contributes to the mammalian atrial action potential. J Mol Cell Cardiol. 2013;59:11–9.
Mathar I, Kecskes M, Van der Mieren G, Jacobs G, Camacho Londoño JE, Uhl S, et al. Increased β-adrenergic inotropy in ventricular myocardium from Trpm4−/− mice. Circ Res. 2014;114:283–94.
Andelfinger G, Tapper AR, Welch RC, Vanoye CG, George AL, Benson DW. KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet. 2002;71:663–8.
Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31.
Acknowledgements
We are indebted to patients for agreeing to participate to this study. Preksha SHUKLA is warmly thanked for improving the edition of this manuscript.
Funding
This study was supported by Projet Hospitalier de Recherche Clinique 2008, France to Patrice Bouvagnet
Availability of data and materials
Individual information, phenotype and variants data were deposited in the databases LOVD (http://databases.lovd.nl/shared/genes) Clinical characteristics (CD, PC and VP) and sequencing data of LQT known genes of patients are available in Nantes (JJS) and Lyon (GM). TRPM4 mutation screening of the whole cohort and sequencing of mutants are available in Lyon (HL, PB). Electrophysiological recordings are available in Caen (TH, LS and RG).
Authors’ contributions
TH performed electrophysiological studies; LH performed the sequencing, mutagenesis and preparation of HEK 293 clones; LS participated in the electrophysiological studies; JJS and GM participated in the design of the sequencing of LQT known genes; CD, PC and VP contributed patients; RG and PB participated in the design of the study and drafted the manuscript; HL and PB conceived the study; All authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the local ethics committees (comité de protection des personnes Ouest IV and Sud-Est II) and is in accordance with the last version of the Declaration of Helsinki. All study participants provided written informed consent to participate to this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hof, T., Liu, H., Sallé, L. et al. TRPM4 non-selective cation channel variants in long QT syndrome. BMC Med Genet 18, 31 (2017). https://doi.org/10.1186/s12881-017-0397-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-017-0397-4