Abstract
Background
The ‘Cytocam’ is a third generation video-microscope, which enables real time visualisation of the in vivo microcirculation. Based upon the principle of incident dark field (IDF) illumination, this hand held computer-controlled device was designed to address the technical limitations of its predecessors, orthogonal polarization spectroscopy and sidestream dark field (SDF) imaging. In this manuscript, we aimed to compare the quality of sublingual microcirculatory image acquisition between the IDF and SDF devices.
Methods
Using the microcirculatory image quality scoring (MIQS) system, (six categories scored as either 0 = optimal, 1 = acceptable, or 10 = unacceptable), two independent raters compared 30 films acquired using the Cytocam IDF video-microscope, to an equal number obtained with an SDF device. Blinded to the origin of the films, the raters were therefore able to score between 0 and 60 for each film analysed. The scores’ distributions between the two techniques were compared.
Results
The median MIQS (95 % CI) given to the SDF camera was 7 (1.5–12), as compared to 1 (0.5–1.0) for the IDF device (p < 0.0001). Of the six categories assessed by the MIQS, nearly one fifth of the SDF videos were scored as unacceptable for pressure (20 %), content (20 %), and stability (17 %), with focus scoring deficiently 13 % of the time. High agreement between the two raters scoring values was evident, with an intra-class correlation coefficient (ICC) of 0.96 (95 % CI: 0.94, 0.98).
Conclusions
These results demonstrate that the quality of sublingual microcirculatory image acquisition is superior in the Cytocam IDF video-microscope, as compared to the SDF video-microscope.
Similar content being viewed by others
Background
Incident dark field (IDF) imaging is an important technique that allows real time visualisation of the microcirculation [1]. Based upon the illumination of microvessels covered by a thin epithelial layer, it may be thought of as the successor to both orthogonal polarization spectroscopy (OPS) [2], and more recently, sidestream dark field (SDF) imaging [3]. Introduced in 2012, this third generation hand-held camera known as the Cytocam IDF video-microscope (Braedius Medical, Huizen, The Netherlands), was developed in an attempt to overcome many of the previous generations devices technical limitations [1]. These included; i) the limitations imposed by analogue video cameras, ii) the inability to achieve automatic microcirculation analysis, iii) pressure-induced microcirculatory alterations (predominantly caused by the heavy weight of the devices (SDF camera weight 320 g), iv) the requirement for hand operated focussing, and v) poor quality of image acquisition [4].
The Cytocam is a lightweight (120 g), fully digitalised pen-like device (length 220 mm, diameter 23 mm) that applies the principle of incident dark field microscopy introduced by Sherman and Cook in 1971 [5]. Blood vessels <100 μm in diameter, and <1000 μm below the surface of an organ or mucosal surface, are visualised in a two-dimensional plane through the process of epi-illumination [5]. Highly illuminating light emitting diodes (LEDs) enable suitable tissue penetration, and to avoid motion induced blurring secondary to fast moving erythrocytes [6], a very short LED pulse time of two milliseconds is utilised. Image delineation is optimised using a 3.5 megapixel high-resolution sensor, an optical magnification factor of four times, and an optical resolution of more than 300 lines/mm - an improvement of 50 % over SDF devices. This is further enhanced with an effective field of view (FOV) almost three times as large as earlier devices (1.55 × 1.16 mm, FOV area = 1.79 mm2), which may be magnified by a factor of 211 times on the display monitor [1]. Improved focussing is achieved through an integrated distance measurement system, which through the means of a manually adjusting the piezo linear motor via the computer interface, can alter the sensor position in steps of two microns. This novel quantitative focusing mechanism results in an accurate and repeatable focus distance, without having to repeatedly adjust the focus depth for every subsequent measurement. Finally, the IDF video-microscope has the capabilities for direct microcirculation analysis where the images are recorded digitally and analysed automatically. Specialised software automatically detects and quantitatively assesses the vessels’ diameters, and the flow velocity of erythrocytes within visualised vessels. Previously analysis of SDF videos required their conversion from analogue to digital images, with subsequent off-line analysis using specialised image processing software [7].
Although the IDF device should have significant superiority, in terms of image quality, over previous technologies this requires confirmation. We therefore set out to directly compare IDF and SDF images in a formalised manner.
Methods
Thirty films of human sublingual microcirculation obtained using an SDF video-microscope (MicroVision Medical, Amsterdam, Netherlands), were compared to thirty comparable films obtained using the Cytocam IDF video-microscope. The films were picked at random from a database of over 800 SDF and IDF films, all of which were obtained from healthy adult volunteers who had given informed consent. Ethical approval for the study had been obtained from University College London Research and Ethics Committee. Two raters (EGK, JC), blinded to the device on which the video was generated, independently graded the films using the Microcirculation image quality score (MISQ) system [8]. In 2007 a consensus statement that outlined five key principles for optimal image acquisition [9]. These were:
-
1.
