Abstract
Background
Bloodstream infections (BSIs) are an important cause of morbidity and mortality in hospitalized patients. We evaluate incidence of community- and hospital-onset BSI rates and outcomes before and during the SARS-CoV-2 pandemic.
Methods
We conducted a retrospective cohort study evaluating patients who were hospitalized for ≥ 1 day with discharge or death between June 1, 2019, and September 4, 2021, across 271 US health care facilities. Community- and hospital-onset BSI and related outcomes before and during the SARS-CoV-2 pandemic, including intensive care admission rates, and overall and ICU-specific length of stay (LOS) was evaluated. Bivariate correlations were calculated between the pre-pandemic and pandemic periods overall and by SARS-CoV-2 testing status.
Results
Of 5,239,692 patient admissions, there were 20,113 community-onset BSIs before the pandemic (11.2/1000 admissions) and 39,740 (11.5/1000 admissions) during the pandemic (P ≤ 0.0062). Corresponding rates of hospital-onset BSI were 2,771 (1.6/1000 admissions) and 6,864 (2.0/1000 admissions; P < 0.0062). Compared to the pre-pandemic period, rates of community-onset BSI were higher in patients who tested negative for SARS-CoV-2 (15.8/1000 admissions), compared with 9.6/1000 BSI admissions among SARS-CoV-2-positive patients. Compared with patients in the pre-pandemic period, SARS-CoV-2-positive patients with community-onset BSI experienced greater ICU admission rates (36.6% vs 32.8%; P < 0.01), greater ventilator use (10.7% vs 4.7%; P < 0.001), and longer LOS (12.2 d vs 9.1 d; P < 0.001). Rates of hospital-onset BSI were higher in the pandemic vs the pre-pandemic period (2.0 vs 1.5/1000; P < 0.001), with rates as high a 7.3/1000 admissions among SARS-CoV-2-positive patients. Compared to the pre-pandemic period, SARS-CoV-2-positive patients with hospital-onset BSI had higher rates of ICU admission (72.9% vs 55.4%; P < 0.001), LOS (34.8 d vs 25.5 d; P < 0.001), and ventilator use (52.9% vs 21.5%; P < 0.001). Enterococcus species, Staphylococcus aureus, Klebsiella pneumoniae, and Candida albicans were more frequently detected in the pandemic period.
Conclusions and relevance
This nationally representative study found an increased risk of both community-onset and hospital-onset BSI during the SARS-CoV-2 pandemic period, with the largest increased risk in hospital-onset BSI among SARS-CoV-2-positive patients. SARS-CoV-2 positivity was associated with worse outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States [1, 2]. Prior to the COVID-19 pandemic, BSIs and septicemia affected 1.5 million Americans annually [3, 4]. Recent studies have reported BSI rates among hospitalized patients with COVID-19 of 7 to 15%, and estimates suggest that up to 50% of patients who die from COVID-19 have a coinfection with an additional pathogen [5, 6].
Data also indicate that patients with COVID-19 and BSI have worse outcomes [6,7,8]. For example, a multicenter, retrospective study reported that adult patients who were hospitalized with severe COVID-19 and secondary BSIs presented with more severe illness and had longer hospital stays and higher mortality rates [7]. Similarly, a study found that SARS-CoV-2-positive patients early in the pandemic had higher rates of hospital-onset infections, greater antimicrobial usage, and extended hospital and intensive-care unit length of stay (LOS), compared with SARS-CoV-2-negative or untested patients [8]. Another study reported a 3.9-fold increase in BSI among hospitalized patients who tested positive for SARS-CoV-2 compared to those who tested negative [6].
However, most studies on the epidemiology of BSI during the COVID-19 pandemic have been of short duration and report on the early period of the pandemic [7,8,9], highlighting a need for data that capture the impact of clinically relevant COVID-19 variants, including the Delta variant, and reflect the use of immunomodulatory agents, which could affect the risk of secondary infections [10].
To address these limitations, we evaluated the epidemiology and outcomes of culture-positive gram-negative, gram-positive, and fungal/yeast pathogens from a blood source in patients hospitalized prior to and during the SARS-CoV-2 pandemic across 271 facilities in the United States.
