Abstract
Purpose
This study aims to analyze whether undergoing amniocentesis during pregnancy in women diagnosed with hepatitis B virus (HBV) infection leads to HBV transmission to newborns.
Methods
Retrospective data collection was conducted from June 2019 to November 2022 on expectant mothers positive for hepatitis B surface antigen (HBsAg) who underwent amniocentesis at The Third Affiliated Hospital of Sun Yat-sen University, along with data on their newborns. The study summarized the HBV infection status of newborns born to mothers with different expressions of hepatitis B e antigen (HBeAg), antiviral treatment versus no treatment, and different HBV DNA viral loads before delivery.
Results
In this study, 346 expectant mothers tested positive for HBsAg, along with 351 newborns (including 5 sets of twins, with 8 infants (2.28%) testing HBsAg-positive at birth. All newborns received dual immunotherapy and were followed up. At 7–12 months, retesting for HBsAg positivity and HBV DNA positivity among infants revealed that out of the infants born with HBsAg positivity, 7 cases had seroconverted to negative, while the remaining infant, who was positive for both HBsAg and HBeAg at birth, tested positive for both HBsAg and HBV DNA at 7–12 months. Thus, one case of vertical transmission of hepatitis B from mother to child occurred in this study. The proportion of infants born with HBsAg + among newborns born to HBeAg-positive mothers (4 cases, 6.06%) was significantly higher than that among newborns born to HBeAg-negative mothers (4 cases, 1.41%) (P < 0.05). The proportion of infants born with HBsAg + showed no significant difference between newborns born to mothers receiving antiviral therapy (2 cases, 2.90%) and those born to mothers not receiving antiviral therapy (6 cases, 2.13%) (P > 0.05). Among expectant mothers with viral load ≥ 6 log 10 IU/mL before delivery, 3 newborns (30.00%) were manifesting HBsAg positivity at birth, significantly higher than the group with viral load < 6 log 10 IU/mL before delivery (5 cases, 1.47%) (P < 0.05).
Conclusion
Among HBsAg-positive expectant mothers, only a small number of infants are infected with the hepatitis B virus at birth, the proportion of which is relatively low. Infants born to mothers who are HBeAg-positive or have a viral load ≥ 6 log10 IU/mL have a higher risk of being born positive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus (HBV) is a DNA virus that targets liver cells, causing hepatitis B infection, which can range from acute to chronic. Chronic HBV infection can lead to severe complications such as cirrhosis and liver cancer [1, 2]. HBV spreads primarily through vertical transmission (mother-to-child transmission, MTCT) and horizontal transmission (such as sexual contact or blood exposure) [3]. Globally, HBV is a major public health issue, with the World Health Organization estimating around 200 million chronic carriers and 780,000 annual deaths from related complications [4,5,6]. Approximately 92 million individuals in China are chronically infected [4] despite recent efforts to enhance vaccination and healthcare measures [7,8,9]. In China, MTCT accounts for 30–50% of HBV infections, representing a significant route for familial transmission [10, 11]. While the use of hepatitis B vaccine and immunoglobulin (HBIG) has been effective in reducing MTCT, 5–10% of HBV-positive expectant mothers still transmit the virus to their infants [12, 13]. Preventing MTCT is crucial for controlling HBV, reducing chronic infections, and mitigating public health burdens. The risk of MTCT after amniocentesis is debated. The procedure may theoretically increase risk by causing placental leakage or contaminating amniotic fluid with maternal blood containing HBV [14, 15]. However, studies show mixed results, with some indicating no increased risk and others suggesting potential risk in mothers with high HBV DNA levels [16,17,18]. The current literature is largely retrospective and lacks large-scale retrospective cohort studies. This study aims to address this gap by retrospectively analyzing data from HBV-infected expectant mothers undergoing amniocentesis at our hospital from 2019 to 2023, to assess its impact on HBV MTCT.
