Abstract
Background
CD4+ T cell counts in certain human immunodeficiency virus (HIV)-infected patients called immunological non-responders (INRs) could not return to a normal level even with sustained antiretroviral therapy (ART) because of persistent immune activation, which is associated with pro-inflammatory cytokines production and an altered intestinal microbiome profile. Changes in gut bacterial composition have been linked to low CD4+ T cell counts in HIV-infected individuals. However, the association between CD4+ T cell counts and gut microbiota community composition and cytokines levels in INRs (CD4+ T cell counts < 500 cells/μL) from Yunnan Province, China, has not been previously investigated.
Methods
To address this issue, we carried out a cross-sectional study of 34 HIV-infected INRs. The patients were divided into CD4 count > 200 cells/μL group and CD4 count < 200 cells/μL group. The gut microbiota composition of each subject was analyzed by 16S rRNA gene sequencing. We also compared CD8+ T cell counts, pro-inflammatory cytokines levels, and nutritional status between the two groups.
Results
Compared to INRs with CD4 count > 200 cells/μL, those with CD4 count < 200 cells/μL had a lower CD4/CD8 ratio, lower nutritional status and higher serum levels of tumor necrosis factor (TNF)-α, interferon-γ-inducible protein (IP)-10 and interleukin (IL)-1α. Ruminococcaceae was less abundant in the CD4 count < 200 cells/μL group than in the CD4 count > 200 cells/μL group, and difference in alpha diversity was observed between the two groups. Moreover, CD4+ T cell counts were negatively associated with TNF-α and IL-1α levels and positively associated with the relative abundance of Ruminococcaceae.
Conclusions
Our study demonstrated that lower CD4+ T cell counts in INRs are associated with a reduced abundance of Ruminococcaceae in the gut and elevated serum pro-inflammatory cytokines levels. Thus, interventions targeting gut microbiota to increase CD4+ T cell counts are a potential strategy for promoting immune reconstitution in HIV-infected INRs.
Similar content being viewed by others
Background
Human immunodeficiency virus (HIV) infection depletes CD4+ T cells, leading to the development of acquired immunodeficiency syndrome (AIDS), AIDS-defining opportunistic infections or cancer. CD4+ T cell counts circulating in the blood are less than 200 cells/μL in AIDS patients [1, 2]. Although plasma viral load declines to undetectable levels after initiation of antiretroviral therapy (ART), immune reconstitution may not occur; about 10–40% of HIV-infected individuals fail to normalize CD4+ T cell counts. These patients, known as immunological non-responders (INRs), have severe immune dysfunction with CD4+ T cell counts < 500 or < 350 cells/μL, or in extreme cases, < 200 cells/μL [3, 4].
HIV infection leads to the destruction of the intestinal integrity and mucosa, imbalance of gut microbial community composition, microbial translocation, systemic inflammation, persistent immune activation, pro-inflammatory cytokines release, immune exhaustion, and organ dysfunction [5,6,7,8,9,10,11]. The failure of CD4+ T cell counts to normalize in INRs may be attributable to immune activation associated with compromised gut barrier immunity and alterations in intestinal microbiome profile [9, 12,13,14,15]. It was also reported that ART-treated HIV patients with poor CD4+ T cell and CD4/CD8 ratio recovery have increased microbial translocation and elevated levels of pro-inflammatory cytokines [16].
Alpha diversity measures reflect the complexity and/or richness of operational taxonomic units (OTUs). Higher alpha diversity is generally considered as a marker of health; HIV infection status was found to be significantly associated with a decrease in alpha diversity [17]. HIV infection causes a shift in gut microbiota community composition from Bacteroides to Prevotella predominance [16,17,18,19]. Independent of gender and sexual behavior, individuals with HIV infection have a distinct gut microbiome profile characterized by Gammaproteobacteria enrichment, Lachnospiraceae and Ruminococcaceae depletion, and decreased alpha diversity [20]. Compared to ART-naïve HIV progressors, elite controllers were found to harbor a larger number of genera (including a greater abundance of Succinivibrio) and had higher richness indices for fecal microbiota [21].
Low CD4+ T cell counts (< 200 cells/μL) were shown to be associated with alterations in the bacterial microbiome in an HIV-infected cohort in Uganda [1], and findings from a study of HIV-infected individuals in Beijing found that the abundance of certain bacterial strains was closely related to CD4+ T cell numbers regardless of ART [16]. However, there have been no detailed and comprehensive studies on the relationship between immunological status, gut microbiome profile, and inflammation in INRs in Yunnan Province, China. To address this issue, this study investigated the association between CD4+ T cell counts (with 200 cells/μL as the cut-off value), gut microbiota community composition and abundance, and serum cytokines levels in HIV-infected INRs in Yunnan.
Methods
Study design
A total of 34 HIV-infected INRs (CD4 count < 500 cells/μL), receiving sustained ART (Table 1) with undetectable (< 50 copies/mL) plasma HIV-1 RNA level for at least 24 months were recruited at the Second People’s Hospital of Dali City in Dali, Yunnan, China from June 2019 to November 2019. The patients were divided into two groups according to CD4+ T cell counts (CD4 count > 200 cells/μL (n = 17) and CD4 count < 200 cells/μL (n = 17) after more than two years of ART, respectively). All subjects were ≥ 18 years old and provided written informed consent before participating in the study, which was approved by the Human Clinical Research Ethics Committee of the First Affiliated Hospital of Kunming Medical University (No. 2018L43). All experiments were performed in accordance with the approved guidelines and regulations according to the principles expressed in the Declaration of Helsinki, and the experimental protocols were approved by the institutional review board of Kunming Medical University. The clinical characteristics of the study participants are shown in Table 1.
