Abstract
Background and objectives
Klebsiella pneumoniae (K. pneumoniae) is the second leading cause of community-acquired and hospital-acquired gram-negative bloodstream infection (BSI). This study aimed to assess the epidemiological and microbial-resistance characteristics and clinical factors associated with K. pneumoniae BSI in Saudi Arabia.
Materials and Methods
Data of 152 K. pneumoniae isolates diagnosed between January 2019 and January 2020 at King Fahad Medical City, Riyadh, Saudi Arabia were evaluated retrospectively. Clinical records of the patients were collected and analysed statistically.
Results
In total, 152 cases of K. pneumoniae BSI were identified. Adult patients (66.4%) were at a higher risk of developing the infection than paediatric patients (33.6%). The rate of infection was slightly higher in women than in men. Neurological disorders were the predominant underlying conditions for the acquisition of K. pneumoniae BSI, at all ages. Most of the deceased patients were adults with multi-organ dysfunction. Klebsiella pneumoniae showed disturbing resistance to amoxicillin-clavulanate and cefuroxime (72.4%), ceftazidime (67.8), cephalothin (76.3%), and to Carbapenems (36.1%).
Conclusions
The impact of K. pneumoniae BSI was seen not only at the patient level, but also at the community level, and was related to multi-drug resistant infection. These findings provide a better understanding of microbial resistance and its association with patient clinical outcomes.
Similar content being viewed by others
Introduction
Klebsiella pneumoniae, a normal commensal organism living on human mucosal surfaces, including the gastrointestinal tract and oropharynx, can invade other tissues and cause severe infections [1]. It is the second leading cause of bloodstream infections (BSI) caused by gram-negative bacteria, after Escherichia coli [2]. BSI can occur as a primary infection with no identifiable source. However, it is typically caused by spread from a known source into the bloodstream. The urinary tract, gastrointestinal tract, intravenous or urinary catheters, and respiratory sites are common sources of secondary BSI [3].
Several investigations have indicated that diabetes, hepatobiliary illness, and neoplastic disease were associated as risk factors to around 50% of community-acquired K. pneumoniae [4, 5] Lin Y et al. (2010), on the other hand, discovered that chronic lung illness was the primary underlying disease, which may be supported by the older age of their research cohort. Prior antibiotic usage, as well as the use of invasive procedures such as an endotracheal catheter, bladder catheter, and intravenous catheter, are key risk factors for developing such infection [6].
Over the past two decades, K. pneumoniae has become clinically significant owing to its increased antibiotic resistance, propensity to develop antibiotic resistance, and ability to produce serious outcomes [7, 8]. More recently, it has been identified as a major source of hospital-acquired pneumonia, accounting for approximately. 10% of all hospital-acquired infections, second among gram-negative bacteria [9]. Multidrug-resistant (MDR) K. pneumoniae is one of the main factors responsible for nosocomial infections, including pneumonia, urinary tract infections, and bloodstream infections (BSIs); its production of extended-spectrum β-lactamases (ESBL) and carbapenemases causes high mortality rates (40–50%), primarily among critically ill and immunocompromised patients [10]. The emergence of extensively drug-resistant (XDR) and pan-drug-resistant (PDR) strains, via continuous accumulation of antibiotic-resistant genes, protects K. pneumoniae from all available antimicrobials [11, 12].
The increasing prevalence of multidrug-resistant K. pneumoniae is a global health concern listed by the World Health Organization (WHO) as a critical priority. Unfortunately, in Saudi Arabia, research findings regarding K. pneumoniae infections are consistent with the global emergence of drug-resistant strains [13, 14]. There have been multiple reports of NDM and OXA-48 carbapenemases producing K. pneumoniae in Saudi Arabia [15, 16], and more recently the first case of KPC producing K. pneumoniae has been reported [17]. Furthermore, a study of ICU patients revealed a high prevalence of drug-resistant Enterobacteriaceae infections, which are related to a high mortality rate. Out of the 227 Enterobacteriaceae cultures included in that study, 60% were either MDR (n = 130) or XDR (n = 8) infections, with no PDR cultures; K. pneumoniae represented (33%) of the MDR/XDR cultures [18].