Materials and methods
Study Population: Data were collected from HBV-infected expectant mothers who underwent amniocentesis at The Third Affiliated Hospital of Sun Yat-sen University from June 2019 to November 2023, along with information on their delivered newborns. Inclusion Criteria: (1) expectant mothers positive for HBV; (2) Absence of pre-existing conditions like hypertension, diabetes, or heart disease before pregnancy; (3) Expectant mothers requiring amniocentesis who underwent the procedure; (4) Women who had successful deliveries resulting in live births.、. Exclusion Criteria: (1) Expectant mothers co-infected with hepatitis C virus, hepatitis D virus, or human immunodeficiency virus; (2) The prolonged use of corticosteroids or immunomodulators; (3) HBsAg-positive partners of expectant mothers; (4) expectant mothers with psychiatric disorders or intellectual disabilities who cannot cooperate with the investigation or refuse to participate; (5) Abnormal termination of pregnancies. A total of 346 HBV-positive expectant mothers were enrolled in the study. The study obtained approval from the Medical Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University and informed consent from the expectant mothers and their families.
Amniocentesis procedure
Before the procedure, proper education is provided to the expectant mother, guiding her to empty her bladder and assisting her into a supine position. The abdominal skin is routinely disinfected, drapes are placed, and the probe (previously disinfected) is used for ultrasound examination. Before the procedure, accurate fetal biometry measurements, such as the biparietal diameter, are taken. The puncture should avoid the area with the most amniotic fluid. If the anterior placenta cannot be avoided, the thinnest edge of the placenta is chosen as the optimal puncture site. Continuous ultrasound imaging guides the depth of needle insertion, rapidly advancing the needle until entering the amniotic cavity. A new syringe is then used to aspirate amniotic fluid for testing. After needle withdrawal, pressure is applied to the puncture site.
Data collection
General demographic data are collected, including age, gravidity, HBV serological markers, HBV DNA viral load, whether antiviral treatment is received, specific medications, indications for amniocentesis, gestational age at amniocentesis, and mode of delivery for expectant mothers. Data for infants include gestational age at delivery, gender, birth weight, HBV serological markers, and HBV DNA viral load in venous blood at birth and during follow-up at 7–12 months old.
Diagnostic criteria for HBV MTCT
The diagnostic criteria for HBV mother-to-child transmission include peripheral blood HBsAg positivity and/or HBV DNA positivity at birth in the newborn, which persists as positive until 7–12 months of age [10].
Treatment protocol
All newborns receive early combined passive and active immunization within 12 h of birth. The specific protocol is as follows: within 6 h of birth, newborns receive treatment with hepatitis B immunoglobulin (produced by Sichuan Yuanda Shuyang Pharmaceutical Co., Ltd., Chengdu, China) via intramuscular injection at a dose of 100 IU. Additionally, within 6 h of birth (first dose), at 1 month (second dose), and 6 months of age (third dose), newborns are vaccinated with 10 µg of yeast-derived recombinant hepatitis B vaccine (HBVac, produced by Beijing Tiantan Biological Products Co., Ltd., Beijing, China). In exceptional circumstances, administering the second dose of the hepatitis B vaccine may be delayed. However, it should not exceed 3 months, while the third dose of the hepatitis B vaccine should still be administered on schedule. Post-discharge follow-up is conducted for both the mother and the newborn. These infants are followed up until 12 months of age, with peripheral venous blood collected at 0, 7 ~ 12 months of follow-up to detect HBV serological markers and HBV DNA levels.
Antiviral Treatment Protocol for expectant mothers: mothers with serum HBV DNA levels ≥ 2.0 × 10^5 IU/mL are referred to a multidisciplinary team (MDT) consisting of obstetricians and hepatologists. The necessity of antiviral treatment and potential side effects are explained to the expectant mothers, assisting them in making treatment decisions. Antiviral treatment is scheduled after obtaining informed consent from the expectant mothers. Typically, nucleoside analogues such as tenofovir and entecavir are initiated for antiviral treatment between gestational weeks 24 and 28. For patients with active hepatitis, cirrhosis, or other related conditions, early initiation of antiviral treatment may be considered.
HBV DNA viral load and HBV serological marker detection methods
HBV DNA viral load detection method: The PCR-fluorescence probe method is employed for HBV DNA viral load detection. The PCR-fluorescence quantification kit for hepatitis B virus utilizes specific detection of nucleic acid fragments of the hepatitis B virus through polymerase chain reaction (PCR), enabling rapid identification of HBV DNA viral load.
Detection methods for serological markers of hepatitis B virus infection: The Abbott reagents are used to perform quantitative tests for the five hepatitis B markers via Enzyme-linked immunosorbent assay (ELISA) or Chemiluminescence immunoassay (CLIA). The five hepatitis B markers include HBsAg (HBsAg ≥ 0.05 IU/ml is positive), HBsAb (HBsAb ≥ 10 IU/ml is positive), HBeAg (HBeAg ≥ 1.00 S/CO is positive), HBeAb (HBeAb ≤ 1.00 S/CO is positive), and HBcAb (HBcAb ≥ 1.00 S/CO is positive).
