Abstract
Background
COVID-19 outbreaks still occur in English care homes despite the interventions in place.
Methods
We developed a stochastic compartmental model to simulate the spread of SARS-CoV-2 within an English care home. We quantified the outbreak risk with baseline non-pharmaceutical interventions (NPIs) already in place, the role of community prevalence in driving outbreaks, and the relative contribution of all importation routes into a fully susceptible care home. We also considered the potential impact of additional control measures in care homes with and without immunity, namely: increasing staff and resident testing frequency, using lateral flow antigen testing (LFD) tests instead of polymerase chain reaction (PCR), enhancing infection prevention and control (IPC), increasing the proportion of residents isolated, shortening the delay to isolation, improving the effectiveness of isolation, restricting visitors and limiting staff to working in one care home. We additionally present a Shiny application for users to apply this model to their facility of interest, specifying care home, outbreak and intervention characteristics.
Results
The model suggests that importation of SARS-CoV-2 by staff, from the community, is the main driver of outbreaks, that importation by visitors or from hospitals is rare, and that the past testing strategy (monthly testing of residents and daily testing of staff by PCR) likely provides negligible benefit in preventing outbreaks. Daily staff testing by LFD was 39% (95% 18–55%) effective in preventing outbreaks at 30 days compared to no testing.
Conclusions
Increasing the frequency of testing in staff and enhancing IPC are important to preventing importations to the care home. Further work is needed to understand the impact of vaccination in this population, which is likely to be very effective in preventing outbreaks.
Similar content being viewed by others
Introduction
Care homes have borne a large burden of the COVID-19 pandemic. A study pooling data from 26 countries found that 41% of all COVID-19 deaths were in care home residents [1]. In England there were an estimated 30,500 excess death registrations in care home residents between the 23rd March and the 19th of June 2020, compared to the same time period during previous years 2017–2019 [2]. During the third week of January 2021, 45% of COVID-19 deaths in England and Wales were in care home residents (2,386 deaths per week related to COVID-19 in care home residents), suggesting the measures in place in care homes were not sufficient to prevent and suppress outbreaks [3]. Following the vaccination campaign in care homes, COVID-19-related deaths decreased to between 7 and 20 deaths per week in June 2021, and ranged from 61 to 107 per week during October 2021 [3].
Approximately 460,000 residents are registered as living in around 15,000 care homes in England, of which around 6,000 provide care exclusively for older people (around 230,000 beds) [4]. This analysis focuses on the latter. Within this manuscript we distinguish between nursing care homes, which provide 24 h nursing care (approximately 35% of care homes that provide care to older people in England [4]), and residential care homes, which do not. In practice, this distinction may not be as clear as some nursing care homes may only provide nursing care to some of their residents.
Many factors put care homes at increased risk of infectious disease outbreaks. Firstly, although some infection prevention and control (IPC) measures such as mask wearing, hand hygiene and cohorting have been beneficial in preventing the transmission of SARS-CoV-2, implementing IPC measures in these settings is difficult. Physical distancing and deep cleaning is challenging since these facilities are residents’ homes and as such frequently contain soft furnishings and shared living spaces. In addition many residents have high levels of personal care needs due to their clinical conditions, which require close and frequent contact with staff, making it impossible for residents and staff to adhere to physical distancing measures. Isolation capability is also limited for residents with ambulatory dementia. During the COVID-19 pandemic in the UK, IPC has been hindered further by varied access to personal protective equipment (PPE) and testing, as well as a lack of staff sick pay [5]. Secondly, in England care homes are closely linked to hospitals, with on average 1 hospital admission per resident per year in 2016/2017 [6], making this setting vulnerable to importations from hospital. Thirdly, care homes are closely linked to the community through care home staff and through visitors. Staff working across several care homes could also enhance the spread of SARS-CoV-2 between care homes [5].
The high burden of COVID-19 mortality seen in care home settings emphasises the need for studies to explore the drivers of SARS-CoV-2 outbreaks as well as to identify effective mitigation and control measures. National COVID-19 guidelines for care homes in England are outlined in Additional file 1. In this study we use a mathematical model to simulate the spread of SARS-CoV-2 in English care homes with baseline interventions in place. Baseline interventions included testing of symptomatic and asymptomatic residents and staff in care homes and upon discharge of residents from hospital into care homes, isolation of test-positive residents, and a decrease of transmission rates once 1 or more cases were detected in the care home. We aim to quantify care home outbreak risk in terms of (i) the baseline scenario, (ii) community prevalence, (iii) the relative contribution of different importation routes, and (iv) the potential reductions provided by additional non-pharmaceutical interventions.
Methods
Model overview
We used a stochastic compartmental model to simulate the transmission of SARS-CoV-2 among residents and staff in English care homes (see Additional file 1 for details). Two types of facilities were considered: a residential care home with 29 beds and 29 members of staff and a nursing care home with 47 beds and 94 members of staff. The resident bed numbers represent the median bed numbers in these facilities in England [4] and assumed staff to resident ratios (see Additional file 1: Figure S1) [7, 8]. We included resident hospitalisation (see schematic in Fig. 1), testing of residents and staff, isolation of residents, absence and replacement of staff (see Additional file 1), and resident death. The pathways by which residents and staff may become infected are shown in Additional file 1: Figure S2. The three routes of SARS-CoV-2 importation into the care home are: from the community or another care home through staff, from the community through visitors, and from hospital through residents. With the exception of the frequency of testing, which was fixed, parameter values were drawn randomly from their respective distributions (see Additional file 1: Table S1). Mortality and hospitalisation dynamics are described in detail in Additional file 1. The number of parameter sets and simulations per parameter set were determined by examining the point at which the model outputs converged (see Additional file 1: Figures S3 and S4).