Such reports emphasise the necessity of continuous monitoring of the antimicrobial resistance of bacterial isolates, as this can be used to provide guidance to clinicians in the application of empirical treatment. This retrospective study investigated K. pneumoniae bacteraemia cases at the King Fahad Medical City (KFMC) in Riyadh, Saudi Arabia. The primary objectives were to evaluate the epidemiological patterns, determine the antimicrobial resistance profiles of the isolates, and clarify their association with patients’ clinical outcomes.
Materials and methods
Study design and setting
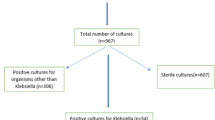
This retrospective study was conducted over one year (January 2019 to January 2020) at KFMC, which has a capacity of 1200 beds. A total of 152 Klebsiella pneumoniae isolates from blood clinical samples were analysed. The clinical history of 152 patients was included in this study.
Data collection
In total, 152 samples of Klebsiella pneumoniae were collected from blood (central and peripheral line blood). The following were the included collection categories: (A) age (paediatric or adult). (B) Ward or clinic to which the patient was admitted (emergency, ICU, ward, or outpatient clinic). (C) blood sample source and location or site; and (D) bacterial-resistance category (susceptible, Extended-spectrum β-lactamases (ESBL), and Carbapenem-Resistant strains). Any growth other than that of K. pneumoniae was excluded from the study. Clinical history was collected from the KFMC database for paediatric and adult patients admitted to ICU. The clinical history collected for ICU patients included different criteria: (1) if present, the type of co-infection; (2) exposure to carbapenem or other antibiotics in the past 14–30 days; (3) renal dialysis at isolation or not; (4) mechanical ventilation or not; (5) chronic diseases such as diabetes mellitus (DM), hypertension, renal disease, or malignancy; (6) presence of clinical symptoms such as fever, gastrointestinal tract (GIT) symptoms, or respiratory symptoms; (7) presence of wound or urinary tract infection; (8) presence of bacteraemia or septicaemia; and (9) clinical outcomes and additional notes, if available.
Klebsiella pneumoniae identification and antimicrobial susceptibility testing
All isolates were presumptively identified as Klebsiella species, using a Phoenix BD instrument (Becton Dickinson Diagnostic Systems, Sparks, MD, USA) for full identification and sensitivity testing. We included only patients whose isolates were definitively identified as K. pneumoniae. Antimicrobial sensitivity testing (AST) was performed for the following antibiotics: ampicillin (AMP), amoxicillin-clavulanate (AMC), piperacillin-tazobactam (TZP), imipenem (IPM), meropenem (MER), ertapenem (ETP), cephalothin (CEF), cefuroxime (CXM), ceftazidime (CTZ), cefoxitin (FOX), cefepime (CFPM), cefotaxime (CTX), ceftriaxone (CRO), ciprofloxacin (CIP), levofloxacin (LVX), gentamicin (GM), amikacin (AMK), tigecycline (TGC), colistin (COL), and trimethoprim-sulfamethoxazole (TMP-SMX). Susceptibility was classified as follows: susceptible, intermediate, or resistant. Confirmation of resistant isolates was performed using Microbroth dilution. Additional tests of disc diffusion or gradient diffusion (Etest) methods were performed using Mueller-Hinton agar which were then incubated in ambient air at 35 ℃ for 16–20 h. For interpretation, CLSI M100 Interpretive Document for Enterobacterales. EUCAST was used for the interpretation of tigecycline activity.
Statistical analysis
The data were analysed using GraphPad Prism version 9.3.1. Descriptive analysis, using contingency tables and graphs, was used to illustrate the following data: age divisions, sex, ward/clinic, sample source, and sample site. The descriptive data are expressed as absolute numbers (n) and percentages. P < 0.05 was considered statistically significant. Relative risk (RR) was computed to demonstrate how much the risk variables raised the risk of mortality following a study by Hafiz et al. (2022) [19].
Ethical approval
This project was approved by the institutional review board (IRB) of KFMC. Consent was obtained from KFMC according to the ICH GCP ethical code (IRB approval number 20-164E). Informed consent was obtained from all the participants and from the legal guardians of the participants who were below 16 years of age.