Methods
Study design
This multicenter, retrospective cohort analysis included all hospitalized adults aged ≥ 18 years from 271 US facilities included in the BD Insights Research Database (Becton, Dickinson and Company, Franklin Lakes, NJ), which includes both small and large medical care facilities in rural and urban areas throughout the United States (Additional file 1: Table S1). This electronic surveillance system and clinical research database, which has been previously described [8, 11, 12], is exempted from patient consent requirements because it uses deidentified data. This retrospective, deidentified data set was approved, and informed consent requirements were waived by the New England Institutional Review Board (Wellesley, MA, USA; IRB No. 120180023). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies to develop this report [13].
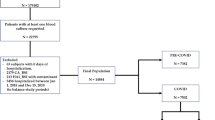
Eligible admissions included subjects with ≥ 1 inpatient stay with a record of discharge or death between July 1, 2019, and September 4, 2021. The pre-pandemic period was defined as July 1, 2019, to February 29, 2020, and the pandemic period was defined as March 1, 2020, to September 4, 2021. All admissions with an incident BSI infection, defined as a non-contaminated first positive blood culture for gram-negative or gram-positive bacterial pathogens, fungal pathogens, or yeast pathogens, were included in the analysis. Microbiology results likely associated with a blood contaminant were excluded by a previously described methodology that uses time of collection, source, microorganism type, and numbers of microorganisms in a culture to flag likely contaminated samples [14]. Monomicrobial BSI with the gram-negative pathogens, Enterobacterales (Citrobacter freundii, Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella aerogenes, Morganella morganii, Proteus mirabilis, Providencia stuartii, Serratia marcescens), Acinetobacter baumannii species, Bacteroides fragilis, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia; the monomicrobial gram-positive pathogens Enterococcus spp, group A Streptococcus, group B Streptococcus, Staphylococcus aureus, and Streptococcus pneumoniae; the monomicrobial fungus or yeast pathogens, non-Candida albicans, Candida albicans, and other Candida were included in the analysis. Polymicrobial results were included cases in which > 1 designated pathogen was obtained from first positive blood culture during the admission. For the purposes of comparison, patients were categorized into 3 groups: (1) SARS-CoV-2 positive, (2) tested and SARS-CoV-2 negative, and (3) SARS-CoV-2 untested. SARS-CoV-2 infection was defined as a positive SARS-CoV-2 antigen or polymerase chain reaction (PCR) test 7 days prior to admission or during hospitalization.
BSIs were defined as community-onset infections (COBSI) if the blood culture was collected ≤ 2 days from admission and as hospital-onset infections (HOBSI) if the blood culture was collected > 2 days from admission. All results from microbiology testing were obtained from analyses performed by local microbiology labs in the hospitals included in the BD Insights Research Database.
Outcomes
The primary outcome was to compare the overall rate of BSI during the pre-pandemic period with the rate of BSI in SARS-CoV-2-positive, SARS-CoV-2-negative, and SARS-CoV-2-untested patients during the pandemic period. Secondary outcomes included the rates of COBSI and HOBSI, the time to collection of HOBSIs, and the characteristics and outcomes of patients with COBSI and HOBSI.
In-hospital mortality was reported in cases with a designation of mortality, presence in the morgue, or death in Admission, Discharge, and Transfer (ADT) data feeds [11]. We evaluated in-hospital mortality (available for patients tested for SARS-CoV-2), hospital LOS, and intensive care unit (ICU) LOS in all patients and stratified by SARS-CoV-2 testing status during the pandemic period. LOS was calculated using hospital ADT data as the difference between admission and discharge dates.
Maximum laboratory values assessed within the first 3 days of admission, except for diabetes, were used as surrogates for comorbidities, defined as severe underlying illnesses or conditions, during the admission period (Additional file 2: Table S2) [11]. We evaluated the impact renal insufficiency or failure, diabetes, sepsis, liver dysfunction, myocardial inflammation or suspected heart failure, and cytokine stimulation (Additional file 2: Table S2).