Five separate image sites per organ
-
2.
Avoidance of pressure artefacts
-
3.
Elimination of secretions
-
4.
Adequate focus and contrast adjustment
-
5.
High quality recording
In 2013, a more formal approach to grading the quality of image acquisition prior to analysis was described, thereby giving a semi-objective measure of its suitability to be entered for computer analysis and quantification [8]. Six key characteristics of image capture were identified and encompassed within the ‘Microcirculation Image Quality Score’ (MIQS) (Table 1).
Each of the six categories is graded as 0 (optimal), 1 (acceptable) or 10 (unacceptable). If the total of the six categories is >10, then the video is unsuitable for Table 1 analysis and discarded. This somewhat peculiar scoring system is used, for if any one category is designated as unacceptable, it enforces that the video is not used [8].
The agreement between the two raters was assessed using the intra-class correlation coefficient and Bland and Altman limits of agreement. Over the range of scores given, Spearman’s correlation coefficient was used to assess the degree of over- or under- estimation of the score by either rater. The Mann–Whitney U-test was used to compare the score’s distribution between the two techniques. The two-tailed significance level was set at 0.05, and R (version 3.1.0) was used for the analyses.
Results
All 60 videos were analysed by both raters and no problems were encountered. The distribution of the individual total scores by rater is shown in Fig. 1.
Very good agreement between raters’ total scores are evident with an intra-class correlation coefficient of 0.96 (95 % CI: 0.94, 0.98). In addition, good agreement is evident in Fig. 2 (mean difference (rater2 – rater1): −0.75), and whilst some individual variation may exist as indicated by the slightly wide limits of agreement ( −4.86; 3.36), no over- or under- estimation trend by either rater was demonstrated (rho = −0.165, p = 0.21). For each device, the breakdown percentages of films scored as optimal, acceptable or unacceptable is presented in Table 2. When comparing the tools, the median score (95 % CI) given to the SDF video-microscope was 7 (1.5; 12), as opposed to 1 (0.5; 1.0) for the IDF video-microscope (p < 0.0001). The distribution of these values may be seen in Fig. 3. Examples of images taken with the Incident Dark Field imaging camera, and Sidestream Dark Field imaging camera can be seen in Additional files 1 and 2.
Discussion
These results demonstrate for the first time, that the Cytocam IDF video-microscope is superior to the SDF video-microscope in terms of the quality of sublingual microcirculatory image acquisition.
High agreement between the two raters scoring values was demonstrated, and whilst it is evident from the Bland Altman plot that some individual variation existed between raters, neither individual demonstrated a trend in over- or under-estimating the score as the total values increased. Using the total score value to determine if an image was deemed suitable for analysis, (i.e. if given a total score ≥10 renders the video as unacceptable), there was 100 % exact agreement (95 % CI: 94 %; 100 %) between the two raters.
As to whether the IDF video-microscope was superior to the SDF video-microscope in terms of providing acceptable images for analysis, the median score of 7 given to the SDF images, as opposed to 1 for the IDF videos, indicates that the SDF camera is more prone to produce unacceptable results. In this instance, 100 % of the images obtained using the IDF video-microscope were judged to be acceptable for data analysis, as opposed to only 50 % of these data collected using the SDF device. Table 2 demonstrates how the individual components of the MIQS system were scored for both cameras. From this we are able to see which categories SDF scored particularly poorly for as compared to IDF. The IDF video-microscope did not receive any scores of 10 from either rater, however nearly a fifth of the SDF videos were scored as unacceptable for stability (17 %), pressure (20 %), and content (20 %), with focus scoring deficiently 13 % of the time. This indicates superior IDF image acquisition for multiple categories, as opposed to in only one area of data capture.
Although 60 films chosen at random from a large database of images were analysed (30 for each device), a weakness in this manuscript was that no power calculation was performed prior to commencing. This said, the strong statistical significance supports the belief that it was adequately powered. Additionally, as the MIQS still relies on observer input to grade images, it is thus subjective in its film assessment. Nevertheless, it is the most formal approach to image grading we have to date, and the high ICC supports its use.
Conclusion
In conclusion, these data demonstrate that the IDF video-microscope provides improved image acquisition of human sublingual microcirculation when compared to the SDF video-microscope. Superior in five out of the six categories comprising the MIQS, the use of IDF offers an advanced insight into the clinical evaluation of the microvasculature.
Abbreviations
- FOV:
-
Field of view
- IDF:
-
Incident dark field
- LED:
-
Light emitting diode
- MIQS:
-
Microcirculation image quality score
- OPS:
-
Orthogonal polarization spectroscopy
- SDF:
-
Sidestream dark field
References
Aykut G IY, Ince C.: A new generation computer controlled imaging sensor based hand held microscope for quantifying bedside microcirculatory alterations. In Annual update in Intensive Care and Emergency Medicine 2014 Edited by Vincent JL. Springer; 2014:pp. 367-pp. 385.