Results
Demographic and clinical characteristics of patients infected with K. pneumoniae BSI
During the study period, 152 incident BSI cases were identified as caused by K. pneumoniae isolated from the central or peripheral venous catheter. Approximately two-thirds (66%) of the study population were aged > 15 years. Females were fairly probable as males to be infected with K. pneumoniae BSI. Among the incident K. pneumoniae bloodstream infections, 53 isolates were classified as ESBL strains, 55 as Carbapenem-Resistant strains, and 44 as susceptible strains. More than half of the K. pneumoniae isolates originated from critical care wards, such as ER and ICU wards (Table 1).
Clinical manifestations among adult and paediatric patients infected with K. pneumoniae BSI
Univariate analysis was conducted to compare clinical manifestations between paediatric and adult patients with K. pneumoniae bloodstream infections (Table 2). Paediatric patients were substantially more likely to develop septicaemia than adults (P < 0.0001, 56.9% vs. 21.8%, respectively). However, septic shock was significantly more frequent in adult patients (P = 0.0092). In adult patients, K. pneumoniae BSI and comorbidities, such as diabetes mellitus, hypertension, malignancy, chronic kidney disease, and ischaemic heart disease, were significantly associated (P < 0.0001, P < 0.0001, P = 0.0078, P = 0.0021, and P = 0.0004, respectively). Notably, paediatric patients with acute respiratory distress syndrome were slightly more vulnerable to K. pneumoniae BSI than adult patients (P = 0.040).
Clinical outcome of patients infected with K. pneumoniae BSI
We conducted a univariate analysis to compare the outcome of all patients with K. pneumoniae bloodstream infection. Table 3 shows the relative risks (RRs) of mortality and 95% confidence intervals, demonstrating the strength of the associations between the risk factors and mortality. To ensure an accurate comparison between patients, we excluded four patients because they were transferred to another medical facility. Of the total patients with K. pneumoniae BSI (n = 148), the overall mortality rate was 32.4% (48/148 patients). Univariate analysis revealed many risk factors associated with mortality (ranked from highest to lowest significance): mechanical ventilation, multi-organ dysfunction, septic shock, gastrointestinal infection, chronic kidney disease, carbapenem resistance, age > 15 years, ischaemic heart disease, and hypertension (P < 0.0001, P = 0.0005, P = 0.0007, P = 0.0061, P = 0.0087, P = 0.0029, P = 0.0169, P = 0.0170 and P = 0.0462, respectively). The risk of mortality was 34% higher in adult patients and approximately 50% lower in paediatric patients (RR = 1.342; 95% CI: 1.063–1.669 vs. RR = 0.508; 95% CI: 0.275–0.889). Patients with multi-organ dysfunction were at a substantially high risk of death from K. pneumoniae BSI (RR = 16.67; 95% CI: 2.801–101.1 Septic shock and chronic kidney disease raised the RR three-fold, whereas ischaemic heart disease, mechanical ventilation, gastrointestinal infection, and carbapenem resistance raised it two-fold (Table 3).
Comparison between ICU and Non-ICU patients with K. pneumoniae BSI
A univariate analysis was conducted to compare the clinical characteristics and risk factors of patients with K. pneumoniae bloodstream infection related to ICU admission. Table 4 demonstrates the strength of the associations between the risk factors and ICU admission among K. pneumoniae BSI patients. Almost 40% of the patients with K. pneumoniae bloodstream infection were admitted to the ICU. Intriguingly, malignancy was significantly associated with non-ICU patients (P < 0.0001). In addition, the susceptible K. pneumoniae strains were significantly isolated from non-ICU patients compared to the ICU patients (P = 0.0108). Carbapenem resistance, mechanical ventilation, respiratory infection, multi-organ dysfunction, mortality, ischemic heart disease, and septic shock were significantly higher among ICU patients than non-ICU patients (P < 0.0001, P < 0.0001, P = 0.0006, P = 0.0039, P = 0.0043, P = 0.0133, and P = 0.0244, respectively).