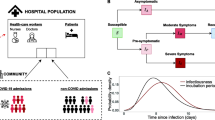
Model schematic of the SARS-CoV-2 infection and disease process in residents and staff. Residents were classified into susceptible (Sr), exposed (Er), infectious asymptomatic (Ia,r), infectious preclinical (Ipc,r), infectious clinical with high infectiousness (Ich,r), infectious clinical with low infectiousness (Icl,r), and recovered (Rr) compartments. Staff were classified into susceptible (Ss), exposed (Es), infectious asymptomatic (Ia,s), infectious preclinical (Ipc,s), infectious clinical with high infectiousness (Ich,s), and recovered (Rs) compartments. Darker shades denote compartments that contribute towards the force of infection. Resident movements are denoted by bold purple arrows, staff movements are denoted by bold green arrows and visiting is denoted by a bold orange arrow. Residents exit the care home due to hospital visits for COVID-19 and non-COVID-19 reasons or as a result of death. Residents enter the care home from the hospital following a COVID-19 admission, a non-COVID-19 admission or as a new admission. Within-hospital transmission dynamics were not modelled explicitly. Flows of new care home residents arriving from the community and care home residents moving into the community are assumed to be negligible during the pandemic and thus are not considered in the model. Staff are assumed to live in the community, and a small proportion of staff work at another care home. Staff may become absent because of COVID-19 symptoms or a positive test, and return to the care home recovered. Absent staff may be replaced by a secondary pool of staff, who in turn leave the care home as the original staff return from their absence
Testing and isolation
In the baseline scenario we assumed that, upon presentation of COVID-19 symptoms, a mean of 90% (95% 81–97%) of residents were tested and (if positive and isolation was possible) were isolated within a day of symptom onset. Not all symptomatic residents were tested due to the inability to swab some residents (e.g. agitated residents with severe dementia). On average, we assumed 85% (95% 76–93%) of residents without symptoms were tested every 28 days and 95% (95% 85–97%) of staff without symptoms were tested every 7 days (reflecting the previous testing policy in England [9]). In addition, all residents who were hospitalised were tested in hospital before returning to the care home.
In the baseline scenario, we assumed all tests carried out were using laboratory real-time polymerase chain reaction (PCR). PCR testing was assumed to have 100% specificity and, a mean of 90% (95% 88–92%) sensitivity when carried out in hospital [10, 11] (ie. it detected on average 90% of infectious individuals). There is no PCR sensitivity data specific to the care home setting. We assumed the mean sensitivity dropped to 80% (95% 72–88%) when carried out in the care home (as healthcare workers are better trained to carry out these tests than care home staff). The mean delay to isolation/absence (if it occurred) in residents/staff without symptoms tested by PCR was assumed to be 2 days (95% 0.7–3.9). This was defined as the delay between entering an infectious state and isolation/absence, therefore comprising the delay to testing and obtaining testing results. The mean delay to isolation/absence in residents with symptoms was assumed to be 1 day (95% 0.3–1.9). Residents tested in hospital and found positive were assumed to be immediately isolated upon their return to the care home if their hospitalisation was for COVID-19, and after a mean of 2 days (95% 0.7–3.9) if they had been hospitalised for a different reason. Lateral flow antigen tests (LFD) were explored in alternative testing scenarios and were assumed to have a mean of 70% sensitivity (95% 61–78%), and a mean delay to isolation of 0.25 days (95% 0.08–0.48, given a delay in relocation logistics) [12].
Of those residents testing positive or developing symptoms, on average 80% (95% 66–92%) were isolated (\({p}_{i}\)). This assumption reflects limited isolation capacity in many care homes [13]. In the baseline scenario, we assumed that isolation reduced the transmission rate from isolated individuals by an average 75% (47–96%), in addition to the abovementioned reduction across all individuals in care homes with detected outbreaks. Care home resident testing and isolation pathways are further described in Additional file 1: Figure S5.
Scenarios, outputs, sensitivity analysis
All scenarios compared nursing and residential care homes. Three community prevalence scenarios were considered: low (mid-July 2020), medium (baseline scenario, late September 2020), and high (early April 2020). The prevalence scenarios infomed the probability of visitors being infectious, the force of infection to staff from the community, the proportion of staff and replacement staff starting the simulation in each infectious state, the probability of residents being infected in hospital, the non-COVID hospitalisation rate, and the probability of another care home experiencing an outbreak (see Additional file 1 for details). We also included a scenario where 50% of staff and residents were immune at the start of the simulations. Key assumptions of the baseline scenario are listed in Table 1.
To assess the relative importance of the routes of entry into the care home, we evaluated four scenarios: eliminating the importation from hospital into the care home, stopping visitors, restricting all original staff to work in one care home, and stopping the importation from staff. To assess the impact of different testing strategies in care homes we varied the frequency of testing, compared PCR and LFD tests, and compared the testing of residents, staff, and both. We also simulated reactive improvements in IPC (modelled through a further reduction in the mean transmissibility in the care home once an outbreak is detected from 50% (95% 31–73%) to 75% (95% 63–85%)), decreasing the delay to isolation/absence to a mean of 0.25 days (95% 0.08–0.48) for residents/staff testing positive or being symptomatic, increasing the mean proportion of residents symptomatic and testing positive being isolated to 95% (95% 76–100%) and the mean effectiveness of isolation to 95% (65–100%), stopping all visiting, and restricting all original staff to work in one care home. The effectiveness of each intervention was calculated as described in Additional file 1.