Based on previously published criteria and a lack of timely data within the database to identify ventilator use, ventilator use was assumed in patients who were (a) started on intravenous (IV) push sedation medications (propofol, lorazepam, midazolam, ketamine, or dexmedetomidine,) or IV opioids (fentanyl, sufentanil, remifentanil, or hydromorphone) with a duration of at least 24 h, and (b) who had at least 2 arterial blood gas results collected at least 24 h apart, the first blood gas collected on the first day of sedation medication [11].
Statistical analysis
Bivariate correlations were calculated between the pre-pandemic and pandemic periods overall and by SARS-CoV-2 testing status for age, gender, prior admission, underlying conditions, LOS, ventilation status, and ICU status. Chi square or Fisher’s exact tests were used to perform bivariate comparisons between categorical subgroups, while continuous variables were analyzed using Kruskal–Wallis tests and Wilcoxon tests were used for pairwise comparisons. Two sample t-tests were used to compare the means of two samples. All statistical tests were conducted using a pre-specified two-tailed alpha level of 0.05. Statistical analyses were performed using R (R Ver. 4.1.2, R Foundation for Statistical Computing, Vienna, Austria), with RStudio (Boston, MA).
Results
There were 1,789,449 patient admissions during the pre-pandemic period (July 1, 2019, to February 29, 2020) and 3,450,243 admissions during the pandemic period (March 1, 2020, to September 4, 2021). Most (65%) hospitals included in the analysis were urban and 75% had 300 or fewer beds (Additional file 1: Table S1).
BSIs were detected in 22,884 patient admissions (12.79/1000 admissions) in the pre-pandemic period and in 46,568 admissions (13.50/1000 admissions) during the pandemic period (P ≤ 0.0001). Both COBSI and HOBSI were observed more frequently during the pandemic than the pre-pandemic period (COBSI: 11.51 vs 11.24 per 1000 admissions, P ≤ 0.0022; HOBSI: 1.99 vs 1.55 per 1000 admissions; P < 0.0001 for both comparisons), as shown in Table 1.
Epidemiology and pathogen distribution of COBSI before and during the SARS-CoV-2 pandemic
Mean ages of patients admitted for COBSI were comparable between the pre-pandemic and pandemic periods (65.1 vs 65.2 years; Table 2). Patients with COBSI during the pandemic period were more likely to be male (53.3% vs 52.0%; P < 0.05), have prior admissions in the previous 90 days (27.0% vs 22.7%; P < 0.001), and have ≥ 1 underlying condition (89.1% vs 86.3%; P < 0.001; Table 2).
There were 39,704 patient admissions with COBSI during the pandemic period. Rates of COBSI were highest among SARS-CoV-2-negative patients (15.8/1000 admissions), followed by SARS-CoV-2-positive patients (9.6/1000 admissions), and those not tested (6.86/1000 admissions).
SARS-CoV-2-positive patients were older (66.2 vs 65.1; P < 0.05) and were more likely to have had ≥ 1 underlying conditions (94.6% vs 86.3%; P < 0.001). During the pandemic period compared to the pre-pandemic period, COBSI rates were significantly higher among SARS-CoV-2-negative patients (15.8 vs 11.2; P < 0.001).
Significantly higher rates of COBSI admissions were seen during the pandemic period compared to the pre-pandemic period for the following pathogens (per 1000 admissions): S aureus (3.05 vs. 2.81), K pneumoniae (1.00 v. 0.93), Enterococcus spp (0.74 vs. 0.64), group B Strep (0.45 vs. 0.42), and polymicrobial pathogens (0.42 vs. 0.36), all P ≤ 0.0404, as shown in Table 3.
Epidemiology and pathogen distribution of HOBSI before and during the SARS-CoV-2 pandemic
There were 6,864 patient admissions with HOBSI during the pandemic period. Rates of HOBSI were highest among SARS-CoV-2-positive patients (7.3/1000 admissions), followed by SARS-CoV-2-negative patients (2.3/1000 admissions), and then those not tested (1.0/1000 admissions; Table 4).