Antimicrobial susceptibility of K. pneumoniae BSI isolates
Figure 1 summarises the results of the antimicrobial susceptibility tests. Klebsiella pneumoniae showed alarming resistance with XXXore than 60% of isolates were resistant to AMC as well as most of the cephalosporins (CEF, CXM, CTZ, CRO, CTX, and CEPM). Approximately half of the isolates were resistant to TMP-SMX. They exhibited lower resistance to the aminoglycosides (GM and AMK) than to the β-lactam antibiotics. Carbapenem-Resistant strains accounted for approximately 36% of all isolates. Interestingly, one-third of the isolates were resistant to tigecycline, a third were moderately sensitive, and a third were sensitive. Only isolates that were Carbapenem-Resistant were reported to have colistin resistance (6.6%), whereas isolates that were susceptible, ESBL, and Carbapenem-Resistant were reported to have tigecycline resistance.
Antibiotic susceptibility of Klebsiella pneumoniae bloodstream isolates (n = 152). AMP, ampicillin; AMC, amoxicillin-clavulanate; AMK, amikacin; CEF, cephalothin; COL, colistin; CTZ, ceftazidime; CXM, cefuroxime; CFPM, cefepime; CTX, cefotaxime; CRO, ceftriaxone; CIP, ciprofloxacin; ETP, ertapenem; FOX, cefoxitin; GM, gentamicin; IPM, imipenem; LVX, levofloxacin; MER, meropenem; TGC, tigecycline; TMP-SMX, trimethoprim-sulfamethoxazole; TZP, piperacillin-tazobactam
Discussion
Klebsiella pneumoniae BSIs are associated with high mortality rates worldwide. The emergence of antibiotic-resistant K. pneumoniae complicates the management of infections caused by these bacteria. This study aimed to evaluate the epidemiology, resistance profiles, and clinical outcomes of K. pneumoniae BSIs. The infection rate was slightly higher in females (52%) than in males (48%). The ICU and ER had the highest prevalence of K. pneumoniae BSI. ICUs are considered factories that create, amplify, and disseminate antibiotic resistance [20, 21]. The high prevalence of antibiotic resistance in ICUs might be due to multiple infections, frequent application of antimicrobials, or the frequent use of invasive procedures. In our study, Klebsiella pneumoniae BSIs in patients admitted to the ICU were significantly associated with multiple risk factors including carbapenem resistance, mechanical ventilation, respiratory infection, multi-organ dysfunction, ischemic heart disease, and septic shock were significantly higher among ICU patients than non-ICU patients. In a recent study by Wang et al. (2023), age over 70 years, admission to ICU, and urinary tract infection were found to be the risk factors for Carbapenem-resistant and ESBL-KP-resistance [22]. In another study by Huang et al. (2023), the risk factors for resistance to carbapenems in K. pneumoniae were ICU admission, respiratory failure, admission from the Emergency, and imipenem use [23].
In addition, the mortality rate was higher among ICU patients and contributed to 45.9% of the death rate. This finding is supportive of the EUROBACT-2 international cohort study on epidemiology and outcomes of hospital-acquired bloodstream infections in ICU patients which revealed predominant Klebsiella spp. (27.9%) bloodstream infection in ICU patients with poor outcomes [24].
Various hospital-based studies have suggested multiple comorbidities, including DM, biliary disease, and liver disease, as risk factors for K. pneumoniae BSI development [25, 26]. Here, we found that neurological disorders were the primary underlying conditions in both age groups, whereas DM was a main risk factor in adults. This conflicts with another study [28], which reported a lower risk associated with DM than with chronic liver disease and cancer. This contrast may reflect differences in the selected populations studied. Increasing age is associated with an increased risk of comorbidities [27, 28]. Here, the risk factors were associated with a 32.4% mortality rate. The risk of dying from K. pneumoniae BSIs was greater for adults than paediatric patients and was high for those with multi-organ failure. The higher mortality rate can be attributed to the greater virulence of the carbapenemase-producing strains, inappropriate antibiotic therapy, the greater toxicity and reduced effectiveness of antibiotics, and severe underlying diseases such as DM and chronic kidney disease [29].
