Abstract
Introduction
The Bacille-Calmette–Guerin (BCG) vaccination remains the primary strategy to prevent severe disseminated TB in young children, particularly in high TB-burden countries such as Ethiopia. Accurate knowledge of vaccination coverage in small geographical areas is critically important to developing targeted immunization campaigns. Thus, this study aimed to investigate the spatiotemporal distributions and ecological level determinants of BCG vaccination coverage in Ethiopia.
Method
Bacille-Calmette–Guerin immunization coverage and geographical information data were obtained from five different Demographic and Health Surveys, conducted in Ethiopia between 2000 and 2019. Data for independent variables were obtained from publicly available sources. Bayesian geostatistical models were used to predict the spatial distribution of BCG vaccination coverage in Ethiopia.
Result
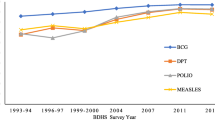
The overall national BCG vaccination coverage between 2000 and 2019 was 65.5%. The BCG vaccine coverage was 53.5% in 2000, 56.9% in 2005, 64.4% in 2011, 79.6% in 2016, and 79.0% in 2019. BCG vaccination coverage increased by 47.6% in Ethiopia from 2000 to 2019, but substantial geographical inequalities in BCG coverage remained at sub-national and local levels. High vaccination coverage was observed in northern, western, and central parts of Ethiopia. Climatic and demographic factors such as temperature, altitude, and population density were positively associated with BCG vaccination coverage. Whereas, healthcare access factors such as distance to health facilities and travel time to the nearest cities were negatively associated with BCG vaccine coverage in Ethiopia.
Conclusion
Despite substantial progress in national BCG vaccination coverage, marked spatial variation in BCG coverage persists throughout the country at sub-national and local levels. Healthcare access and climatic and demographic factors determined the spatial distribution of BCG vaccination coverage. Maintaining a high level of vaccination coverage across geographical areas is important to prevent TB in Ethiopia.
Similar content being viewed by others
Introduction
The Bacille-Calmette–Guerin (BCG) vaccine is the only vaccine currently available for the prevention of tuberculosis (TB), a bacterial disease that kills more than one million people globally every year [1, 2]. The BCG vaccine contains a live attenuated Mycobacterium bovis that can prevent severe forms of TB such as meningitis and disseminated TB [3, 4]. BCG vaccination is universally provided for all newborn babies in high TB-burden countries such as India and Ethiopia. In low TB incidence countries, it is targeted to specific TB risk groups [5, 6].
Although various efforts have been made to improve access and coverage of BCG vaccination, TB remains a major health problem among children in developing countries including Ethiopia [7, 8]. The World Health Assembly endorsed the Global Vaccine Action Plan 2011–2020 as a framework for maximizing the advantages of vaccination and realizing a vision of universal access to vaccines and immunization with 90% coverage [9]. As part of this global plan, Ethiopia has made several efforts to improve the national BCG vaccination coverage. In the last few decades, BCG vaccination coverage has been enhanced through the combined efforts of the Reaching Every District approach, a health extension program, and the introduction of Enhanced Routine Immunization Activities in Ethiopia [10]. However, despite all these tremendous efforts, the BCG vaccination coverage remains low in the country [11].
The coverage of BCG vaccination can be influenced by various factors including the socio-demographic and economic status of the mother [12,13,14]. Previous studies conducted in developed countries showed that the rate of BCG vaccination was associated with family-related characteristics such as wealth index, literacy rate, and healthcare access [15, 16]. Few primary studies have been also conducted in Ethiopia to examine the determinants of BCG vaccination coverage. However, all the studies did not consider geographic variation in the estimation of BCG vaccination uptake [12, 13, 17]. Previous studies were not also considered ecological level factors associated with BCG vaccination at the subnational and local levels. However, accurate knowledge of vaccination coverage in small geographical areas is critically important for designing targeted immunization campaigns. Identifying areas with low vaccination coverage is important for immunization programs, particularly in Ethiopia and other low-income countries for the rational allocation of resources. The information is also important to measure the efforts and achievements of vaccination programs at the local level. Thus, this study aimed to investigate the spatial pattern and drivers of BCG vaccination among children in Ethiopia,
Methods
Study setting
The study was conducted in Ethiopia using an ecological study design. Ethiopia is the second-most populous country in Africa, with an estimated population size of more than 115 million people in 2020 [18]. There are marked differences in population structure, socioeconomic conditions, disease burden, and climatic conditions across the country. Ethiopia has a surface area of approximately 1.1 million square kilometers and a population density of more than 215 people per square kilometer [18]. It has a variety of geographical features altitudes ranging from 125 m below sea level in Afar depression to 4533 m above sea level in Ras Dejen, Amhara region. Ethiopia has a three-tier health system through which immunizations and other health care services are delivered. More than 84% of the population resides in rural parts of the country with low healthcare access. Universal BCG vaccination at birth is part of the national health program in Ethiopia.