Statistical methods
The database is established using Microsoft Office Excel 2013 to input and cross-check the data. IBM SPSS Statistics 26.0 is utilized for statistical analysis. For categorical data, frequencies (n) and percentages (%) are used for representation, and chi-square tests are conducted for comparisons. For continuous data, the Kolmogorov-Smirnov test is employed to check for normality. Data conforming to a normal distribution are presented as mean ± standard deviation, while those not conforming are presented as median (Interquartile Range) -- M (IQR). P < 0.05 indicates statistical significance.
Results
The research procedure (Fig. 1)
Basic characteristics of HBsAg-positive expectant mothers
Within the time frame of case inclusion, a total of 353 HBsAg-positive pregnant women underwent amniocentesis during pregnancy. Among them, 5 pregnant women opted for pregnancy termination due to fetal genetic abnormalities, 1 pregnant woman experienced a spontaneous miscarriage at 24 + 2 weeks due to stage 5 chronic kidney disease, and 1 pregnant woman underwent induced labor at 33 + 4 weeks due to intrauterine fetal demise. As per the inclusion criteria, 346 HBsAg-positive expectant mothers were gathered as the study cohort. Basic demographic data during the perinatal period were collected, including age, gravidity, parity, serum index test results, HBV DNA quantification, information related to amniocentesis, and mode of delivery.
Among the 346 HBsAg-positive expectant mothers, ages ranged from 23 to 42 years, and the median age of the participants was 33 years. The study further analyzed the gravidity and parity of women in the sample. The results revealed that out of the total, 86 women (24.86%) had only been pregnant once, 105 women (30.35%) had been pregnant twice, 73 women (21.10%) had been pregnant thrice, 45 women (13.01%) had been pregnant four times, and 37 women (10.69%) had been pregnant five times or more. Regarding parity, 107 women (30.92%) were nulliparous, 196 women (56.65%) had experienced one childbirth, 40 women (11.56%) had experienced two childbirths, and only 3 women (0.09%) had experienced three childbirths. Serological marker testing for hepatitis B virus infection revealed that among these 346 expectant mothers, 65 exhibited HBeAg positivity, accounting for 18.79%. Additionally, 67 expectant mothers (19.36%) received or had received antiviral treatment. Among these 67 expectant mothers receiving antiviral treatment, 20 (29.85%) underwent treatment before pregnancy, while the remaining 47 (70.15%) chose to undergo antiviral treatment during pregnancy after receiving counseling. Among the 67 cases, 58 (86.57%) received TDF as antiviral medication, 5 (7.46%) received entecavir, and the remaining 4 (5.97%) received tenofovir alafenamide. The study tested HBV DNA quantification on expectant mothers at different times. The results showed that the HBV DNA quantification for all expectant mothers during the first prenatal examination was 7.00 (3.00) log10 IU/mL. By the time of amniocentesis, there was a significant reduction in HBV DNA levels among expectant mothers receiving antiviral treatment, leading to an overall HBV DNA level decreased to 4.00 (1.00) log10 IU/mL. The results of testing before delivery indicated that the overall HBV DNA levels in expectant mothers remained at 4.00 (1.00) log10 IU/mL. A summary analysis of indications for amniocentesis for all expectant mothers revealed that many required the procedure due to high-risk Down syndrome (DS), ultrasound abnormalities, advanced maternal age, thalassemia, Non-Invasive Prenatal Testing (NIPT) abnormalities, and other indications. Among these, DS high-risk accounted for 62.14% (215 cases), advanced maternal age accounted for 16.47% (57 cases), ultrasound abnormalities accounted for 5.49% (19 cases), thalassemia accounted for 7.23% (25 cases), NIPT abnormalities accounted for 2.89% (10 cases), and other indications accounted for 5.20% (18 cases). It is worth mentioning that one case (0.29%) had DS high-risk combined with ultrasound abnormalities, and another case (0.29%) was advanced maternal age combined with ultrasound abnormalities. The median gestational age at amniocentesis for these expectant mothers was 19.79 weeks, with an Interquartile Range (IQR) of 4.39 and a range of 17.14 to 26.29 weeks. The median duration from amniocentesis to delivery was 128.50 days, with an IQR of 25.25 and a range of 73 to 157 days. Finally, the mode of delivery for the expectant mothers was recorded and classified, with 212 cases (61.27%) undergoing vaginal delivery, 131 cases (37.86%) undergoing cesarean section, and the remaining 3 cases (0.87%) delivered via forceps or vacuum extraction. See Table 1 for detailed characteristics.