Compared to patients during the pre-pandemic period, SARS-CoV-2-positive patients with a HOBSI infection were significantly older (63.4 y vs 62.0 y, P < 0.05) and less likely to have had a prior 30- or 90-day admission, more likely to have ≥ 1 underlying condition (98.7% vs 91.5%; P < 0.001), and had a longer time from admission to hospital (HO) blood culture collection (17.5 vs 11.0 days; P < 0.001).
During the pandemic period, the rate of HOBSI was significantly higher among SARS-CoV-2-positive patients (7.3 per 1000 admissions) and SARS-CoV-2-negative patients (2.3 per 1000 admissions) compared to the rate during the pre-pandemic period (1.5 per 1000 admissions; P < 0.001).
As seen with COBSI, significantly higher rates of HOBSI per 1000 admissions attributable to S aureus (0.58 vs. 0.45), Enterococcus spp (0.25 vs. 0.16), K pneumoniae (0.17 v. 0.14), non-C albicans (0.15 vs. 0.10), C albicans (0.13 vs. 0.08), and P aeruginosa (0.11 vs. 0.08), all P ≤ 0.0093, were observed during the pandemic compared to the pre-pandemic period.
Although the median time from admission to collection of HOBSI events was significantly longer among SARS-CoV-2-positive patients during the pandemic (14 days, P < 0.001) compared to the pre-pandemic period (7 days), the median time to HO culture collection was similar among SARS-CoV-2–negative patients (7 days) and among patients not tested (7 days) (Table 5).
Outcomes
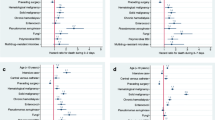
Overall, outcomes among patients diagnosed with BSI in the pre-pandemic period differed from those during pandemic period (Tables 2 and 4). These differences were largely driven by differences between SARS-CoV-2-positive patients and those who were negative or not tested. Among patients with COBSI, SARS-CoV-2-positive patients had significantly higher rates of ICU admissions (36.6% vs 32.8%; P < 0.01) and mechanical ventilation (10.7% vs 4.7%; P < 0.01), with significantly longer overall and ICU LOS (overall LOS: 12.2 vs 9.1 days; P < 0.001; ICU LOS; 6.5 vs 4.5 days; P < 0.001) compared to patients in the pre-pandemic period. Among the subset of patients during the pandemic period for whom mortality data were available (n = 20,064, 50.5%), mortality was 20.7% among SARS-CoV-2-positive patients and 7.0% among SARS-CoV-2-negative patients.
Among patients with HOBSI, rates of ICU admission were similar between the pre-pandemic and pandemic periods; however, during the pandemic period, rates of ventilator use (29.3% vs 21.5%; P < 0.001), hospital LOS (28.0 vs 25.5 days; P < 0.001) and ICU LOS were higher (16.1 vs 12.3 days; P < 0.001). These differences were likely driven by SARS-CoV-2-positive patients, who, compared with patients during the pre-pandemic period, had higher rates of ICU admission (72.9% vs 55.4%; P < 0.001), ventilator use (52.9% vs 21.5%; P < 0.001), and longer hospital LOS and ICU LOS (hospital LOS: 34.8 vs 25.5 days; P < 0.001; ICU LOS, 23.5 vs 12.3; P < 0.001). Among the subset of patients with HOBSI during the pandemic for whom mortality data were available (n = 3252), mortality rates were significantly higher among SARS-CoV-2-positive patients (48.6%) compared to SARS-CoV-2–negative patients (19.7%; P < 0.001) (Table 4).
Discussion
This study showed a significantly higher blood culture positive rate for both COBSI and HOBSI in the pandemic period compared to the pre-pandemic period. The higher rate of COBSIs during the pandemic was primarily driven by higher rates in SARS-CoV-2-negative patients whereas the higher rates of HOBSIs were primarily driven by rates in SARS-CoV-2-positive patients. These findings are consistent with those from a recent, large meta-analysis of 46 studies (published through April 19, 2021) including 42,694 patients [15], which reported that 7.3% of patients hospitalized with COVID-19 developed a BSI (95% CI, 4.7–1.1%) and that BSIs were nearly 3 times more likely in COVID-19-positive patients compared to those without COVID-19 (OR 2.77; 95% CI, 1.53–5.02; P < 0.001) [15].