Data source
Data for BCG immunization coverage was obtained from the Ethiopian Demographic and Health Survey (EDHS). The EDHS contains five different surveys which have been conducted every 5 years between 2000 and 2019. The survey was conducted using nationally representative samples that provided estimates for urban and rural areas. Global Positioning System (GPS) coordinates for each survey location were also available in the EDHS.
Geospatial covariate data were obtained from several publicly available sources with a resolution of 1 km2. Climatic variables such as mean monthly temperature and mean monthly precipitation were accessed from the WorldClim website [19] and altitude data was obtained from the Shuttle Radar Topography Mission (SRTM) [20]. Travel time to cities in minutes and access to health care facilities were retrieved from the Malaria Atlas Project (MAP) [21]. Population density and distance to waterbody data were accessed from WorldPop [22] and Global Lakes and Wetlands Database (GLWD), respectively. The covariates were selected based on potential associations with BCG vaccination coverage, as obtained from previous literature. The choice of these variables was also based on the availability of high-resolution country-wide data. The polygon shapefile for the Ethiopian administrative boundaries was obtained from the Global Administrative Areas (GADEM) free online database.
Data processing and analysis
The dependent variable for this study was BCG vaccination coverage, which was operationally defined as the number of children who received the BCG vaccine divided by the total number of children born within five years preceding the survey. Descriptive statistics were first carried out to summarize BCG immunization coverage at the regional level.
Spatial analysis
The Bayesian model-based geostatistics was used to generate a spatially continuous estimate of the national BCG immunization coverage mapped at a resolution of 1 km2. The binomial regression model was fitted within the Bayesian framework to the BCG immunization coverage of both fixed effects and geostatistical random effects. Five models were constructed separately for each year of BCG immunization coverage data (i.e., 2000, 2005, 2011, 2016, and 2019). The model for immunization coverage was the same for all five data sets. A spatial binomial regression model was fitted for immunization coverage using geostatistical random effects and covariate fixed effects for mean annual temperature, mean annual precipitation, altitude, travel time to the nearest city, distance to the nearest health facilities, distance to the water body, and population density [23]. The BCG immunization coverage was taken at each surveyed location j as the outcome variable, which was assumed to follow a binomial distribution;
where \({Y}_{j}\) is the observed immunization coverage, \({n}_{j}\) is the total number of vaccinated and unvaccinated children and \({p}_{j}\) is the predicted BCG immunization coverage at location\(j\). Mean predicted BCG immunization coverage was modeled via a logit link function to a linear predictor defined as: .
where α is the intercept, β is a matrix of covariate coefficients, \({\varvec{X}}\) is a design matrix of \(z\) covariates, and \({\zeta }_{j}\) are spatial random effects modeled using a zero-mean Gaussian Markov random field (GMRF) with a Matérn covariance function. The covariance function was defined by two parameters: the range \(\rho\), which represents the distance beyond which correlation becomes negligible (about 0.1 km), and \(\sigma\), which is the marginal standard deviation [24, 25]. Non-informative priors were used for α (uniform prior with bounds – ∞ and ∞), and we set normal priors with mean = 0 and precision (the inverse of the variance) = 1 × 10–4 for each β. We used default priors for the parameters of the spatial random field [26]. Parameter estimation was done using the Integrated Nested Laplace Approximation (INLA) approach in R (R-INLA) [24, 25]. Sufficient values (i.e., 150,000 samples) from each simulation run for the variables of interest were stored to ensure full characterization of the posterior distributions.