Maternal and neonatal outcomes
As shown in Table 1, a total of 212 expectant mothers underwent vaginal delivery, while 131 expectant mothers delivered via cesarean section, and the remaining 3 expectant mothers underwent delivery using forceps or vacuum extraction. Among the 346 expectant mothers, there were 351 newborns delivered (5 cases of twins, all delivered by cesarean section), including 188 male infants and 163 female infants. All pregnancies resulted in successful deliveries without any significant adverse events.
Neonatal characteristics
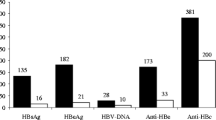
Among the 351 newborns were 188 male infants (53.56%) and 163 female infants (46.44%). Birth weights were measured and recorded at birth, with a median birth weight of 3.20 kg, an interquartile range (IQR) of 0.20, and a weight range of 1.30 ~ 4.25 kg. According to the results of the hepatitis B virus (HBV) five-item test, 8 infants (2.28%) tested positive for HBsAg at birth, 68 infants (19.37%) tested positive for HBeAg, 147 infants (41.88%) tested positive for HBeAb, 6 infants (1.71%) tested positive for HBcAb, and 8 infants (2.28%) tested positive for HBV DNA. All newborns received dual immunotherapy after birth, and their conditions were followed up. HBsAg and HBV DNA were retested at 7–12 months of age. The follow-up at 7–12 months showed that only 1 infant tested positive for HBsAg, with an HBV DNA level of 5 log10 IU/mL. This infant was delivered by cesarean section and tested positive for HBsAg, HBeAg, and HBcAb at birth but tested negative for HBV DNA (Table 2). To ensure comprehensive monitoring of neonatal health and HBV infection status, additional follow-up included ultrasound examinations at 7–12 months of age. This follow-up aimed to assess liver function and screen for any persistent HBV-related issues.
Analysis of neonatal infection in HBeAg positive and negative expectant mothers groups
Among the 346 HBeAg-positive expectant mothers, 65 exhibited HBeAg positivity during prenatal screening (including 1 case of twins), and 281 exhibited HBeAg negativity (including 4 cases of twins). A total of 351 neonates were included in this analysis. Among infants born to HBeAg + mothers, 4 cases manifested HBsAg positivity at birth, accounting for 6.06% of the total. Among infants born to HBeAg- -mothers, 4 cases also exhibited HBsAg + at birth, accounting for 1.41% of the total. The proportion of newborns born to HBeAg + mothers showing HBsAg + at birth was significantly higher than that of newborns born to HBeAg- mothers (P < 0.05). Detailed comparisons are shown in Table 3. In addition to initial infection testing at birth and the 7–12-month follow-up, infants underwent ultrasound examinations and liver sample analyses to evaluate liver health and the effectiveness of prevention measures. These assessments helped to monitor for any delayed or residual HBV-related complications.
Analysis of neonatal infection in expectant mothers with and without antiviral therapy
A total of 346 HBeAg-positive expectant mothers, including 67 cases (including 2 sets of twins) who received antiviral therapy during pregnancy or before pregnancy, and 279 cases (including 3 sets of twins) who did not receive antiviral therapy, were included in this analysis. A total of 351 newborns were included in this analysis. Expectant mothers undergoing antiviral treatment gave birth to 2 newborns with HBsAg + status, accounting for 2.90% of the total. In contrast, among expectant mothers not receiving antiviral treatment, 6 newborns were HBsAg+, representing 2.13% of the total. The proportions of newborns with HBsAg-positive status in both groups are similar (P > 0.05). Refer to Table 4 for further details.
Analysis of neonatal infection in expectant mothers with different viral loads before delivery
A total of 346 expectant mothers underwent HBV DNA quantitative testing before delivery. The results showed that 10 cases had a viral load ≥ 6 log10 IU/mL before delivery, among whom 3 cases (30.00%) HBsAg + at birth. Among the expectant mothers with a viral load < 6 log10 IU/mL, there were 336 cases, including 5 cases of twin births. Among the 341 newborns born to these women, 5 cases (1.47%) of HBsAg + at birth. The difference between the two groups was statistically significant (P < 0.05). Detailed results are provided in Table 5.