Antimicrobial resistance is one of the most urgent public health concerns worldwide. Based on a comprehensive global analysis, it caused 1.27 million deaths in 2019, more than those caused by HIV/AIDS or malaria [30], and it could lead to 10 million deaths by 2050 unless a global effort to control it is implemented [31]. Increased prescription rates, and the extensive use of antibiotics, have led to the emergence of resistance against last-resort drugs, including carbapenems and colistin, especially among medically important bacteria such as E. coli and K. pneumoniae. It has been estimated that resistance to fluoroquinolones and β-lactam antibiotics, including carbapenems, cephalosporins, and penicillins, is responsible for more than 70% of deaths attributable to antimicrobial resistance [30]. The emergence and spread of MDR K. pneumoniae pose a global public health concern. In Saudi Arabia, the rate of K. pneumoniae resistance has increased substantially in the last few years, reaching 100% resistance in some regions [32]. We believe that the local pattern of antibiotic prescription is comparable to the national pattern of the Hafiz et al. (2023) study, which looks at the impact of improper antibiotic therapy on drug-resistant Gram-negative bacteria and indicates that it is strongly associated with poor outcomes [33]. Furthermore, according to worldwide research, inappropriate treatment is related to poor outcomes [34, 35].
ESBLs, produced primarily by gram-negative bacteria, mediate resistance to a wide range of β-lactam antibiotics, including extended-spectrum cephalosporins and the monobactam aztreonam. Most ESBL-encoding genes are carried by mobile genetic elements, facilitating the spread of resistance genes among bacteria. Several national and international studies have reported an increase in the prevalence of ESBL production among clinical isolates, reaching approximately 74% in some countries [36,37,38]. A study conducted in Saudi Arabia [39] revealed that, among the gram-negative bacteria, ESBL-producing K. pneumoniae was second only to E. coli as a cause of BSI. In the current study, 34.87% of the K. pneumoniae isolates produced ESBL, whereas this rate was 21.3% for the Makkah region (western Saudi Arabia) [32]. Similarly, the rate of ESBL-producing isolates was higher for Riyadh (central Saudi Arabia) than for Abha (an eastern region) and Al-Khobar (a central region) [40]. This regional variation in the prevalence of ESBL-producing K. pneumoniae in Saudi Arabia is presumably related to factors such as antibiotic prescription rates and resistance-related reporting. The rapid development of resistance is associated with membrane permeability, efflux pump activity, and β-lactamase production [41].
Our findings revealed high resistance (64–76%) to cephalosporins. Overall, our observed cephalosporin resistance rates were lower than those previously reported for Saudi Arabia: resistance to third- and fourth-generation cephalosporins increased substantially from 2011 to 2021, reaching 84.9%, 85.1% and 85.8% for ceftazidime, cefotaxime, and cefepime, respectively [32].
In this study, 36.18% of the isolates were Carbapenem-Resistant strains. Resistance to carbapenems is a major public health problem, as they are the last line of drugs for treating severe bacterial infections caused by ESBL-producing gram-negative bacteria. In Saudi Arabia, K. pneumoniae is the most common of the carbapenem-resistant Enterobacteriaceae found in hospitalised patients [42]. Unfortunately, the prevalence of carbapenem-resistant K. pneumoniae has significantly increased in Saudi Arabia: K. pneumoniae resistance to imipenem increased from 6.6% to 2011 to 59.9% in 2021, suggesting an increase in the consumption of antibiotics [32].
In 2019, K. pneumoniae was the second most common pathogen after E. coli, responsible for the most deaths attributable to antimicrobial resistance, causing > 600,000 antimicrobial resistance-associated deaths globally. In our study, mortality rates were higher for patients infected with ESBL- and Carbapenem-Resistant strains K. pneumoniae than for those infected with susceptible K. pneumoniae strains. BSI caused by ESBL-producing K. pneumoniae isolates were associated with 33.3% mortality, whereas higher mortality (52.1%) was associated with BSIs caused by Carbapenem-Resistant K. pneumoniae (52.1%), consistent with prior national studies [43, 44]. Globally, carbapenem-resistant K. pneumoniae was responsible for 55,700 deaths in 2019, followed by carbapenem-resistant Acinetobacter baumannii. Higher mortality is associated with infections caused by carbapenem-resistant K. pneumoniae than with those caused by carbapenem-sensitive K. pneumoniae [29]. A case-controlled study of critically ill patients found that carbapenem-susceptible K. pneumoniae BSI was responsible for 41% of in-hospital mortality, compared to 79% in those infected with Carbapenem-Resistant K. pneumoniae [45].