Predictions of BCG immunization coverage at unsampled locations were made at 1 km2 resolution by interpolating the spatial random effects and adding them to the sum of the products of the coefficients for the spatially variant fixed effects at each prediction location [27]. The intercept was added, and the overall sum was back-transformed from the logit scale to the prevalence scale, providing prediction surfaces that show the estimated immunization coverage for all prediction locations.
The Watanabe Applicable Information Criterion (WAIC) statistic was used to select the best-fitting model.
Result
National and regional coverage of BCG vaccine in Ethiopia
The national and regional coverage of the BCG vaccine in Ethiopia between 2000 and 2019 is described in (Table 1). The average national BCG vaccine coverage for all five EDHS data was 65.5%. The coverage for each EDHS was 53.5% in 2000, 56.9% in 2005, 64.4% in 2011, 79.6% in 2016, and 79.0% in 2019. While high regional coverage of the BCG vaccine was observed in Addis Ababa, Tigray, and Dire Dawa, low coverage was observed in Somali and Afar regions (Table 1).
Trends of BCG vaccine coverage
The temporal trends of the BCG vaccine coverage in Ethiopia from 2000 to 2019 are described in Fig. 1. In the first four surveys, the coverage was increased from 53.5% in 2000 to 79.6% in 2016, with a faster increment between 2011 and 2016. However, BCG vaccine coverage slightly declined from 79.6% in 2016 to 79% in 2019 (Fig. 1).
Spatial clustering of BCG vaccine coverage
Significant spatial variations of BCG vaccine coverage were observed at sub-national and local levels in Ethiopia. High BCG vaccine coverage was observed in Northern (Tigray region), Central (Addis Ababa), and Eastern (Dire Dawa), whereas lower BCG vaccine coverage was observed in Eastern (Afar and Somali), Western (Gambela and Benshangul_gumuz), and Southern (Oromia and SNNPR) parts of Ethiopia (Fig. 2).
The predicted BCG vaccine coverage for the overall and each individual EDHSs were delineated in Fig. 3. Highest predicted BCG vaccine coverage was observed in Northern, Northwestern, and central parts of Ethiopia, and lower BCG vaccine coverage was observed in Southern, Eastern, and Northeastern parts of the country (Fig. 3).
Drivers of BCG immunization coverage
The Bayesian geostatistical model was used to investigate the association between BCG vaccine coverage and covariates. Temperature was positively associated with BCG vaccine coverage in 2000 [mean regression coefficient (β): 0.63; 95% credible interval (95% CrI): 0.11, 1.15] and 2019 (β: 2.23; 95% CrI: 1.20, 3.27). Altitude was also positively associated with BCG vaccine coverage in 2019 (β: 2.61; 95% CrI: 1.57, 3.66). Furthermore, the population density was positively associated with BCG vaccine coverage in all EDHSs. Whereas travel time to the nearest cities in minutes and distance to health facilities were negatively associated with BCG vaccine coverage in all EDHS (Table 2).
Widely Applicable Information Criterion (WAIC) statistic was used to check the model fitness, the model which contains all the covariates was the best-fitting model for BCG immunization coverage in all EDHS surveys (see Additional file 1: Table S3).
Discussion
This study found that BCG vaccination coverages were substantially varied at national, subnational, and local levels in Ethiopia. The spatial distribution of BCG vaccination coverage was also temporally varied. The overall BCG vaccination coverage in Ethiopia varies from53.5% in 2000, to 79.6% in 2016 and 79.0% in 2019, which indicates a 33% increase in the past two decades. The increment in the BCG vaccination coverage is partially due to the expansion and implementation of the health extension program p in Ethiopia. While the health extension program has several packages that promote disease prevention and control through household and community empowerment, immunization services including BCG vaccination were the core of the program [28]. Despite this effort, a slight decrement was observed between 2016 and 2019. These could be due to the internal instabilities and displacements of populations in some parts of the country, which might affect immunization services. The BCG vaccination coverage in our study is low as compared to the 2021 WHO report for Southeast Asia countries (87%) [29]. The low vaccination coverage in Ethiopia might be due to the difference in the socio-economic developments and healthcare systems for immunization programs. The recent COVID-19 pandemic might also affect immunization coverage in Ethiopia [30, 31].