Our study is among the first reports to compare BSI rates and causative pathogens during the pandemic with those observed prior to the pandemic. A particularly interesting finding from our analysis is the substantially higher rates of COBSI among SARS-CoV-2-negative patients (15.8 per 1000 admissions) compared with SARS-CoV-2-positive patients (9.6 per 1000 admissions).
Higher HOBSI rates during the pandemic may have reflected high levels of patient severity and long ICU and hospital lengths of stay. It is possible that intrinsic factors may also have adversely impacted outcomes in patients without SARS-CoV-2, resulting in higher HOBSI rates. For example, overworked hospital staff, widespread during the pandemic period [16] could have led to suboptimal care, contributing to increased HOBSI rates. Short staffing has been associated with a higher incidence of HO infections and data from the CDC’s National Safety Health Network indicate that the higher rates of several important HO infections in 2020 versus 2019 [17]. In fact, the CDC analysis demonstrated that the rate of central-line associated bloodstream infections (CLABIs) were nearly 50% higher in the latter half of 2020 and methicillin-resistant Staphylococcus aureus (MRSA) infections increased by 34% compared with 2019 [17]. Moreover, because prior studies have shown that central line-associated infection is a common cause of BSIs in patients with COVID-19, a fear of prolonged patient contact, the practice of pronation for ventilated patients [18], and aerosolization of SARS-CoV-2 could be a barrier to catheter hygiene and maintenance [7]. Finally, the pandemic has revealed staffing constraints and supply chain weaknesses that limit the ability of microbiology labs and infectious disease specialists to fully support infection prevention and antimicrobial stewardship policies, which also may affect BSI rates and outcomes [19, 20].
The increased rate of COBSI admissions could also be attributable to delays in care [21]. A survey of 5,412 US adults revealed that approximately 41% delayed or avoided medical care during the pandemic due to concerns about COVID-19, including 12% who avoided urgent or emergency care and 32% who avoided routine care. Results from a retrospective analysis of Medicare beneficiaries reported early increases in hospitalizations and 30-day mortality, suggesting that patients were presenting with more advanced illness, possibly related to delays in care [22, 23].
Differences were also identified in blood-positive pathogens during the pre-pandemic and pandemic periods. For example, rates of Enterococcus were higher during the pandemic, a finding that confirms the previous results that have shown an unexpectedly high prevalence of enterococcal BSIs among patients with COVID-19 [24,25,26,27,28]. Compared to the pre-pandemic period, we also found higher rates of HO fungal BSI caused by Candida species during the pandemic, confirming data suggesting high fungal BSI rates among SARS-CoV-2-positive patients [7, 29]. In our study, C albicans was the third most common pathogen and seen in 10.5% of SARS-CoV-2-positive patients but were not observed as a top 5 pathogen among SARS-CoV-2-negative patients or in the pre-pandemic period (Table 3). A significant 35% increase in HOBSI risk associated with Candida species during the pandemic period has also been observed in a study of 69 US hospitals [30], possibly driven by risk factors frequently present in critically ill patients with COVID-19, including the use of mechanical ventilation, indwelling devices, glucocorticoids, and broad-spectrum antibiotic therapy [31,32,33]. Our results also showed a significantly longer time for culture collection in HOBSI pathogens in SARS-CoV-2-positive patients (14 days median) compared to SARS-CoV-2-negative patients, patients not tested, and the pre-pandemic period (median 7 days for all 3 groups).
Key strengths of our study include its demographic and geographic diversity, and its duration, including data from multiple phases within the first 18 months of the pandemic. These strengths address the limitations of previous studies, which have generally been smaller, less geographically or demographically representative of the US population, and of a relatively short duration early in the pandemic [6, 25, 28, 34, 35]. Lastly, our study included a comparison with pre-COVID-19 patients to examine potential differences between the pre- and post-pandemic period. Accordingly, we found significantly higher hospital-onset BSI rates/1000 admissions during the pandemic period compared to the pre-pandemic period for several pathogens, including S aureus, Enterococcus spp, K pneumoniae, non-C albicans, C albicans, and P aeruginosa. Further evaluation of prevalence of these pathogens in SARS-2-CoV-associated BSI is warranted, particularly for Candida and Enterococcus.