Univariate sensitivity analyses were conducted for all parameters (see Additional file 1). We also estimated the outcomes for \({R0}_{a}\)(\({R0}\) for the symptomatic pathway (a)) of 1 and 3. These are in line with the R0 estimated for SARS-CoV-2 in healthcare facilities with 5–10 average contacts per day [16].
Shiny application COS-LTCF
The transmission model has been developed into a freely-available Shiny application found here: https://cmmid-lshtm.shinyapps.io/cos-ltcf/. COS-LTCF enables the user to explore alternative care home characteristics, outbreak characteristics and interventions to those considered here.
Results
Outbreak risk and role of community prevalence in driving outbreaks
Under baseline assumptions (Table 1), COVID-19 outbreaks were probable in both residential and nursing care homes despite the testing strategies and non-pharmaceutical interventions already in place (see Additional file 1: Figures S6-S13 for outbreak dynamics in patients and staff). The median behaviour of the model predicts an outbreak in which approximately 40% of residential care home residents become infected and recover by day 90 (35% in nursing care homes), with a cumulative median of two deaths due to COVID-19 (none in residential care homes).
The probability of a care home experiencing an outbreak varied greatly depending on the community prevalence assumed (Fig. 2). By day 30, a cumulative 10% (95% 4–27%) of nursing care homes had experienced outbreaks under low community prevalence, 42% (95% 16–83%) under medium prevalence, and 90% (95% 48–100%) under high prevalence. Outbreaks in residential care homes were somewhat less likely to occur by day 30 (respectively, 5%, 95% 2–14%, 23%, 95% 9–54%, 72%, 95% 37–98% by day 30). By day 90, a cumulative 24% (95% 1–65%) of nursing care homes had experienced large outbreaks under low community prevalence, 65% (95% 2–99%) under medium prevalence, and 89% (95% 8–100%) under high prevalence (in residential care homes, respectively, 6%, 95% 0–27%, 19%, 95% 1–79%, 42%, 95% 2–93%). In univariate sensitivity analyses, these outcomes were generally most sensitive to changes to assumed transmission rates and durations of infectiousness (Additional file 1: Figures S14, S15). Overall, simulations are consistent with the dynamics observed in England, in which care homes are experiencing outbreaks despite the interventions in place [3].
The cumulative probability of an outbreak (a), large outbreak (b) and number of residents symptomatic (c) over time is dependent on community prevalence (high = dark blue, medium (baseline) = light blue, low = turquoise) in both nursing care homes (left panels) and residential care homes (right panels). The coloured line represents the median and the shaded area represents the 25–75%. The vertical dashed lines show the thresholds (30 and 90 days) at which the cumulative probability of an outbreak and a large outbreak (respectively) were assessed in subsequent analysis
In the scenario where 50% of residents and staff were assumed to be immune at the start of the simulation, the probability of an outbreak by day 30 in a nursing care home decreased to 5% (95% 2–13%) under low community prevalence, 18% (95% 6–48%) under medium community prevalence and 47% (95% 19–90%) under high community prevalence. This was lower in residential care homes (10% under medium community prevalence, 95% 3–28%). The probability of a large outbreak by day 90 was 0.2% (95% 0–9%) in nursing homes under low community prevalence, 0.8% (95% 0–29%) under medium community prevalence, and 1.5% (95% 0–35%) under high community prevalence.
Routes of importation to the care home
We considered three routes of importation to the care home: from the community through staff, from the community through visitors, and from hospital through residents. We assumed staff had the same risk of acquiring SARS-CoV-2 outside of the care home as any other individual in the community, (except those working at more than one care home who had an additional risk). In the baseline scenario, each resident visited hospital on average 0.5 times per year (details in Additional file 1) and received 88 visitors per year (see Additional file 1: Table S1). Compared to the baseline scenario, where all of these routes of importation are included, eliminating importation from hospital into the care home and stopping visitors had a small impact on the probability of an outbreak at 30 days (Fig. 3). The most influential route of importation was through staff from the community. This ranking is robust to different community prevalence scenarios, across both types of care homes considered, and in a scenario where 50% of staff and residents were immune at the start of the simulations (S16).
The cumulative probability of an outbreak at 30 days under low community prevalence (top panels), medium community prevalence (middle panels) and high community prevalence (bottom panels) over time for different importation scenarios (dark brown = baseline, dark red = no importation from hospital, light red = no importation from staff working at another care home, orange = no visitors, purple = no importation from staff), in both nursing care homes (left panels) and residential care homes (right panels)
Testing strategy
Under baseline assumptions, in nursing care homes, the most effective testing strategy of those explored in reducing the cumulative probability of an outbreak at 30 days was daily LFD testing in staff and in residents (Fig. 4). This strategy was 42% (95% 21–58%) effective in preventing outbreaks at 30 days compared to no testing under otherwise baseline scenario assumptions (i.e. it reduced the relative probability of an outbreak at 30 days by 42% compared to no testing). Eliminating testing in residents altogether when staff were tested daily by LFD yielded similar results (39% effective, 95% 18–55%, respectively). The effectiveness of PCR testing strategies was lower than for equivalent frequency LFD strategies (Fig. 5).