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999;5:1209–12.
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15:15101–14.
Mik EG, Johannes T, Fries M. Clinical microvascular monitoring: a bright future without a future? Crit Care Med. 2009;37:2980–1.
Sherman H, Klausner S, Cook WA. Incident dark-field illumination: a new method for microcirculatory study. Angiology. 1971;22:295–303.
Cerny V. Sublingual microcirculation. Appl Cardiopulm Pathophysiol. 2012;16:229–48.
Dobbe JSG, Atasever B, Van Zijderveld R, Ince C. Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput. 2008;46:659–70.
Massey MJ, Larochelle E, Najarro G, Karmacharla A, Arnold R, Trzeciak S, et al. The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care. 2013;28:913–7.
De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101.
Acknowledgements
The members of the Caudwell Xtreme Everest Research Group are as follows:
V. Ahuja, G. Aref-Adib, R. Burnham, A. Chisholm, K. Clarke, D. Coates, M. Coates, D. Cook, M. Cox, S. Dhillon, C. Dougall, P. Doyle, P. Duncan, M. Edsell, L. Edwards, L. Evans, P. Gardiner, M. Grocott, P. Gunning, N. Hart, J. Harrington, J. Harvey, C. Holloway, D. Howard, D. Hurlbut, C. Imray, C. Ince, M. Jonas, J. van der Kaaij, M. Khosravi, N. Kolfschoten, D. Levett, H. Luery, A. Luks, D. Martin, R. McMorrow, P. Meale, K. Mitchell, H. Montgomery, G. Morgan, J. Morgan, A. Murray, M. Mythen, S. Newman, M. O’Dwyer, J. Pate, T. Plant, M. Pun, P. Richards, A. Richardson, G. Rodway, J. Simpson, C. Stroud, M. Stroud, J. Stygal, B. Symons, P. Szawarski, A. Van Tulleken, C. Van Tulleken, A. Vercueil, L. Wandrag, M. Wilson, J. Windsor.
Scientific Advisory Group: B. Basnyat, C. Clarke, T. Hornbein, J. Milledge, J. West.
Members of the Xtreme Everest 2 Research Group are as follows:
S Abraham, T Adams, W Anseeuw, R Astin, B Basnyat, O Burdall, J Carroll, A Cobb, J Coppel, O Couppis, J Court, A Cumptsey, T Davies, S Dhillon, N Diamond, C Dougall, T Geliot, E Gilbert-Kawai, G Gilbert-Kawai, E Gnaiger, M Grocott, C Haldane, P Hennis, J Horscroft, D Howard, S Jack, B Jarvis, W Jenner, G Jones, J van der Kaaij, J Kenth, A Kotwica, R Kumar BC, J Lacey, V Laner, D Levett, D Martin, P Meale, K Mitchell, Z Mahomed, J Moonie, A Murray, M Mythen, P Mythen, K O’Brien, I. Ruggles-Brice, K Salmon, A Sheperdigian, T Smedley, B Symons, C Tomlinson, A Vercueil, L Wandrag, S Ward, A Wight, C Wilkinson, S Wythe.
Scientific Advisory Board: M Feelisch, E Gilbert-Kawai, M Grocott (chair), M Hanson, D Levett, D Martin, K Mitchell, H Montgomery, R Moon, A Murray, M Mythen, M Peters.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interest
Can Ince has developed SDF imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center (AMC). He has been a consultant for MVM in the past, but has not been involved with this company for more than 5 years now, except that he still holds shares. Braedius Medical, a company owned by a relative of Can Ince, has developed and designed a hand held microscope called CytoCam-IDF imaging. Dr Ince has no financial relation with Braedius Medical of any sort; he had never owned shares, or received consultancy or speaker fees from Braedius Medical. The authors declare that they had no competing interests.
Authors’ contributions
E G-K: Design of study, conduct of study, analysis of data, writing manuscript. JC: Conduct of study, analysis of data, writing manuscript. VB: Analysis of data, writing manuscript. CI: Design of study, writing manuscript. DM: Analysis of data, writing manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1:
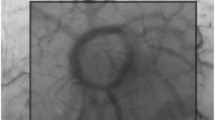
Two examples of images obtained using the incident dark field video-microscope. (PNG 1659 kb)
Additional file 2:
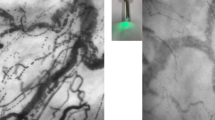
Two examples of images obtained using the sidestream dark field video-microscope. (PNG 1188 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gilbert-Kawai, E., Coppel, J., Bountziouka, V. et al. A comparison of the quality of image acquisition between the incident dark field and sidestream dark field video-microscopes. BMC Med Imaging 16, 10 (2016). https://doi.org/10.1186/s12880-015-0078-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-015-0078-8