Our study also had several limitations. First, we did not evaluate the impact of medications and immunosuppressive therapies on hospital-onset BSI rates. A multicenter study of hospitalized adults with COVID-19 found that the combined use of corticosteroids and tocilizumab in these patients was associated with BSI rates, independent of other risk factors [36]. However, results from a small single-center study suggested that nosocomial use of dexamethasone had no effect on BSI rates in the critically ill [10]. Further studies are needed to establish any definitive pathophysiologic role for immunosuppressive therapy in the development of BSIs in patients with COVID-19. We were also unable to evaluate or document markers of hospital stressors, including staffing limitations, lack of ICU space, and shortages in personal protective equipment, which might affect BSI rates or patient outcomes. Finally, we cannot draw conclusions regarding the impact of the pandemic on BSI-related mortality because pre-pandemic mortality data were not available.
In conclusion, overall rates of BSIs have appeared to significantly increase during the COVID-19 pandemic, largely resulting from an increase in COBSI rates among SARS-CoV-2-negative patients and HOBSI rates among both SARS-CoV-2- positive and negative patients. The additive burden of BSI and COVID-19 highlights a need for interventions to both prevent and effectively treat hospital-acquired BSI in SARS-CoV-2-positive patients. Identification and evaluation of such interventions during later stages of the pandemic is warranted.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request. The data sharing policy of Merck Sharp and Dohme Corp, a subsidiary of Merck and Co, Inc, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the study data can be submitted through the EngageZone site or via email to moc.kcrem@sseccaatad.
References
Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501–9. https://doi.org/10.1111/1469-0691.12195.
Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014;27(4):647–64. https://doi.org/10.1128/CMR.00002-14.
Lester D, Hartjes T, Bennett A. CE: a review of the revised sepsis care bundles. Am J Nurs. 2018;118(8):40–9. https://doi.org/10.1097/01.NAJ.0000544139.63510.b5.
Xu JQ, Murphy SL, Kochanek KD, Arias E. Deaths: final data for 2019. Natl Vital Stat Rep. 2021;70(8):1–87. https://doi.org/10.15620/cdc:106058.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Shukla BS, Warde PR, Knott E, et al. Bloodstream infection risk, incidence, and deaths for hospitalized patients during coronavirus disease pandemic. Emerg Infect Dis. 2021;27(10):2588–94. https://doi.org/10.3201/eid2710.210538.
Bhatt PJ, Shiau S, Brunetti L, et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2021;72(12):e995–1003. https://doi.org/10.1093/cid/ciaa1748.
Puzniak L, Finelli L, Yu KC, et al. A multicenter analysis of the clinical microbiology and antimicrobial usage in hospitalized patients in the US with or without COVID-19. BMC Infect Dis. 2021;21(1):227. https://doi.org/10.1186/s12879-021-05877-3.
Zhu N, Rawson TM, Mookerjee S, et al. Changing patterns of bloodstream infections in the community and acute care across two COVID-19 epidemic waves: a retrospective analysis using data linkage. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab869.
Rothe K, Lahmer T, Rasch S, et al. Dexamethasone therapy and rates of secondary pulmonary and bloodstream infections in critically ill COVID-19 patients. Multidiscip Respir Med. 2021;16(1):793. https://doi.org/10.4081/mrm.2021.793.
Finelli L, Gupta V, Petigara T, Yu K, Bauer KA, Puzniak LA. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw Open. 2021;4(4): e216556. https://doi.org/10.1001/jamanetworkopen.2021.6556.
McCann E, Srinivasan A, DeRyke CA, et al. Carbapenem-nonsusceptible gram-negative pathogens in ICU and non-ICU Settings in US hospitals in 2017: a multicenter study. Open Forum Infect Dis. 2018;5(10):ofy241. https://doi.org/10.1093/ofid/ofy241.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X.