Discussion
With the continuous development of medical technology, interventional ultrasound is gradually being widely used in clinical medicine. Under ultrasound guidance, fetal specimens can be effectively and accurately extracted for examinations, such as the most basic molecular genetics and cytogenetics. This enables timely and accurate prenatal diagnosis of genetic diseases, intrauterine infections, and even abnormalities in high-risk fetuses, thereby reducing the birth of defective children and improving population quality [19, 20]. Amniocentesis is a classic clinical practice technique and a representative invasive diagnostic technique. However, there is some controversy regarding whether amniocentesis should be performed in HBV-positive expectant mothers [21]. On one hand, amniocentesis may increase the risk of MTCT of HBV because the procedure involves puncturing the amniotic membrane and amniotic fluid, which presents a possibility of virus entry into the maternal bloodstream [22]. On the other hand, for expectant mothers requiring amniocentesis, not performing the procedure may result in missing the opportunity for timely diagnosis of whether the fetus has a genetic disorder, thereby affecting prenatal management and intervention measures [23].
Previous studies have reported on the potential risk of increased mother-to-child transmission (MTCT) of HBV associated with amniocentesis. Ko et al. early case-control study observed no significant difference in MTCT rates when comparing HBV surface antigen-positive mothers who underwent amniocentesis with those who did not [18]. Similarly, Alexander et al. reported similar findings in a study of 21 mothers with chronic hepatitis B who underwent amniocentesis (except for one mother, all mothers were HBeAg-negative), with their paired infants receiving appropriate immunoprophylaxis [24]. Towers et al. recent study assessed the MTCT rate of HBV in mothers based on HBsAg positivity or detectable HBV DNA in newborn cord blood samples [25]. They concluded that the MTCT rate in newborns of 30 mothers who underwent amniocentesis was consistent with the rate in newborns of 72 mothers who did not undergo amniocentesis. However, the aforementioned exploratory studies are mostly retrospective and limited by relatively small sample sizes, lacking retrospective studies. To address this research gap, this study retrospectively collected 346 cases of HBsAg + expectant mothers and analyzed the impact of amniocentesis on the MTCT of HBV.
This study included 346 HBsAg + expectant mothers and 351 newborn babies, among which 8 newborns tested HBsAg + at delivery, with a proportion of 2.28%. Notably, most infants who tested HBsAg + at delivery had converted to negative status between 7 and 12 months after receiving combined immunotherapy and vaccination. Only one infant who was HBsAg + and HBeAg + at delivery tested positive for HBsAg and HBV DNA between 7 and 12 months. This result indicates that after receiving immunotherapy and vaccination, most infants who tested HBsAg-positive at birth had converted to negative status between 7 and 12 months. This demonstrates the effectiveness of immunotherapy and vaccination in preventing HBV transmission. However, for a small number of infants who tested positive for both HBsAg and HBeAg at birth, the transition to negative status may not be as smooth as for other infants, and there may still be detection of HBV DNA. This may indicate that the HBV infection status of these infants is more complex, requiring closer monitoring and management to ensure timely measures are taken to prevent virus transmission. It also underscores the importance of more detailed monitoring and management for high-risk infants, especially those born to mothers who are carriers of both HBsAg and HBeAg [26]. Further analysis reveals that the proportion of infants born HBsAg + from HBeAg + mothers (6.06%) is significantly higher than the proportion of babies born HBeAg + from HBeAg- mothers (1.41%) (P < 0.05). This suggests that maternal HBeAg + status may play a crucial role in MTCT. HBeAg-positive status is typically associated with higher HBV viral loads, which may contribute to an increased risk of MTCT. A high viral load can increase the likelihood of the fetus being exposed to the virus in the uterus, thereby increasing the risk of being born with HBsAg. Additionally, HBeAg + status may also be associated with increased virus activity and transmission. HBeAg is generally considered a marker of HBV activity, as its presence indicates active virus replication and release. Therefore, maternal HBeAg + status may increase virus exposure during fetal development, increasing the risk of being born with HBsAg [27, 28]. This result emphasizes the importance of special attention and management for HBeAg + expectant mothers and their infants. For these expectant mothers, closer monitoring and care may be necessary to ensure that their babies are as protected as possible from HBV infection at birth. Additionally, this highlights the need for regular monitoring and assessment during pregnancy for expectant mothers who are carriers of HBV. This allows for appropriate preventive measures to be taken based on their viral load and other relevant factors.