Our sample size was small, comprising only data collected over one year at KFMC. Using a larger sample will increase the precision of our findings with regard to statistical analysis. Additionally, further analysis is essential to examine the effects of the COVID-19 pandemic on the resistance profile of K. pneumoniae.
Conclusions
K. pneumoniae isolates with a significant incidence of resistance were found in the institution. These results help shed light on microbial resistance and its relation to patient clinical outcomes. The high mortality rate among ICU patients is significantly associated with several risk factors, highlighting the importance of further investigating each factor and revising policies that might decrease the probability of such an outcome. Additionally, the observed resistance to colistin and tigecycline is concerning, particularly considering that a similar recent local study found a high prevalence of tigecycline resistance in ESBL-producing K. pneumoniae [36]. This may support the possibility that the selection pressure imposed by excessive antibiotic usage played a role in the development of the observed resistance.
Data Availability
Data is available in the KFMC institute data system and could be available for the public upon special request through the correspondence author.
References
Bagley ST. Habitat association of Klebsiella species. Infect Control. 1985;6. https://doi.org/10.1017/S0195941700062603. no. 2.
Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4). https://doi.org/10.1128/cmr.11.4.589.
Montgomerie JZ, Ota JK. “Klebsiella Bacteremia Forty-one patients with Klebsiella bacteremia admitted.” [Online]. Available: http://archinte.jamanetwork.com/.
Ko WC, et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8(2). https://doi.org/10.3201/eid0802.010025.
Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med. 2002;162(9). https://doi.org/10.1001/archinte.162.9.1021.
Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect Dis. 2010;10. https://doi.org/10.1186/1471-2334-10-307.
Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9. https://doi.org/10.1016/S1473-3099(09)70054-4. no. 4.
da Lima AM, de Melo MES, Alves LC, Brayner FA, Lopes ACS. Investigation of class 1 integrons in Klebsiella pneumoniae clinical and microbiota isolates belonging to different phylogenetic groups in Recife, State of Pernambuco. Rev Soc Bras Med Trop. 2014;47(2). https://doi.org/10.1590/0037-8682-0021-2014.
Abdelsalam MFA, Abdalla MS, El-Abhar HSED. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin–meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist. 2018;15. https://doi.org/10.1016/j.jgar.2018.07.003.
Martin RM, Bachman MA. “Colonization, infection, and the accessory genome of Klebsiella pneumoniae,” Frontiers in Cellular and Infection Microbiology, vol. 8, no. JAN. 2018. https://doi.org/10.3389/fcimb.2018.00004.
Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41(3). https://doi.org/10.1093/femsre/fux013.
Xu J, Zhao Z, Ge Y, He F. Rapid emergence of a pandrug-resistant Klebsiella pneumoniae ST11 isolate in an inpatient in a teaching hospital in China after treatment with multiple broad-spectrum antibiotics. Infect Drug Resist. 2020;13. https://doi.org/10.2147/IDR.S243334.
Al Bshabshe A, et al. Rising Klebsiella pneumoniae infections and its Expanding Drug Resistance in the Intensive Care Unit of a Tertiary Healthcare Hospital, Saudi Arabia. Cureus. 2020. https://doi.org/10.7759/cureus.10060.
Al-Zalabani A, AlThobyane OA, Alshehri AH, Alrehaili AO, Namankani MO, Aljafri OH. Prevalence of Klebsiella pneumoniae antibiotic resistance in Medina, Saudi Arabia, 2014–2018. Cureus. 2020. https://doi.org/10.7759/cureus.9714.
Alotaibi F. Carbapenem-Resistant Enterobacteriaceae: an update narrative review from Saudi Arabia. J Infect Public Health. Jul. 2019;12(4):465–71. https://doi.org/10.1016/J.JIPH.2019.03.024.
Al-Abdely H et al. “Molecular characterization of carbapenem-resistant Enterobacterales in thirteen tertiary care hospitals in Saudi Arabia,” Ann Saudi Med, vol. 41, no. 2, pp. 63–70, Mar. 2021, https://doi.org/10.5144/0256-4947.2021.63.
Alghoribi MF, et al. Genomic analysis of the first KPC-producing Klebsiella pneumoniae isolated from a patient in Riyadh: a new public health concern in Saudi Arabia. J Infect Public Health. 2020;13(4). https://doi.org/10.1016/j.jiph.2020.01.003.
