Abstract
Background
Fusobacterium nucleatum (F. nucleatum) is a resident anaerobic bacterium, which in rare cases may invade blood from the head and neck or the digestive tract to cause bacteremia and induce venous thrombosis. F. nucleatum is closely related to abdominal tumors, but it has not been reported in relation to renal tumors. We report herein a possible case.
Case presentation
This patient had kidney cancer with thrombosis in the right renal vein but had no sign of infection. After radical nephrectomy, thrombi formed in his left renal vein, and when removed, severe sepsis occurred. He did not respond to treatment with antibiotics and died, but the blood culture done confirmed that he had F. nucleatum bacteremia.
Conclusion
F. nucleatum may also be associated with kidney cancer, and could cause post-operative renal vein thrombosis, and sepsis or septic shock after thrombectomy.
Similar content being viewed by others
Background
Fusobacterium nucleatum (F. nucleatum) is a gram-negative anaerobe that exists in the upper respiratory tract, gastrointestinal tract, and female urogenital tract [1]. It is an opportunistic pathogen. The first case reported with bacteremia followed suppurative thrombophlebitis of the internal jugular vein associated with oropharyngeal infection [2]. This suggested that thrombosis may be a complication of bacterial infection. Subsequently, thrombosis was reported in other veins, such as portal vein [3], hepatic vein [4], inferior vena cava [5], and dural venous sinus [6], but renal vein thrombosis (RVT) has been reported in only one case [7]. F. nucleatum is often associated with abdominal tumors, such as rectal and ovarian cancers [8], but there is no report of its association with kidney cancers. We report the case of a patient who had RVT after radical nephrectomy and developed severe sepsis due to the spread of F. nucleatum following thrombectomy. Whether kidney cancer is also associated with F. nucleatum infection, thereby increasing the risk of thrombectomy, is an issue that clinicians, particularly surgeons, would need to be aware of.
Case presentation
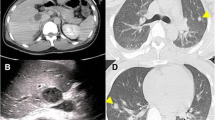
This Chinese patient was 59 years old. He was healthy before, without history of thrombosis. When he was admitted to our hospital, CT examination showed a mass in the right kidney, and there was no sign of infection. However, the subsequent CT angiography showed that the mass in the right kidney had abundant blood supply, and there was thrombosis in the right renal vein. Near the left renal vein there was another mass, but there were no thrombi in the left renal vein and portal vein (Figs. 1 and 2). The entire right kidney and the mass in the left kidney were excised by surgeons, and histological examination suggested WHO/ISUP grade-3 clear cell carcinoma. Post-operatively, he developed acute kidney injury (AKI) as evidenced by decreased urine volume (0.27 ml/h/kg for 3 h) and increased serum creatinine (75 mol/l higher than the preoperative level), and selective left renal venography showed a 2 cm filling defect in the left renal vein, suggesting thrombosis (Fig. 3). After the thrombus was removed, we performed continuous venovenous hemodiafiltration (CVVHDF) on the patient. Within the first 12 h, the patient was conscious, with stable vital signs. In addition, he had no fever, and the urine volume exceeded 40 ml/h, indicating that AKI was prerenal AKI caused by the thrombus in his left renal vein. Thus, he improved quickly after thrombectomy.
However, in the following 12 h, the patient showed signs of infection. His consciousness became poor, while body temperature and heart rate increased, and blood pressure, urine volume and oxygenation index decreased. The high CRP (71.82 mg/l) and PCT (22.82 ng/ml) levels also suggested that the patient might be infected. Based on the 2016 SSC guidelines, the patient had septic shock. In addition, SOFA score and laboratory test results were deteriorating (Table 1). Therefore, we immediately started fluid resuscitation, drew the patient's blood for culture, and empirically commenced meropenem and teicoplanin for treatment of the suspected sepsis.
Unfortunately, he continued to deteriorate such that by the second day his respiratory and circulatory systems collapsed and he required ventilation with almost pure oxygen. He died on the third day of respiratory and circulatory failure, and the result of the blood culture, which was received two days later, showed that he had F. nucleatum bacteremia, sensitive to penicillin, cefoxitin, piperacillin/tazobactam, cefoperazone/sulbactam, imipenem/cilastatin, meropenem, clindamycin and metronidazole, intermediate to ceftriaxone, and resistant to none.