Moreover, low predicted BCG vaccination coverage was observed in Afar, Benishangul, Gambela, and Somali regions [32]. Spatial clustering of TB was reported in these parts of the country in previous studies [33, 34]. This might indicate that in the area with low BCG vaccination there might be a high rate of TB incidence, an important finding to strengthen the BCG vaccination program in this part of the county. The possible reason for low BCG vaccination in Afar, Benishangul, Gambela, and Somali regions could be due to low healthcare access in these regions [35, 36]. Implementing a vaccination program and providing health education in these regions is also difficult as the people living in these areas are nomadic and seasonally move from place to place [37]. Therefore, special vaccination programs (such as mobile health services and immunization comping) that are well fitted with the lifestyle of the people are needed in this part of the country to increase the BCG vaccination coverage.
Our study also indicated that population density was positively associated with BCG vaccination coverage. Areas with high population density are mostly urban and health care access including immunization services is relatively good in urban areas [38]. Moreover, in areas with high population density mothers would have a better awareness of the benefits of childhood immunization services [39].
Seasonal variation is postulated as one of the factors related to immunization coverage. Healthcare-seeking behavior of the people would be low, particularly during the rainy and harvest season which may also affect the vaccination programs [40]. Routine healthcare services including immunization services would be low during the rainy seasons and cloudy environments, because of the difficulties in transportation as a result of flooding and muddy roads.
In line with previous studies conducted in Africa and other low and middle-income countries [41,42,43], distance to health facilities was negatively associated with BCG vaccination coverage in our study. This finding might indicate that poor infrastructure and low transportation services to access health facilities might be a bottleneck to improving vaccination coverage. [44,45,46,47]. In Ethiopia, approximately half of the population lives more than 10 km far from the nearest health facilities and has no access to immunization programs [48]. This inaccessibility of healthcare facilities would be a major barrier to immunization services improvements.
BCG vaccination coverage is also negatively associated with travel time to the nearest city, which is supported by studies conducted in rural Ethiopia [49], Madagascar [40], and Sudan [50]. As the travel time to the nearest cities increases, the accommodation and transportation expenses also increase, which would reduce the vaccination uptake of children.
This study has paramount implications for policymakers and health program designers to come up with evidence-based program enhancement to improve BCG vaccination coverage. However, the study had some important limitations; first, since, the study was based on secondary data, we could not include some important covariates such as literacy rate, and ANC coverage which might have an impact on the prediction of the BCG vaccine coverage. The other limitation of this study is the different data collection periods for the dependent and independent variables. Moreover, the BCG vaccine efficacy was not analyzed in this study due to the nature of the data and as it was beyond the scope of this study.
Conclusion
The national BCG vaccination coverage was increased over time and varies substantially at the sub-national and local levels in Ethiopia. Low BCG vaccination coverage was observed in Southern, Southeastern, Northeastern, and Eastern parts of Ethiopia. Access to health facilities and travel time to the nearest cities were negatively associated with BCG vaccination coverage. Maintaining a high level of vaccination coverage across geographical areas is important to prevent TB in Ethiopia.
References
Tafreshi SJVR. BCG vaccine and pulmonary tuberculosis. Vaccine Res. 2017;4(1):1–5.
Roth A, Garly ML, Jensen H, Nielsen J, Aaby P. Bacillus Calmette–Guérin vaccination and infant mortality. Expert Rev Vaccines. 2006;5(2):277–93.
Woolf SH, Chapman DA, Lee JH. COVID-19 is the leading cause of death in the United States. JAMA. 2021;325(2):123–4.
Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–30.
hebdomadaire WHOJWERRé. Weekly Epidemiological Record, 2004, vol. 79, 28 [full issue]. 2004;79 (28):253–64.
Olusanya BOJHrp, systems. Pattern and determinants of BCG immunization delays in a sub-Saharan African community. 2010;8 (1):1–10.
Organization WH. State of inequality: reproductive maternal newborn and child health: interactive visualization of health data: World Health Organization; 2015.
Victora CG, Requejo JH, Barros AJ, Berman P, Bhutta Z, Boerma T, et al. Countdown to 2015: a decade of tracking progress for maternal, newborn, and child survival. 2016;387 (10032):2049–59.
Mihigo R, Okeibunor J, Masresha B, Mkanda P, Poy A, Zawaira F, et al. Immunization and vaccine development: progress towards high and equitable immunization coverage in the Africa region. 2018 (1):1.