Alkofide H, et al. Multidrug-resistant and extensively drugresistant enterobacteriaceae: prevalence, treatments, and outcomes – a retrospective cohort study. Infect Drug Resist. 2020;13. https://doi.org/10.2147/IDR.S283488.
Hafiz TA et al. “Stenotrophomonas maltophilia Epidemiology, Resistance Characteristics, and Clinical Outcomes: Understanding of the Recent Three Years’ Trends,” Microorganisms, vol. 10, no. 12, Dec. 2022, https://doi.org/10.3390/microorganisms10122506.
Hu Y, Ping Y, Li L, Xu H, Yan X, Dai H. A retrospective study of risk factors for carbapenem-resistant klebsiella pneumoniae acquisition among ICU patients. J Infect Dev Ctries. 2016;10(3). https://doi.org/10.3855/jidc.6697.
Li Y, et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob Resist Infect Control. 2020;9(1). https://doi.org/10.1186/s13756-020-00728-3.
Wang N et al. “Long Term Characteristics of Clinical Distributioand Resistance Trends of Carbapenem-Resistant and Extended-Spectrum β-Lactamase Klebsiella pneumoniae Infections: 2014–2022,” Infect Drug Resist, vol. 16, pp. 1279–1295, Mar. 2023, https://doi.org/10.2147/IDR.S401807.
Huang W, et al. Analysis of risk factors associated with healthcare-associated carbapenem-resistant Klebsiella pneumoniae infection in a large general hospital: a case-case-control study. Eur J Clin Microbiol Infect Dis. Mar. 2023;1–13. https://doi.org/10.1007/S10096-023-04578-W/TABLES/5.
Tabah A, et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: the EUROBACT-2 international cohort study. Intensive Care Med. Feb. 2023;49(2):178–90. https://doi.org/10.1007/S00134-022-06944-2/FIGURES/1.
Meatherall BL, Gregson D, Ross T, Pitout JDD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae Bacteremia. Am J Med. 2009;122(9). https://doi.org/10.1016/j.amjmed.2009.03.034.
Wang LS, Lee FY, Cheng DL, Liu CY, Hinthorn DR, Jost PM. Klebsiella pneumoniae bacteremia: analysis of 100 episodes, J Formos Med Assoc, vol. 89, no. 9, 1990.
Uslan DZ, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167(8). https://doi.org/10.1001/archinte.167.8.834.
James MT, Laupland KB, Tonelli M, Manns BJ, Culleton BF, Hemmelgarn BR. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med. 2008;168(21). https://doi.org/10.1001/archinte.168.21.2333.
Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. Carbapenem Resistance, initial antibiotic therapy, and Mortality in Klebsiella pneumoniae Bacteremia: a systematic review and Meta-analysis. Infect Control Hosp Epidemiol. 2017. https://doi.org/10.1017/ice.2017.197.
Murray CJ, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325). https://doi.org/10.1016/S0140-6736(21)02724-0.
O’Neill J. “Tackling drug-resistant infections globally: final report and recommendations: the review on antimicrobial resistance; 2016 [Available from: https://amr-review. org,” Publications. html, no. May, 2019.
Jalal NA, et al. Prevalence and Antibiogram Pattern of Klebsiella pneumoniae in a Tertiary Care Hospital in Makkah, Saudi Arabia: an 11-Year experience. Antibiotics. Jan. 2023;12(1). https://doi.org/10.3390/antibiotics12010164.
Hafiz TA et al. “A two-year retrospective study of multidrug-resistant Acinetobacter baumannii respiratory infections in critically Ill patients: Clinical and microbiological findings,” J Infect Public Health, vol. 16, no. 3, pp. 313–319, Mar. 2023, https://doi.org/10.1016/j.jiph.2023.01.004.
Bodro M, et al. Epidemiology, antibiotic therapy and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in cancer patients. Support Care Cancer. 2014;22(3). https://doi.org/10.1007/s00520-013-2012-3.
Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66(5). https://doi.org/10.1016/j.jinf.2012.10.028.