Effectiveness of testing strategies in preventing outbreaks in nursing care homes at 30 days (top panels) and large outbreaks at 90 days (bottom panels) by testing intervention and under low (left panels), medium (baseline, middle panels) and high (right panels) community prevalence. In red, the 25–75%, in pink, the 5–95%. Testing interventions include PCR testing (triangles) and LFD testing (dots). R stands for resident and S for staff
Effectiveness of interventions in preventing outbreaks in nursing care homes at 30 days (top panels) and large outbreaks at 90 days (bottom panels) by low (left panels), medium (baseline, middle panels) and high (right panels) community prevalence. In red, the 25–75%, in pink, the 5–95%. R stands for resident and S for staff
Testing only residents daily showed no significant efficacy (2%, 95% -8–14%, by PCR and 4%, 95% -7–16% by LFD). The impact of the baseline testing strategy (PCR once a week in staff, and once a month in residents) compared to no testing was also negligible (3%, 95% -7–14%). The effect of increasing the frequency of testing in staff to twice or three times a week was small (8% effective, 95% -4–23%, and 12% effective, 95% -2–31%, respectively by PCR and 13% effective, 95% 2–26%, and 20% effective, 95% 7–34%, respectively by LFD).
Similar patterns of testing effectiveness were observed in residential care homes (see Additional file 1: Figure S17). Assuming a higher mean R0 for pathway (a) reduced the effectiveness of all testing strategies considered (see Additional file 1: Figure S18).
Other IPC strategies
Under medium community prevalence, reactive improvements in IPC in nursing care homes (decreasing the transmission rate by 75% vs. 50% once an outbreak was detected) were 75% effective (95% 3–100%) in averting large outbreaks at 90 days compared to baseline measures (see Additional file 1: Figure S19 for residential care homes). Proactive improvements in IPC are also important, as shown by the differences in outcome estimated for different assumptions of R0. When the mean R0 in pathway (a) was decreased from 2 to 1, the probability of an outbreak at 30 days decreased from 42% (95% 16–83%) to 22% (95% 8–58%) and the probability of a large outbreak at 90 days from 65% (95% 2–99%) to 8% (95%0–72%).
Decreasing the delay to isolation or absence to a mean of 0.25 days for residents or staff testing positive or symptomatic (from a mean of 1 and 2 days, respectively, in the baseline scenario) was 10% effective (95% – 1–54%) in averting large outbreaks at 90 days. Increasing the mean proportion of symptomatic and test positive residents being isolated to 95% (from a mean of 80%) and the mean effectiveness of isolation to 95% (from a mean of 75%), restricting all visitors to the care home (from a mean frequency of 0.24 per resident per day), and limiting all original staff to working in one care home (from a mean of 1% working at more than one site) had a small effectiveness in averting large outbreaks at 90 days.
Baseline immunity
Our preliminary analysis shows that when 50% of residents and staff were immune at the start of the simulations, 57% (95% 42–62%) of outbreaks and 99% (95% 69–100%) of large outbreaks were averted in nursing care homes, compared to baseline, which is the most effective intervention considered. When 50% of staff and residents are immune at the start of the simulation, the most favorable testing strategies are qualitatively the same, however, they are overall more likely to prevent large outbreaks at 90 days (see Additional file 1: Figure S20). At this level of immunity, daily LFD testing of staff and residents, on average, eliminates the probability of a large outbreak occurring in a nursing home at 90 days. The effectiveness of other interventions is similar to when no immunity is present, with the exception of preventing 75% of transmission once the outbreak is detected, which, on average, eliminates the probability of a large outbreak occurring in a nursing home at 90 days (Additional file 1: Figure S21).
Discussion
Our study shows that COVID-19 outbreaks are probable in both residential and nursing care homes in England despite the non-pharmaceutical interventions in place, suggesting that additional measures are necessary to prevent and contain outbreaks in this setting. This is consistent with COVID-19 deaths in care homes rising during the third wave of the pandemic [3]. We show that community prevalence, through staff importation, determines to a large extent the probability of outbreaks at 30 days. Importation through visitors at pre-pandemic levels and through the infection of residents during a hospital admission (after which all residents are now tested), are less likely to cause outbreaks. This is in agreement with recent studies in Wales and in Scotland finding that the risk of care home outbreaks was not significantly increased in the period following a hospital discharge to the care home and that staff are the main route of importation [18, 19]. These findings suggest that reducing importations via care home staff should be the main focus of interventions aiming to prevent the importation of SARS-CoV-2 to care homes.
The most effective testing strategies involve daily testing of staff. Whilst some of the testing capability limitations in England could be addressed simply, others, such as the time pressure on staff (who are already often overstretched) to carry out additional tests remain problematic. We find that testing strategies involving only residents are ineffective in preventing outbreaks. LFD testing had a marginal benefit over PCR due to the lower delay of turnaround of the test, despite poorer sensitivity. This is in line with an evaluation of point-of-care testing carried out in the UK [20]. However, we did not account for false negative tests contributing to a false sense of security that could lead to increased transmission. Our qualitative findings on the frequency, type of test and the best population to test are in line with those from recent mathematical models describing SARS-CoV-2 transmission in care homes examining testing strategies in other countries [21,22,23,24,25]. However, the particular testing frequency needed to substantially reduce the probability of an outbreak is context-specific, and heavily dependent on the modelling assumptions made (e.g. baseline considered, contact rates assumed, infectious period, proportion of staff and residents asymptomatic, delay to isolation).