FMoH E. Ethiopia national expanded program on immunization comprehensive multi-year plan 2016–2020. FMOH; 2015.
Unies N. World population prospects: The 2017 revision. New York: United Nations, Department of Economic and Social Affairs, Population Division; 2017.
Tsehay AK, Worku GT, Alemu YMJBo. Determinants of BCG vaccination coverage in Ethiopia: a cross-sectional survey. 2019;9 (2):e023634
Bobo FT, Hayen AJBo. Decomposition of socioeconomic inequalities in child vaccination in Ethiopia: results from the 2011 and 2016 demographic and health surveys. 2020;10 (10):e039617.
Mbengue MAS, Sarr M, Faye A, Badiane O, Camara FBN, Mboup S, et al. Determinants of complete immunization among Senegalese children aged 12–23 months: evidence from the demographic and health survey. 2017;17 (1):1–9.
Li J, Menzies D, Landry JS, Benedetti A, Rousseau M-CJPm. Determinants of Bacillus Calmette–Guérin (BCG) vaccination among Québec children. 2014;66:87–94.
Guthmann J, Antoine D, Fonteneau L, Che D, Lévy-Bruhl DJE. Assessing BCG vaccination coverage and incidence of pediatric tuberculosis following two major changes in BCG vaccination policy in France. 2011;16 (12):19824
Lakew Y, Bekele A, Biadgilign SJBph. Factors influencing full immunization coverage among 12–23 months of age children in Ethiopia: evidence from the national demographic and health survey in 2011. 2015;15 (1):1–8.
Shen AK, Fields R. The future of routine immunization in the developing world: challenges and opportunities. Global Health. 2014;2(4):381–94.
WorldClim. Global climate and weather data. 2020.
Smith B, Sandwell D. Accuracy and resolution of shuttle radar topography mission data. Geophys Res Lett. 2003;30(9):1–3.
Project TMA. Explore global malaria data using our custom mapping tools. 2021.
WorldPop. Open spatial demographic data and research. 2021.
Diggle PJ, Tawn JA, Moyeed R. Model-based geostatistics. J R Stat Soc Ser C. 1998;47(3):299–350.
Rue H, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Ser B. 2009;71(2):319–92.
Lindgren F, Rue H, Lindström J. An explicit link between Gaussian fields and Gaussian Markov random fields: the stochastic partial differential equation approach. J R Stat Soc Ser B. 2011;73(4):423–98.
Fuglstad G-A, Simpson D, Lindgren F, Rue H. Constructing priors that penalize the complexity of Gaussian random fields. J Am Stat Assoc. 2019;114(525):445–52.
Thomas A, Best N, Lunn D, Arnold R, Spiegelhalter D. GeoBUGS User Manual. Cambridge: Medical Research Council Biostatistics Unit; 2004.
Health LM. Last Mile Health partners with the Government of Ethiopia to Upskill Community Health Workers. JAN 26, 2021.
WHO. Immunization coverage estimates by WHO region. 2021.
Mohapatra PR, Mishra B, Behera B. BCG vaccination induced protection from COVID-19. Indian J Tuberculosis. 2021;68(1):119–24.
Li WX. Worldwide inverse correlation between Bacille Calmette-Guérin (BCG) immunization and COVID-19 mortality. Infection. 2021;49(3):463–73.
Agegnehu CD, Alem AZ. Exploring spatial variation in BCG vaccination among children 0–35 months in Ethiopia: a spatial analysis of Ethiopian Demographic and Health Survey 2016. BMJ Open. 2021;11(4): e043565.
Alene KA, Elagali A, Barth DD, Rumisha SF, Amratia P, Weiss DJ, et al. Spatial distribution of HIV, tuberculosis, and malaria in Ethiopia. BMJ Glob Health. 2022;7(2): e007599.
Alene KA, Clements ACA. Spatial clustering of notified tuberculosis in Ethiopia: a nationwide study. PLoS ONE. 2019;14(8): e0221027.
Mullan Z. Transforming health care in Ethiopia. Lancet Glob Health. 2016;4(1): e1.
Chaya N. Poor access to health services: ways Ethiopia is overcoming it. Res Comment. 2007;2(2):1–6.