Brossette SE, Hacek DM, Gavin PJ, et al. A laboratory-based, hospital-wide, electronic marker for nosocomial infection: the future of infection control surveillance? Am J Clin Pathol. 2006;125(1):34–9. https://doi.org/10.1309/502AUPR8VE67MBDE.
Ippolito M, Simone B, Filisina C, et al. Bloodstream infections in hospitalized patients with COVID-19: a systematic review and meta-analysis. Microorganisms. 2021;9(10):2016. https://doi.org/10.3390/microorganisms9102016.
Boyle P. Hospital innovate amid dire nursing shortages. AAMC. 2021. https://www.aamc.org/news-insights/hospitals-innovate-amid-dire-nursing-shortages. Accessed 14 Feb 2022.
Evans G. Healthcare-associated infections increase dramatically during pandemic. Hospital Control & Prevention. 2021. https://www.reliasmedia.com/articles/148560-healthcare-associated-infections-increase-dramatically-during-pandemic. Accessed 14 Feb 2022.
Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8(1): e48.
Tsai JM, Tolan NV, Petrides AK, et al. How SARS-CoV-2 transformed the clinical laboratory: challenges and lessons learned. J Appl Lab Med. 2021;6(5):1338–54. https://doi.org/10.1093/jalm/jfab034.
Catalán P, Alonso R, Alcalá L, et al. The challenge of COVID-19 for a clinical microbiology department. Diagn Microbiol Infect Dis. 2021;101(2): 115426. https://doi.org/10.1016/j.diagmicrobio.2021.115426.
Czeisler M, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–7. https://doi.org/10.15585/mmwr.mm6936a4.
Smulowitz PB, O'Malley AJ, Khidir H, Zaborski L, McWilliams JM, Landon BE. National trends in ED visits, hospital admissions, and mortality for medicare patients during the COVID-19 pandemic. Health Aff (Millwood). 2021;40(9):1457–1464. https://doi.org/10.1377/hlthaff.2021.00561
Aung S, Vittinghoff E, Nah G, et al. Emergency activations for chest pain and ventricular arrhythmias related to regional COVID-19 across the US. Sci Rep. 2021;11(1):23959. https://doi.org/10.1038/s41598-021-03243-6.
Bonazzetti C, Morena V, Giacomelli A, et al. Unexpectedly high frequency of Enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49(1):e31–40. https://doi.org/10.1097/CCM.0000000000004748.
Buetti N, Ruckly S, de Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–7. https://doi.org/10.1007/s00134-021-06346-w.
Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10): e13319. https://doi.org/10.1111/eci.13319.
Grasselli G, Scaravilli V, Mangioni D, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–65. https://doi.org/10.1016/j.chest.2021.04.002.
Giacobbe DR, Labate L, Tutino S, et al. Enterococcal bloodstream infections in critically ill patients with COVID-19: a case series. Ann Med. 2021;53(1):1779–86. https://doi.org/10.1080/07853890.2021.1988695.
Brikman S, Dori G, Kasher C, et al. Candida bloodstream infection, a dire complication in hospitalized COVID-19 patients: three cases from a single center in Northern Israel. Isr Med Assoc J. 2021;23(10):615–7.
Sturm LK, Saake K, Roberts P, Masoudi F, Fakih MG. Impact of COVID-19 pandemic on hospital onset bloodstream infections (HOBSI) at a large health system. Am J Infect Control. 2021;S0196-6553(21)00852-X. https://doi.org/10.1016/j.ajic.2021.12.018
Ghosh A, Sarkar A, Paul P, Patel P. The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID19. Fungal Biol Rev. 2021;38:67–91. https://doi.org/10.1016/j.fbr.2021.09.003.
Basile K, Halliday C, Kok J, Chen SC. Fungal infections other than invasive Aspergillosis in COVID-19 patients. J Fungi (Basel). 2022;8(1):58. https://doi.org/10.3390/jof8010058.
Arastehfar A, Carvalho A, Nguyen MH, et al. COVID-19-associated Candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi (Basel). 2020;6(4):211. https://doi.org/10.3390/jof6040211.