We assessed the effectiveness of other IPC interventions when added to the current strategies in place (baseline). We show that decreasing transmission rates within the care home, whether proactively or reactively, was very effective in averting outbreaks at 30 days and large outbreaks at 90 days. Further research is needed to quantify the single and combined effect of measures such as deep cleaning, PPE, enhanced hand hygiene, ventilation on decreasing transmission rates in this setting. Cohorting is another intervention that could be important to decrease transmission rates, as shown in a recent mathematical modelling study set in a Scottish care home [26].
Our findings suggest that when 1% of staff work at more than one care home, limiting all original staff to working at one site is ineffective; however, this may be useful for care homes with a higher proportion of staff working across various sites, as SARS-CoV-2 has been shown to spread between care homes. Although the proportion of care homes with staff working at multiple care homes has been described [5], only one survey of Thames Valley care home managers has estimated the proportion of staff working across multiple care homes (1%, C Watson, personal communication, June 2020). Staff constitute an important route of importation into care homes, therefore, it is necessary to better understand their working patterns and behaviours.
We found restricting visitors in English care homes was also ineffective. A recent rapid review of the literature found no evidence of an impact of visitors on COVID-19 infections in care homes, but an increase in depression, loneliness and a potential impact on the quality of care of residents due to the absence of informal care [27]. Three mathematical modelling studies of Scottish care homes also found the impact of visiting on COVID-19 outbreaks to be small or negligible [19, 21, 26]. Together, these findings suggest visiting restrictions may provide more harm than benefit to residents, provided baseline IPC measures are in place. However; to our knowledge, no studies to date have published visiting patterns for care homes in England. In addition to relatives, professionals also visit care home residents (e.g. GPs, physiotherapists). We did not account for these visits in our model and may therefore underestimate the overall frequency and impact of visits. We also did not account for the uniqueness and correlation between visitors.
Another key data limitation is the need to better understand the contact patterns within care homes. We assumed that transmission rates were the same between residents, between residents and staff, and between staff. Contrasting our findings, a recent study set in a rehabilitation centre where the contacts between residents were common and prolonged found that testing residents was more effective than testing staff [28]. This shows the importance that contact matrices may have on determining appropriate interventions in care homes. We also assumed transmission rates were the same in nursing and residential care homes; however, nursing care homes may have more staff-resident contact due to the higher care needs of this population, whilst resident-resident contacts may be lower. Another limitation of this work is that we do not consider the effect of staff absence on the rates of transmission within the care home, which are likely to increase due to remaining staff being overstretched and therefore more likely to carry out sub-standard IPC. We also did not consider indirect transmission within the care home, which could play an important role in the dynamics of SARS-CoV-2 transmission in closed settings, but for which there is currently very limited data available.
To the best of our knowledge, this is the first model to explicitly evaluate the relative importance of all SARS-CoV-2 importation routes to care homes, including the hospitalisation of residents, and the first study to assess the impact of a range of non-pharmaceutical interventions against SARS-CoV-2 in English care homes. Upon searching medRxiv/bioRxiv and PubMed for articles with the search terms ("COVID-19" OR "SARS-CoV-2" OR "coronavirus") AND ("care home" OR "LTCF" OR "long term care facility" OR "nursing home") AND ("model") in November 2021, we found 16 studies explicitly modelling SARS-CoV-2 transmission dynamics within care homes and quantifying the impact of specific care home interventions on care home outbreaks [19,20,21, 26, 28,29,30,31,32,33,34,35,36,37,38,39]. Most of these were based in the USA, where care homes are generally larger in capacity and thus where SARS-CoV-2 transmission dynamics may be different. Four studies were set in the UK (three in Scotland [19, 21, 26], one in the UK [20]). Three other studies aimed to replicate population-level dynamics of SARS-CoV-2 with consideration of specific care home elements in Belgium, the UK and the Stockholm area, and looked at the effects of generic interventions in the population such as physical distancing measures [40,41,42].
We developed the COS-LTCF app to enable decision makers to explicitly tailor the care home, outbreak and intervention characteristics to their particular setting of interest. Our study highlights the high risk of a COVID-19 outbreak occurring in English care homes under baseline IPC interventions. Our model indicates that community prevalence, through staff importation, is key in determining the probability of care home outbreaks at 30 days, and that more frequent testing is needed. Our preliminary analysis of vaccination suggests that vaccination, even if only partially effective, will provide a substantial effect in reducing the burden of disease in care homes. Future work will explore the dynamics of vaccination in this setting.
Conclusions
In order to prevent COVID-19 deaths in the extremely vulnerable care home population, it is crucial to understand the SARS-CoV-2 importation dynamics to care homes and to determine the most effective interventions. We found that community prevalence, through staff importations, was the main driver of outbreaks in care homes at 30 days, not importation from hospital visits nor by visitors. In line with this, we found daily testing of staff to be the most effective testing strategy in preventing outbreaks. We show the previous testing strategy (PCR testing residents once every 28 days and staff once a week) to be ineffective in preventing outbreaks and suggest that more frequent testing of staff is required. Restricting visitors bore little effect on the probability of an outbreak occurring by day 30. Interventions focusing on decreasing the transmission of SARS-CoV-2 in the care home were the most effective in reducing the frequency of outbreaks. We provide a Shiny application for users to explore alternative care home characteristics, outbreak characteristics and interventions.
Availability of data and materials
The code is available here: https://github.com/rmjlros/COVID19_care_home_NPIs.