Zepro NB, Ahmed AT. Determinants of institutional delivery service utilization among pastorals of Liben Zone, Somali Regional State, Ethiopia, 2015. Int J Women’s Health. 2016;8:705.
Nolna SK, Bonono C-R, Moncher MN, Bindé T, Nolna D, Zogo PO. Factors influencing the performance of routine immunization in urban areas: a comparative case study of two cities in Cameroon: Douala and Yaounde. Vaccine. 2018;36(49):7549–55.
Hathi P, Haque S, Pant L, Coffey D, Spears D. Place, and child health: the interaction of population density and sanitation in developing countries. Demography. 2017;54(1):337–60.
Mensah K, Heraud J-M, Takahashi S, Winter A, Metcalf CJE, Wesolowski A. Seasonal gaps in measles vaccination coverage in Madagascar. Vaccine. 2019;37(18):2511–9.
Sato R. Association between access to a health facility and continuum of vaccination behaviors among Nigerian children. Hum Vaccin Immunother. 2020;16(5):1215–20.
Phillips DE, Dieleman JL, Lim SS, Shearer J. Determinants of effective vaccine coverage in low and middle-income countries: a systematic review and interpretive synthesis. BMC Health Serv Res. 2017;17(1):1–17.
Friede AM, Waternaux C, Guyer B, De Jesus A, Filipp LC. An epidemiological assessment of immunization program participation in the Philippines. Int J Epidemiol. 1985;14(1):135–42.
Schellenberg JRA, Mrisho M, Manzi F, Shirima K, Mbuya C, Mushi AK, et al. Health and survival of young children in southern Tanzania. BMC Public Health. 2008;8(1):1–17.
Jahn A, Floyd S, Mwinuka V, Mwafilaso J, Mwagomba D, Mkisi R, et al. Ascertainment of childhood vaccination histories in northern Malawi. Tropical Med Int Health. 2008;13(1):129–38.
Abdulraheem I, Onajole A, Jimoh A, Oladipo A. Reasons for incomplete vaccination and factors for missed opportunities among rural Nigerian children. J Public Health Epidemiol. 2011;3(4):194–203.
Ndiritu M, Cowgill KD, Ismail A, Chiphatsi S, Kamau T, Fegan G, et al. Immunization coverage and risk factors for failure to immunize within the Expanded Programme on Immunization in Kenya after introduction of new Haemophilus influenzae type b and hepatitis b virus antigens. BMC Public Health. 2006;6(1):1–8.
Ababa A. Ethiopia. Abstract ht**tps://wfphaconfexcom/wfpha/2012/webprogram/Paper10587html. 2013.
Kiros G-E, White MJ. Migration, community context, and child immunization in Ethiopia. Soc Sci Med. 2004;59(12):2603–16.
Ibnouf A, Van den Borne H, Maarse J. Factors influencing immunization coverage among children under five years of age in Khartoum State, Sudan, South Africa. Fam Practice. 2007;49(8):14.
Acknowledgements
We thank the MEASURE DHS program for providing us with the data for this study.
Funding
There is no fund received.
Author information
Authors and Affiliations
Contributions
KAAtalell conceived and designed the study, run the analysis, and drafted the manuscript. TGA, AW writes the draft with KAAtalell. DZA, NWT, GMB, MAA, and KAAlene reviewed the design and the drafted manuscript. All authors critically reviewed the manuscript for important intellectual content and contributed to the final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was not needed as we used secondary data through a formal request from the Demographic and Health Survey (DHS) programs.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Uncertainty maps of BCG vaccination coverage among children under the age of five years in Ethiopia: A) 2000-2019, B) 2000, C) 2005, D) 2011, E) 2016 and F) 2019. Table S1. Covariate correlation result of variables included in this study. Table S2. Odds ratio with 95% Confidence Intervals (CI) of covariates included in a Bayesian spatial model with Binomial response for the BCG vaccination coverage in Ethiopia. Table S3. Watanabe-Akaike information criterion (WAIC) values corresponding to different model specifications. Table S4. Data sources and definitions of covariates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Atalell, K.A., Alemayehu, M.A., Teshager, N.W. et al. Mapping BCG vaccination coverage in Ethiopia between 2000 and 2019. BMC Infect Dis 22, 569 (2022). https://doi.org/10.1186/s12879-022-07547-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07547-4