Discussion and conclusion
This was a rapidly progressive case of septic shock due to F. nucleatum. The patient had no signs of infection before the operation, and full aseptic precautions were observed in all the operations. Therefore, we speculate that the F. nucleatum infection was associated with the patient's kidney cancer, as suggested by thrombosis in the right renal vein. The subsequent sepsis and thrombosis in the left renal vein were accompanied by F. nucleatum bacteremia.
The original source of the infection is unknown, but the reported sources are mainly concentrated in the head and neck and the abdominal cavity [9], although the bacteria from these sources have not been reported to cause RVT. Forming venous thrombosis after blood stream invasion is the prerequisite for F. nucleatum to disseminate septic emboli, because it promotes the aggregation of platelets [10].
Each year, only 5.5 to 7.6 people out of 1,000,000 develop F. nucleatum bacteremia, but the mortality rate is as high as 10–15% [6, 11]. Compared with women, men are more prone to F. nucleatum bacteremia, and the mortality rate of patients above 40 years old is much higher [12, 13]. Tumors also increase the risk of infection [14]. The main cause of death is the dissemination of septic emboli and the formation of abscesses in special parts [15].
Our patient was male and over 40 years old. The kidney cancer may have compromised his local immunity, and subsequently F. nucleatum invaded the blood to form a thrombus in the renal vein. There is no evidence that the bacterium is from the head and neck or the abdominal cavity, but the thrombus in the renal vein suggests that the bacterium might have originated from the urinary tract.
F. nucleatum is closely related to abdominal tumors and its detection rate is highest in patients with colorectal cancer [16]. However, the mechanism by which it induces tumor formation is unknown [17]. There are no reports about the association of F. nucleatum with kidney cancer (Table 2), but we speculate that the bacterium could also be associated with the occurrence of renal tumors. Combining the 13 cases in Table 2 and the 22 cases collected by Yusuf et al. [18], we summarize the characteristics of the 35 cases reported so far in Table 3.
There have so far been no specific recommendations on the antibiotic therapy of infections due to F. nucleatum. Three case reports put forward that F. nucleatum is resistant to penicillin, amoxicillin, amox-clav [5], and metronidazole [19], but there is no evidence for the 2–6-week treatment [7] recommended by most doctors.
We treated our patient empirically in accordance with the 2016 SSC guidelines, and the subsequent in vitro susceptibility test of the isolate showed that meropenem was effective. Our patient might have died from the complications associated with the infection rather than from failure of antibiotic therapy.
We conclude that as with other abdominal tumors, F. nucleatum may also be associated with kidney tumors, and that septic thrombo-embolization and severe sepsis could complicate the post-operative management of such cases.
Availability of data and materials
The data and materials, including all the clinical data of the patients are included within the article.
Abbreviations
- CT:
-
Computed tomography
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- SOFA:
-
Sepsis-related Organ Failure Assessment
- SSC:
-
Surviving Sepsis Campaign
References
Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases: expert consult premium edition—enhanced online features and print. Mycopathologia. 2014;149(1):47–8.
Lemierre A. On certain septicaemias due to anaerobic organisms. Lancet. 1936;227(5874):701–3.
Rahmati E, She RC, Kazmierski B, Geiseler PJ, Wong D. A case of liver abscess and fusobacterium septicemia. IDCases. 2017;9:98–100.
Zheng L, Giri B. Gastrointestinal variant of Lemierre syndrome: Fusobacterium nucleatum Bacteremia-associated hepatic vein thrombosis: a case report and literature review. Am J Ther. 2016;23(3):e933–6.
Pett E, Saeed K, Dryden M. Fusobacterium species infections: clinical spectrum and outcomes at a district general hospital. Infection. 2014;42(2):363–70.
Huggan PJ, Murdoch DR. Fusobacterial infections: clinical spectrum and incidence of invasive disease. J Infect. 2008;57(4):283–9.