Abbreviations
- IPC:
-
Infection prevention and control
- LFD:
-
Lateral flow antigen testing
- PCR:
-
Polymerase chain reaction
- PPE:
-
Personal protective equipment
References
Comas-Herrera A, Zalakaín J, Litwin C, Hsu AT, Lemmon E, Henderson D, et al. Mortality associated with COVID-19 outbreaks in care homes: international evidence. LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE. 2021.
The Health Foundation. Adult social care and COVID-19: Assessing the impact on social care users and staff in England so far. 2020.
Number of deaths in care homes notified to the Care Quality Commission, England. Office for National Statistics; 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/numberofdeathsincarehomesnotifiedtothecarequalitycommissionengland
Care Quality Commission. Active locations for providers registered under the Health and Social Care Act (HSCA). 2020. https://www.cqc.org.uk/about-us/transparency/using-cqc-data. Accessed 8 Apr 2021.
Office for National Statistics. Impact of coronavirus in care homes in England. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/impactofcoronavirusincarehomesinenglandvivaldi/26mayto19june2020. Accessed 8 Apr 2021.
Wolters A, Santos F, Lloyd T, Lilburne C, Steventon A. Emergency admissions to hospital from care homes: how often and what for? The Health Foundation; 2019.
Ladhani SN, Chow JY, Janarthanan R, Fok J, Crawley-Boevey E, Vusirikala A, et al. Investigation of SARS-CoV-2 outbreaks in six care homes in London. EClinicalMedicine. 2020;26:100533.
Kennelly SP, Dyer AH, Martin R, Kennelly SM, Martin A, O’Neill D, et al. Asymptomatic carriage rates and case-fatality of SARS-CoV-2 infection in residents and staff in Irish nursing homes. Age Ageing. 2021;50:89.
Department of Health and Social Care. Coronavirus (COVID-19): getting tested. GOV.UK. 2021. https://www.gov.uk/guidance/coronavirus-covid-19-getting-tested. Accessed 8 Apr 2021.
Wikramaratna P, Paton RS, Ghafari M, Lourenco J. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. medRxiv. 2020;:2020.04.05.20053355–2020.04.05.20053355.
Chau NVV, Lam VT, Dung NT, Yen LM, Minh NNQ, Hung LM, et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. medRxiv. 2020;:2020.04.27.20082347–2020.04.27.20082347.
Mahase E. Covid-19: Innova lateral flow test is not fit for “test and release” strategy, say experts. BMJ. 2020;371:m4469.
Rajan S, Comas-Herrera A, McKee M. Did the UK Government Really Throw a Protective Ring Around Care Homes in the COVID-19 Pandemic? J Long-Term Care. 2020;3:185–95.
Davies NG, Kucharski AJ, Eggo RM, Gimma A, Edmunds WJ, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Health. 2020;5:E375–85.
Public Health England. Discharge into care homes: designated settings. GOV.UK. 2020. https://www.gov.uk/government/publications/designated-settings-for-people-discharged-to-a-care-home/discharge-into-care-homes-designated-settings. Accessed 22 Dec 2020.
Temime L, Gustin M-P, Duval A, Buetti N, Crépey P, Guillemot D, et al. A Conceptual Discussion About the Basic Reproduction Number of Severe Acute Respiratory Syndrome Coronavirus 2 in Healthcare Settings. Clin Infect Dis. 2021;72:141–3.
Evans S, Stimson J, Pople D, Bhattacharya A, White P, Robotham J. Quantifying the contribution of pathways of nosocomial acquisition of COVID-19 in English hospitals. BMJ. 2020;89:8.
Emmerson C, Adamson JP, Turner D, Gravenor MB, Salmon J, Cottrell S, et al. Risk factors for outbreaks of COVID-19 in care homes following hospital discharge: a national cohort analysis. medRxiv. 2020;2020.08.24.20168955.
Baister M, McTaggart E, McMenemy P, Megiddo I, Kleczkowski A. COVID-19 in Scottish care homes: A metapopulation model of spread among residents and staff. 2021.
Stevenson M, Metry A, Messenger M. Modelling of hypothetical SARS-CoV-2 point of care tests for routine testing in residential care homes: rapid cost-effectiveness analysis. Health Technol Assess. 2021;25:1–74.
Nguyen LLK, Howick S, McLafferty D, Anderson GH, Pravinkumar SJ, Van Der Meer R, Evaluating intervention strategies in controlling coronavirus disease, et al. (COVID-19) spread in care homes: An agent-based model. Infect Control Hosp Epidemiol. 2019;2020:1–11.
Holmdahl I, Kahn R, Hay J, Buckee CO, Mina M. Frequent testing and immunity-based staffing will help mitigate outbreaks in nursing home settings. medRxiv. 2020;:2020.11.04.20224758.
Schmidt AJ, García Y, Pinheiro D, Reichert T, Nuño MA. Using Non-Pharmaceutical Interventions and High Isolation of Asymptomatic Carriers to Contain the Spread of SARS-CoV-2 in Nursing Homes. medRxiv. 2021. https://doi.org/10.1101/2021.01.22.21249308.
Vilches TN, Nourbakhsh S, Zhang K, Juden-Kelly L, Cipriano LE, Langley JM, et al. Multifaceted strategies for the control of COVID-19 outbreaks in long-term care facilities in Ontario. Canada medRxiv. 2020. https://doi.org/10.1101/2020.12.04.20244194.
Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu S, Lo NC. Frequency of Routine Testing for Coronavirus Disease, (COVID-19) in High-risk Healthcare Environments to Reduce Outbreaks. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciaa1383.