However, our study highlights that maternal viral load, rather than the amniocentesis procedure itself, is the key determinant of the risk of HBV transmission to the infant. The findings indicate that the proportion of HBsAg-positive infants at birth is significantly influenced by the maternal viral load, with higher rates of infection associated with viral loads ≥ 6 log10 IU/mL. This underscores that the primary factor affecting MTCT is the level of HBV replication in the mother rather than the invasive nature of the amniocentesis procedure. This is consistent with recent findings that emphasize the role of maternal viral load in determining the risk of HBV transmission [29, 30]. Although our study provides valuable insights, it is limited by its scope compared to other studies, such as Jin Zhou et al., who investigated a larger cohort of 353 HBsAg-positive pregnant women. Their research underscores the need for broader retrospective studies to validate our findings [31] further.
Although our study represents the largest case report on the MTCT of HBV after amniocentesis and is retrospective, there are still some limitations. Given the title change to focus specifically on mothers who underwent amniocentesis, the limitation related to the absence of a control group for comparison with mothers who did not undergo amniocentesis is acknowledged but not addressed in this study. Additionally, the limitations of sample size and variability in antiviral treatment initiation have been considered.
In this study, the follow-up care for the newborns of HBsAg-positive mothers was managed by a multidisciplinary team, including pediatricians and infectious disease specialists. Pediatricians conducted routine health checks, while contagious disease specialists oversaw the management of any HBV-related complications and provided antiviral therapy when necessary.
Moreover, the monitoring of these high-risk infants was supported by a national program designed to track the health outcomes of newborns exposed to HBV. This program facilitated comprehensive follow-up care, ensuring each infant received appropriate immunotherapy and regular testing to detect and manage HBV infection. The program’s systematic approach aids in reducing the risk of HBV transmission and improving long-term outcomes for affected infants.
Our study provides valuable insights into the risk of vertical transmission of HBV associated with prenatal invasive procedures. Given the significance of our findings, we believe that they could inform the development of international guidelines for managing HBV-infected pregnant women undergoing amniocentesis. By incorporating our results, clinical guidelines can be enhanced to better address these patients’ risks and management strategies globally.
In summary, the impact of amniocentesis on the MTCT of HBV in this study indicates a significant increase in the infection rate among infants born to HBeAg-positive mothers. At the same time, antiviral therapy seems to have no significant effect on the infection rate. Additionally, expectant mothers with viral loads ≥ 6 log10 IU/mL before delivery pose a higher transmission risk. These study findings underscore the importance of considering maternal viral load and HBeAg status when formulating prevention strategies, providing valuable information for interventions aimed at preventing HBV mother-to-child transmission.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
26 September 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12879-024-09936-3
References
Zamor PJ, Lane AM. Interpretation of HBV serologies. Clin Liver Dis. 2021;25(4):689–709. https://doi.org/10.1016/j.cld.2021.06.012.
Liu Z, Li Y, Wang Y, Bai X, Zhang Y. Exosomes in HBV infection. Clin Chim Acta. 2023;538:65–9. https://doi.org/10.1016/j.cca.2022.11.012.
Pollicino T, Caminiti G. HBV-Integration studies in the clinic: role in the natural history of infection. Viruses. 2021;13(3):368. https://doi.org/10.3390/v13030368.
Yue T, Zhang Q, Cai T, Xu M, Zhu H, Pourkarim MR, De Clercq E, Li G. Trends in the disease burden of HBV and HCV infection in China from 1990–2019. Int J Infect Dis. 2022;122:476–85. https://doi.org/10.1016/j.ijid.2022.06.017.
Wan X, Young KH, Bai O. HBV-associated DLBCL of poor prognosis: advance in pathogenesis, immunity and therapy. Front Immunol. 2023;14:1216610. https://doi.org/10.3389/fimmu.2023.1216610.
Mazzaro C, Adinolfi LE, Pozzato G, Nevola R, Zanier A, Serraino D, Andreone P, Fenoglio R, Sciascia S, Gattei V, Roccatello D. Extrahepatic manifestations of chronic HBV infection and the role of antiviral therapy. J Clin Med. 2022;11(21):6247. https://doi.org/10.3390/jcm11216247.
Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, Chen W, Bai S, Wu J. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):811. https://doi.org/10.1186/s12879-019-4428-y.
Liu Z, Lin C, Mao X, Guo C, Suo C, Zhu D, Jiang W, Li Y, Fan J, Song C, Zhang T, Jin L, De Martel C, Clifford GM, Chen X. Changing prevalence of chronic hepatitis B virus infection in China between 1973 and 2021: a systematic literature review and meta-analysis of 3740 studies and 231 million people. Gut. 2023;72(12):2354–63. https://doi.org/10.1136/gutjnl-2023-330691.
Li M, Zu J, Shen M, Zhuang G, Chen S, Wang F, Zheng H, Zhang G. Evaluating the independent influence of sexual transmission on HBV infection in China: a modeling study. BMC Public Health. 2021;21(1):388.
Jing W, Liu J, Liu M. Eliminating mother-to-child transmission of HBV: progress and challenges in China. Front Med. 2020;14(1):21–9. https://doi.org/10.1007/s11684-020-0744-2.
Yang M, Qin Q, Fang Q, Jiang L, Nie S. Cesarean section to prevent mother-to-child transmission of hepatitis B virus in China: a meta-analysis. BMC Pregnancy Childbirth. 2017;17(1):303. https://doi.org/10.1186/s12884-017-1487-1.
Lu H, Cao W, Zhang L, Yang L, Bi X, Lin Y, Deng W, Jiang T, Sun F, Zeng Z, Lu Y, Zhang L, Liu R, Gao Y, Wu S, Hao H, Chen X, Hu L, Xu M, Xiong Q, Dong J, Song R, Li M, Xie Y. Effects of Hepatitis B virus infection and strategies for preventing mother-to-child transmission on maternal and fetal T-cell immunity. Front Immunol. 2023;14:1122048. https://doi.org/10.3389/fimmu.2023.
Zheng H, Walsh N, Lesi O, Cui F. New progress towards elimination of mother-to-child transmission of hepatitis B virus in China. Hepatol Int. 2022;16(6):1273–81. https://doi.org/10.1007/s12072-022-10400-0.
Yi W, Pan CQ, Hao J, Hu Y, Liu M, Li L, Liang D. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol. 2014;60(3):523–9.
Han Z, Zhang Y, Bai X, Yin Y, Xu C, Hou H. Mother-to-child transmission of hepatitis B virus after amniocentesis: a retrospective matched cohort study. Prenat Diagn. 2019;39(6):431–40.
Cimpoca B, Panaitescu AM, Gica N, Veduta A, Ciobanu A. Risk of vertical transmission of chronic viral infections after invasive prenatal procedures. Ginekol Pol. 2022 Jan;24. https://doi.org/10.5603/GP.a2021.0196.
Han Z, Zhang Y, Zhou J, Wang Q, Huang Y, Hou H. Risk of mother-to-child transmission of hepatitis B virus after fetal blood sampling: a report of six cases. BMC Infect Dis. 2021;21(1):716. https://doi.org/10.1186/s12879-021-06423-x.
Ko TM, Tseng LH, Chang MH, Chen DS, Hsieh FJ, Chuang SM, Lee TY. Amniocentesis in mothers who are hepatitis B virus carriers does not expose the infant to an increased risk of hepatitis B virus infection. Arch Gynecol Obstet. 1994;255(1):25–30. https://doi.org/10.1007/BF02390671.
Chatzakis C, Sotiriadis A, Dinas K, Ville Y. Neonatal and long-term outcomes of infants with congenital cytomegalovirus infection and negative amniocentesis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2023;61(2):158–67. https://doi.org/10.1002/uog.26128.
Wertheimer A, Decter D, Borovich A, Trigerman S, Bardin R, Hadar E, Krispin E. Amniocentesis in twin gestation: the association between gestational age at procedure and complications. Arch Gynecol Obstet. 2022;305(5):1169–75. https://doi.org/10.1007/s00404-021-06242-0.
Nassr AA, Hessami K, D’Alberti E, Giancotti A, Meshinchiasl N, Evans MI, Di Mascio D, Shamshirsaz AA. Obstetrical outcomes following amniocentesis performed after 24 weeks of gestation: a systematic review and meta-analysis. Prenat Diagn. 2023;43(11):1425–32. https://doi.org/10.1002/pd.6435.