Yu D, Ininbergs K, Hedman K, Giske CG, Strålin K, Özenci V. Low prevalence of bloodstream infection and high blood culture contamination rates in patients with COVID-19. PLoS ONE. 2020;15(11): e0242533. https://doi.org/10.1371/journal.pone.0242533.
Zhang J, Lan P, Yi J, et al. Secondary bloodstream infection in critically ill patients with COVID-19. J Int Med Res. 2021;49(12):3000605211062783. https://doi.org/10.1177/03000605211062783.
Khatri A, Malhotra P, Izard S, et al. Hospital-acquired bloodstream infections in patients hospitalized with severe acute respiratory syndrome coronavirus 2 infection (Coronavirus Disease 2019): association with immunosuppressive therapies. Open Forum Infect Dis. 2021;8(7):ofab339. https://doi.org/10.1093/ofid/ofab339.
Acknowledgements
Not applicable.
Disclosures: Drs Bauer, Finelli, and Moise reported being employees of Merck Sharpe and Dohme, a subsidiary of Merck and Co, Inc, and they may own stock and/or hold stock options in Merck and Co, Inc. Dr. Puzniak was an employee of Merck and Co, Inc, at the time of the analysis and may own stock and/or hold stock options in in Merck and Co, Inc. Drs Gupta, Ai, Watts, and Yu reported being employees of Becton, Dickinson and Company (BD), and Drs. Gupta and Yu may own stock and/or hold stock options in Becton, Dickson and Company.
Funding
Funding for this research was provided by Merck Sharp and Dohme, a subsidiary of Merck and Co, Inc. Drs Bauer, Puzniak, Finelli, and Moise are or were employees of the funding source and played a role in the design and conduct of the study; interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
VG and KAB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: KAB, LF, VG, KCY, LAP. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: KAB, LF, VG, KCY, LAP. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: VG, JAW. Obtained funding: VG. Administrative, technical, or material support: VG, CA, JAW, KAB, LAP. Supervision: KAB, VG, LAP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This electronic surveillance system and clinical research database, which has been previously described, is exempted from patient consent requirements because it uses deidentified data. This retrospective, deidentified data set was approved, and informed consent requirements were waived by the New England Institutional Review Board (Wellesley, MA, USA; IRB No. 120180023). This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies [13]. The study was performed in accordance with all relevant guidelines and regulations, including the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
LP was an employee of Merck Sharp and Dohme Corp., a subsidiary of Merck and Co., Inc., Kenilworth, NJ, USA (MSD), at the time the study was conducted and may own stock and/or hold stock options in Merck and Co., Inc., Kenilworth, NJ, USA. LF, PM, and KAB are current employees of MSD, who may own stock and/or hold stock options in Merck and Co., Inc., Kenilworth, NJ, USA. VG, KCY, and CA are employees of Becton, Dickinson and Company (BD), Franklin Lakes, NJ, USA; KCY and VG may own stock and/or hold stock options in BD. BD was paid to perform the analyses presented. Drs Bauer, Finelli, and Moise reported being employees of Merck Sharpe and Dohme, a subsidiary of Merck and Co, Inc, and they may own stock and/or hold stock options in Merck and Co, Inc. Dr. Puzniak was an employee of Merck and Co, Inc, at the time of the analysis and may own stock and/or hold stock options in in Merck and Co, Inc. Drs Gupta, Ai, Watts, and Yu reported being employees of Becton, Dickinson and Company (BD), and Drs. Gupta and Yu may own stock and/or hold stock options in Becton, Dickson and Company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Distribution of study hospitals.
Additional file 2: Table S2.
Laboratory criteria used as surrogates for admission-period clinical conditions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bauer, K.A., Puzniak, L.A., Yu, K.C. et al. Epidemiology and outcomes of culture-positive bloodstream pathogens prior to and during the SARS-CoV-2 pandemic: a multicenter evaluation. BMC Infect Dis 22, 841 (2022). https://doi.org/10.1186/s12879-022-07810-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07810-8