Nguyen LKN, Howick S, McLafferty D, Anderson GH, Pravinkumar SJ, Van Der Meer R, et al. Impact of visitation and cohorting policies to shield residents from covid-19 spread in care homes: an agent-based model. Am J Infect Control. 2021;49:1105–12.
Comas-Herrera A, Salcher-Konrad M, Baumbusch J, Farina N, Goodman C, Lorenz-Dant K, et al. Rapid review of the evidence on impacts of visiting policies in care homes during the COVID-19 pandemic. LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE. 2020.
Smith DRM, Duval A, Pouwels KB, Guillemot D, Fernandes J, Huynh B-T, et al. Optimizing COVID-19 surveillance in long-term care facilities: a modelling study. BMC Med. 2020;18:386.
Holmdahl I, Kahn R, Slifka KJ, Dooling K, Slayton RB. Modeling the impact of vaccination strategies for nursing homes in the context of increased SARS-CoV-2 community transmission and variants. 2021.
Lucia-Sanz A, Magalie A, Rodriguez-Gonzalez R, Leung C-Y, Weitz JS. Modeling shield immunity to reduce COVID-19 transmission in long-term care facilities. 2021.
Blumberg S, Lu P, Hoover CM, Lloyd-Smith JO, Kwan AT, Sears D, et al. Mitigating outbreaks in congregate settings by decreasing the size of the susceptible population. 2021.
Love J, Wimmer MT, Toth DJA, Chandran A, Makhija D, Cooper CK, et al. Comparison of antigen- and RT-PCR-based testing strategies for detection of SARS-CoV-2 in two high-exposure settings. PLoS ONE. 2021;16:e0253407.
Gómez-Vázquez JP, García Y, Schmidt AJ, Martínez-López B, Nuño M. Testing and Vaccination to Reduce the Impact of COVID-19 in Nursing Homes: An Agent-Based Approach. 2021.
Kahn R, Holmdahl I, Reddy S, Jernigan J, Mina MJ, Slayton RB. Mathematical modeling to inform vaccination strategies and testing approaches for COVID-19 in nursing homes. medRxiv. 2021;:2021.02.26.21252483.
Love J, Keegan LT, Angulo FJ, McLaughlin J, Shea KM, Swerdlow DL, et al. Continued need for non-pharmaceutical interventions after COVID-19 vaccination in long-term-care facilities | medRxiv. medRxiv. 2021.
See I, Paul P, Slayton RB, Steele MK, Stuckey MJ, Duca L, et al. Modeling effectiveness of testing strategies to prevent COVID-19 in nursing homes —United States, 2020. 2021.
Holmdahl I, Kahn R, Hay JA, Buckee CO, Mina MJ. Estimation of Transmission of COVID-19 in Simulated Nursing Homes With Frequent Testing and Immunity-Based Staffing. JAMA Netw Open. 2021;4:e2110071–e2110071.
Jackson ML. Low-Impact Social Distancing Interventions to Mitigate Local Epidemics of SARS-CoV-2. 2020.
Tsoungui HCJ, Adil Mahmoud Yousif N, Alawam Nemer L, NgougoueNgougoue PM, Ngwa GA, Teboh-Ewungkem M, et al. Preventing COVID-19 spread in closed facilities by regular testing of employees-An efficient intervention in long-term care facilities and prisons? PLoS ONE. 2021;16:e0249588.
Franco N. COVID-19 Belgium: Extended SEIR-QD model with nursing homes and long-term scenarios-based forecasts. Epidemics. 2021;37:100490.
Oberhammer J. Social-distancing effectiveness tracking of the COVID-19 hotspot Stockholm. 2020.
Knock ES, Whittles LK, Lees JA, Perez-Guzman PN, Verity R, FitzJohn RG, et al. Key epidemiological drivers and impact of interventions in the 2020 SARS-CoV-2 epidemic in England. Sci Transl Med. 2021;13:eabg4262.
Acknowledgements
We would like to thank Ian Hall, as well as the rest of the Care Home Working Group for helpful conversations as well as William Laing, Claire Goodman, Des Kelly and Julie Robotham for their insight; and Carl Pearson and Joel Hellewell for their helpful inputs. This work uses data provided by patients and collected by the NHS as part of their care and support.