Shahar-Nissan K, Pardo J, Peled O, Krause I, Bilavsky E, Wiznitzer A, Hadar E, Amir J. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396(10253):779–85. https://doi.org/10.1016/S0140-6736(20)31868-7.
Du X, Zhang L, Liu Z, Qian Y, Zhang X, Hu T, Liu S, Wang H, Zhang Z. Risk of mother-to-child transmission after amniocentesis in pregnant women with hepatitis B virus: a retrospective cohort study. Am J Obstet Gynecol. 2024;230(2):249. https://doi.org/10.1016/j.ajog.2023.07.032.
Alexander JM, Ramus R, Jackson G, Sercely B, Wendel GD Jr. Risk of hepatitis B transmission after amniocentesis in chronic hepatitis B carriers. Infect Dis Obstet Gynecol. 1999;7(6):283–6. https://doi.org/10.1002/(sici)1098-0997(1999)7:6%3C283::aid-idog6%3E3.0.co;2-t.
Towers CV, Asrat T, Rumney P. The presence of hepatitis B surface antigen and deoxyribonucleic acid in amniotic fluid and cord blood. Am J Obstet Gynecol. 2001;184(7):1514-8; discussion 1518-20. https://doi.org/10.1067/mob.2001.114866
Funk AL, Lu Y, Yoshida K, Zhao T, Boucheron P, van Holten J, Chou R, Bulterys M, Shimakawa Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(1):70–84. https://doi.org/10.1016/S1473-3099(20)30586-7.
He R, Wen P, Xiong M, Fan Z, Li F, Luo D, Xie X. Cesarean section in reducing mother-to-child HBV transmission: a meta-analysis. J Matern Fetal Neonatal Med. 2022;35(18):3424–32. https://doi.org/10.1080/14767058.2020.1819229.
Taye BW, Ayenew GM, Wasie Taye Z, Balew M, Bishaw Taye E. The risk of mother-to-child transmission of hepatitis B virus infection in Ethiopia: a systematic review and meta-analysis. J Infect Dev Ctries. 2023;17(6):744–51. https://doi.org/10.3855/jidc.17931.
Shimakawa Y, Veillon P, Birguel J, Pivert A, Sauvage V, Guillou-Guillemette HL, Roger S, Njouom R, Ducancelle A, Amta P, Huraux JM, Adoukara JP, Lunel-Fabiani F. Residual risk of mother-to-child transmission of hepatitis B virus infection despite timely birth-dose vaccination in Cameroon (ANRS 12303): a single-centre, longitudinal observational study. Lancet Glob Health. 2022;10(4):e521–9. https://doi.org/10.1016/S2214-109X(22)00026-2.
Zhou M, Li L, Han L, Sun F, Yi N. Breast-feeding is not a risk factor of Mother-to-child transmission of Hepatitis B Virus. Int J Gen Med. 2021;14:1819–27. https://doi.org/10.2147/IJGM.S289804.
Zhang Y, Wei W, Huang Y. Correlation between maternal hepatitis B virus viral load and risk of mother-to-child transmission: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):572. https://doi.org/10.1186/s12879-020-05212-2.
Chen L, Zhao L, Ma Y. The role of maternal viral load in predicting HBV transmission risk: evidence from longitudinal studies. J Viral Hepatitis. 2022;29(1):85–92. https://doi.org/10.1111/jvh.13514.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
We declare that all the listed authors have participated actively in the study and all meet the requirements of the authorship. Dr. JZ & PZZ designed the study and wrote the paper, Dr. ZMT performed research, Dr. CL & LY managed the literature searches and analyses, Dr. ZYH & YZY contributed to the correspondence and paper revision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study obtained approval from the Medical Ethics Committee of The Third Affiliated Hospital of Sun Yat-sen University and informed consent from the expectant mothers and their families.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Following publication of the original article, we have been notified that the equal contribution note was incorrect. Originally published note: “Zhenyan Han and Yuzhu Yin First author contributed equally.” Correct note: “Jin Zhou and Peizhen Zhang are co-first authors who contributed equally to this work.”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, J., Zhang, P., Tan, Z. et al. Results of mother-to-child transmission in hepatitis B-positive mothers who underwent amniocentesis. BMC Infect Dis 24, 957 (2024). https://doi.org/10.1186/s12879-024-09848-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09848-2