Aldrazi FA, et al. ESBL expression and antibiotic resistance patterns in a hospital in Saudi Arabia: do healthcare staff have the whole picture? J Infect Public Health. 2020;13(5). https://doi.org/10.1016/j.jiph.2019.12.001.
Ijsrp “September. 2015 Online Print Version International Journal of Scientific and Research Publications,” 2015, [Online]. Available: http://www.ijsrp.org/e-journal.html.
Zowawi HM, Balkhy HH, Walsh TR, Paterson DL. β-lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin Microbiol Rev. 2013;26(3). https://doi.org/10.1128/CMR.00096-12.
Al-Agamy MHM, Shibl AM, Tawfik AF. Prevalence and molecular characterization of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in Riyadh, Saudi Arabia. Ann Saudi Med. 2009;29(4). https://doi.org/10.4103/0256-4947.55306.
Brady M, Cunney R, Murchan S, Oza A, Burns K. Klebsiella pneumoniae bloodstream infection, antimicrobial resistance and consumption trends in Ireland: 2008 to 2013. Eur J Clin Microbiol Infect Dis. 2016;35(11). https://doi.org/10.1007/s10096-016-2727-4.
Al-Zahrani IA, Alasiri BA. The emergence of carbapenem-resistant Klebsiella pneumoniae isolates producing OXA-48 and NDM in the Southern (Asir) Province, Saudi Arabia. Saudi Med J. 2018;39(1). https://doi.org/10.15537/smj.2018.1.21094.
Alotaibi F. Carbapenem-Resistant Enterobacteriaceae: an update narrative review from Saudi Arabia. J Infect Public Health. 2019;12. https://doi.org/10.1016/j.jiph.2019.03.024. no. 4.
Alraddadi BM, et al. Molecular epidemiology and outcome of carbapenem-resistant Enterobacterales in Saudi Arabia. BMC Infect Dis. Dec. 2022;22(1). https://doi.org/10.1186/s12879-022-07507-y.
Garbati MA, Sakkijha H, Abushaheen A. Infections due to Carbapenem resistant Enterobacteriaceae among saudi arabian hospitalized patients: a matched case-control study. Biomed Res Int. 2016;2016. https://doi.org/10.1155/2016/3961684.
Mouloudi E, et al. Bloodstream infections caused by Metallo- β -Lactamase/ Klebsiella pneumoniae carbapenemase–producing K. pneumoniae among Intensive Care Unit Patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol. 2010;31(12). https://doi.org/10.1086/657135.
Acknowledgements
The authors would like to thank Deputyship for Research and Innovation, ‘’Ministry of Education’’ in Saudi Arabia for funding this research (IFKSUOR3–218–2). Authors express their appreciation to the Microbiology Department, King Fahad Medical City, for facilitating data collection, and to Abeer AlMazyed, who contributed to data collection. The authors thank the Prince Naif Health Research Center, Investigator Support Unit, for the language editing service.
Funding
This research was funded by the Deputyship for Research and Innovation, ‘’Ministry of Education’’ in Saudi Arabia through project no. (IFKSUOR3–218–2).
Author information
Authors and Affiliations
Contributions
T.A.H. conceived and designed the study, reviewed the literature, acquired the data, provided critical comments, and wrote the original draft of the manuscript. S.A. contributed to the conceptualization and design of the study, interpreted the results, wrote the manuscript, performed critical revision, and edited the final version of the manuscript. W.A. managed the data collection and analysis. S.S.A. interpreted the results and edited the final version of the manuscript. S.R.A. contributed to the Discussion section. M.A.M. and K.B. commented critically and edited the final version of the manuscript. F.A. and N.M. contributed to the study design, managed data collection, and interpreted the results. All of the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of King Fahad Medical City and Research Centre, Saudi Arabia (approval number 20-164E). Informed consent was obtained from all the participants and from the legal guardians of the participants who were below 16 years of age.
Consent for publication
Not Applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hafiz, T.A., Alanazi, S., Alghamdi, S.S. et al. Klebsiella pneumoniae bacteraemia epidemiology: resistance profiles and clinical outcome of King Fahad Medical City isolates, Riyadh, Saudi Arabia. BMC Infect Dis 23, 579 (2023). https://doi.org/10.1186/s12879-023-08563-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08563-8