Members of the Centre for Mathematical Modelling of Infectious Diseases (CMMID) COVID-19 modelling working group (random order): Carl A B Pearson, Thibaut Jombart, Simon R Procter, Yung-Wai Desmond Chan, Graham Medley, Joel Hellewell, Billy J Quilty, Yang Liu, Stefan Flasche, Sam Abbott, Rosalind M Eggo, Rachel Lowe, Yalda Jafari, Christopher I Jarvis, Naomi R Waterlow, James D Munday, Amy Gimma, Nikos I Bosse, Petra Klepac, Akira Endo, Adam J Kucharski, Rosanna C Barnard, C Julian Villabona-Arenas, Sebastian Funk, Kevin van Zandvoort, W John Edmunds, William Waites, Emily S Nightingale, Sophie R Meakin, Frank G Sandmann, Fabienne Krauer, Nicholas G. Davies, Alicia Showering, Samuel Clifford, Katherine E. Atkins, Matthew Quaife, Kaja AbbasTimothy W Russell, Fiona Yueqian Sun, Mark Jit, Mihaly Koltai, Kiesha Prem, Jiayao Lei, Anna M Foss, Jack Williams, Kathleen O'Reilly, Damien C Tully, Hamish P Gibbs, Oliver Brady
CMMID COVID-19 modelling working group funding statements: CABP (B&MGF: NTD Modelling Consortium OPP1184344, FCDO/Wellcome Trust: Epidemic Preparedness Coronavirus research programme 221303/Z/20/Z), TJ (Global Challenges Research Fund: ES/P010873/1, UK Public Health Rapid Support Team, NIHR: Health Protection Research Unit for Modelling Methodology HPRU-2012-10096, UK MRC: MC_PC_19065), SRP (B&MGF: INV-016832), GFM (B&MGF: NTD Modelling Consortium OPP1184344), JH (Wellcome Trust: 210758/Z/18/Z), BJQ (NIHR: 16/137/109, NIHR: 16/136/46, B&MGF: OPP1139859), YL (B&MGF: INV-003174, NIHR: 16/137/109, European Commission: 101003688, UK MRC: MC_PC_19065), SFlasche (Wellcome Trust: 208812/Z/17/Z), SA (Wellcome Trust: 210758/Z/18/Z), RME (HDR UK: MR/S003975/1, UK MRC: MC_PC_19065, NIHR: NIHR200908), RL (Royal Society: Dorothy Hodgkin Fellowship), YJ UKRI: MR/V028456/1, CIJ (Global Challenges Research Fund: ES/P010873/1), NRW(MRC: MR/N013638/1), JDM(Wellcome Trust: 210758/Z/18/Z), AG (European Commission: 101003688), NIB (HPRU: NIHR200908), PK (Royal Society: RP\EA\180004, European Commission: 101003688), AE (Nakajima Foundation), AJK (Wellcome Trust: 206250/Z/17/Z, NIHR: NIHR200908), RCB (European Commission: 101003688), Sfunk (Wellcome Trust: 210758/Z/18/Z), KvZ (Elrha R2HC/UK FCDO/Wellcome Trust/NIHR, FCDO/Wellcome Trust: Epidemic Preparedness Coronavirus research programme 221303/Z/20/Z), WJE (European Commission: 101003688, UK MRC: MC_PC_19065, NIHR: PR-OD-1017-20002), WW (MRC: MR/V027956/1), ESN (B&MGF: OPP1183986), SRM (Wellcome Trust: 210758/Z/18/Z), FGS (NIHR: NIHR200929), FK (Innovation Fund: 01VSF18015, Wellcome Trust: UNS110424), NGD (UKRI Research England, NIHR: NIHR200929, UK MRC: MC_PC_19065), SC (Wellcome Trust: 208812/Z/17/Z, UK MRC: MC_PC_19065), MQ (ERC Starting Grant: #757699, B&MGF: INV-001754), KA (BMGF: INV-016832; OPP1157270), TWR (Wellcome Trust: 206250/Z/17/Z), FYS (NIHR: 16/137/109), MJ (B&MGF: INV-003174, NIHR: 16/137/109, NIHR: NIHR200929, European Commission: 101003688), MK (Wellcome Trust 221303/Z/20/Z), KP (B&MGF: INV-003174, European Commission: 101003688), JYL Gates (INV-003174), KO'R (B&MGF: OPP1191821), HPG (EDCTP2: RIA2020EF-2983-CSIGN, UK DHSC/UK Aid/NIHR: PR-OD-1017-20001), OJB (Wellcome Trust: 206471/Z/17/Z)
Funding
AR acknowledges funding from the National Institute for Health Research (Grant: PR-OD-1017–20002). RCB acknowledges funding from the European Union Horizon 2020 Research and Innovation programme, project EpiPose (101003688), throughout the duration of the study. DS is supported by a Canadian Institutes of Health Research Doctoral Foreign Study Award (Funding Reference Number 164263) and the French National Research Agency project SPHINX-17-CE36-0008–01. GMK is supported by the UK Medical Research Council (Grant: MR/P014658/1). The development of the Shiny application COS-LTCF was funded by the World Health Organization. The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all of the data and the final responsibility to submit for publication.
Author information
Authors and Affiliations
Consortia
Contributions
WJE and AR designed the study and the model. AR led the development and analysis of the transmission model, and writing of the manuscript. WJE, RCB, DRMS, GMK, and The Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group contributed to shaping the analysis and writing the manuscript. NGD and SE contributed outputs from the community and hospital transmission models they respectively developed, FG and SRD led the analysis of SUS data and contributed to writing the manuscript. AR and RCB developed the Shiny application. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was a secondary data analysis using published data, with the exception of the use of Secondary Uses Service (SUS) data. The SUS data were analysed by FG in the Health Foundation secure data environment, which is accredited to the NHS Digital Data Security and Protection Toolkit and the ISO27001 Information Security Management standard. Summary statistics were shared with co-authors only after statistical disclosure control. SUS data are routinely collected, retrospective and pseudonymised data made available through an agreement approved by NHS England with the Health Foundation. Therefore, consent to participate nor ethics approval was required for this study.
Consent for publication
Not applicable.
Competing interests
AR is a participant of the SAGE Social Care Working Group. AR, WJE, RCB, NGD, SE, and GMK are participants of the SAGE Scientific Pandemic Influenza Group on Modelling. WJE is a participant of the Scientific Advisory Group for Emergencies. All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rosello, A., Barnard, R.C., Smith, D.R.M. et al. Impact of non-pharmaceutical interventions on SARS-CoV-2 outbreaks in English care homes: a modelling study. BMC Infect Dis 22, 324 (2022). https://doi.org/10.1186/s12879-022-07268-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07268-8