Almohaya AM, Almutairy TS, Alqahtani A, Binkhamis K, Almajid FM. Fusobacterium bloodstream infections: a literature review and hospital-based case series. Anaerobe. 2020;62: 102165.
Nohrström E, Mattila T, Pettilä V, Kuusela P, Carlson P, Kentala E, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6–7):463–70.
Goldstein EJ. Anaerobic bacteremia. Clin Infect Dis. 1996;23(S1):S97-101.
Forrester LJ, Campbell BJ, Berg JN, Barrett JT. Aggregation of platelets by Fusobacterium necrophorum. J Clin Microbiol. 1985;22(2):245–9.
Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264.
Park Y, Choi JY, Yong D, Lee K, Kim JM. Clinical features and prognostic factors of anaerobic infections: a 7-year retrospective study. Korean J Intern Med. 2009;24(1):13–8.
Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2(11):659–66.
Fabbian F, De Giorgi A, Boari B, Misurati E, Gallerani M, Cappadona R, et al. Infections and internal medicine patients: could a comorbidity score predict in-hospital mortality? Medicine. 2018;97(42): e12818. https://doi.org/10.1097/MD.0000000000012818.
Mellor TE, Mitchell N, Logan J. Lemierre’s syndrome variant of the gut. BMJ Case Rep. 2017. https://doi.org/10.1136/bcr-2017-221567.
Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11.
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microb. 2013;14(2):207–15.
Yusuf E, Wybo I, Pierard D. Case series of patients with Fusobacterium nucleatum bacteremia with emphasis on the presence of cancer. Anaerobe. 2016;39:1–3.
Moore C, Addison D, Wilson JM, Zeluff B. First case of Fusobacterium necrophorum endocarditis to have presented after the 2nd decade of life. Tex Heart Inst J. 2013;40(4):449–52.
Garcia-Carretero R. Bacteraemia and multiple liver abscesses due to Fusobacterium nucleatum in a patient with oropharyngeal malignancy. BMJ Case Rep. 2019. https://doi.org/10.1136/bcr-2018-228237.
Cao SA, Hinchey S. Identification and management of Fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12): e12303. https://doi.org/10.7759/cureus.12303.
Ahmed Z, Bansal SK, Dhillon S. Pyogenic liver abscess caused by Fusobacterium in a 21-year-old immunocompetent male. World J Gastroenterol. 2015;21(12):3731–5.
Le Roux K, Seve P, Gomard E, Boibieux A, Beziat C, Stankovic K, et al. Lemierre syndrome variant: Hepatic abscesses and hepatic venous thrombosis due to Fusobacterium nucleatum septicemia. Rev Med Intern. 2006;27(6):482–6.
Iwata N, Komiya N, Uchiyama-Nakamura F, Ohnishi K. Lemierre syndrome: a Japanese patient returning from Thailand. J Infect Chemother. 2010;6(3):213–5.
Nia A, Ungersboeck A, Uffmann M, Leaper D, Assadian O. Septic hip abscess due to Fusobacterium nucleatum and Actinomyces turicensis in an immunocompetent SARS-CoV-2 positive patient. Anaerobe. 2021;71: 102420. https://doi.org/10.1016/j.anaerobe.2021.102420.
Williams MD, Kerber CA, Tergin HF. Unusual presentation of Lemierre’s syndrome due to Fusobacterium nucleatum. J Clin Microbiol. 2003;41(7):3445–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CL conceived the study; CL and QJ performed the experiments; CL and LW analyzed the data; CL and DY investigate the study. CL wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures carried out in this study were in accordance with the ethical standards of the institutional and national responsible committee on human experimentation and the Helsinki Declaration of 1964 and its later amendments or equivalents. This study was approved by the Ethics Committee of Affiliated Tumor Hospital of Zhengzhou University. Informed consent was obtained from the patient included in the study.
Consent for publication
Written informed consent was obtained from the patient's next of kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., Jia, Q., Wang, L. et al. A case report of severe Fusobacterium nucleatum sepsis secondary to nephrectomy. BMC Infect Dis 22, 309 (2022). https://doi.org/10.1186/s12879-022-07294-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07294-6