Subjects who had used antibiotics, probiotics, or prebiotics or had experienced diarrhea or digestive symptoms within the previous 3 months were excluded, as were those with active infection or co-infection with hepatitis B virus (HBV) and HCV.
Clinical data
Fasting blood was collected and the serum was immediately separated by centrifugation and stored at − 80°C until analysis. The samples were used for measurement of systemic inflammation markers and laboratory tests including blood routine and blood biochemical tests.
A heparin anticoagulation vacuum tube was used to collect about 5 mL of venous blood. The absolute numbers of CD3+, CD4+, and CD8+ T lymphocytes were determined using the BD Multitest IMK Kit with BD Trucount tubes on a BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Assessment of nutritional status
Nutritional status was determined by the multiple-frequency bioelectrical impedance method using a body composition analyzer (InBody S10; Biospace, Seoul, Korea). The parameters, which included segmental water, segmental lean, and body mass index (BMI), were recorded immediately after measurement.
Multiplex assay
The levels of ten pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-1α, tumor necrosis factor (TNF)-α, TNF-β, interferon-gamma-inducible protein (IP)-10, monocyte chemotactic protein (MCP)-1, soluble cluster of differentiation 14 (sCD14), D-dimer and C-reactive protein (CRP) were quantified with the FlexMAP 3D with MILLIPLEX Analyst (Millipore, Billerica, MA, USA) and the Human Premixed Multi-Analyte Kit (R&D Systems) according to the manufacturer’s instructions.
DNA extraction and sequencing
Fecal samples were sampled in sterile containers by the patients and immediately stored at – 80 °C until processing. Bacterial DNA was extracted using the QIAamp PowerFecal DNA kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The concentration of DNA extracted from the samples was measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and was adjusted to 12 ng/μL for experiments. Extracted genomic DNA was PCR-amplified with degenerate PCR primers (515F, 5′-GTCCAGCMGCCGCGGTAA-3′; 806R, 5′-GGACTACHVGGGTWTCTAAT-3′) targeting the V4 region of the bacterial 16S rRNA gene. Both forward and reverse primers were tagged with adapter, pad, and linker sequences. PCR enrichment was performed using equal amounts (30 ng) of DNA from each sample in a 50 μL reaction. PCR products were purified using the Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA) and recovered in the elution buffer. Libraries were quantified using Agilent 2100 Bioanalyzer and sequencing was performed at the Beijing Genomics Institute (BGI, Wuhan, China) on a HiSeq 2500 platform (Illumina, San Diego, CA, USA) according to the manufacturer’s standard protocol, generating 250-bp paired-end reads. Raw sequence data generated for this study are available in the Sequence Read Archive under BioProject accession PRJNA695425.
Analysis of sequencing data
Raw reads were filtered to cut off adaptors and low-quality and ambiguous bases, and paired-end reads were merged using the Fast Length Adjustment of Short reads v1.2.11 program (https://ccb.jhu.edu/software/FLASH/) and filtered under specific conditions to obtain high-quality clean tags with FQTRIM v0.94 (https://ccb.jhu.edu/software/fqtrim/). Chimeric sequences were compared with those in the Gold database using UCHIME v4.2.40 (https://drive5.com/usearch/manual/uchime_algo.html). After dereplication using DADA2 (https://benjjneb.github.io/dada2/index.html), we obtained the feature table and feature sequence. Representative OTU sequences were taxonomically assigned using the GreenGenes database v201305 (https://greengenes.secondgenome.com/). Alpha and beta diversity were calculated using QIIME2 (https://qiime2.org/). Using the SILVA (release 132) classifier (https://www.arb-silva.de/), feature abundance was normalized using the relative abundance of each sample. Alpha diversity was used to analyze the complexity of species diversity in each sample based on five indices including Chao1, Observed species, Goods coverage, Shannon, and Simpson. BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used for sequence alignment, and the feature sequences were annotated with the SILVA database for each representative sequence. Diagrams were generated using R package v3.5.2 (https://www.r-project.org/).
Statistical analysis
Alpha diversity measures (Shannon and Simpson diversity indices) were calculated using R package V3.5.2 and compared with the Wilcoxon matched-pairs signed-rank test. Beta diversity analyses based on unweighted UniFrac distance matrices were performed, and differences in microbial community composition between groups were evaluated by Analysis of Similarities (ANOSIM). Principal coordinates analysis (PCoA) or nonmetric multidimensional scaling (NMDS) was used as an ordination method to visualize unweighted UniFrac dissimilarity values in the two groups. Relative taxa abundance (at the family level) and various parameters were compared between the two groups with the Mann–Whitney test. Data are reported as mean ± standard deviations (SD) and were analyzed using Prism 8.0 software (GraphPad, La Jolla, CA, USA). Statistical significance was defined as P < 0.05.
Results
Characterization of the study cohort
A total of 34 HIV-infected participants, aged 33–69 years, were enrolled in this study. No differences were observed between groups in terms of mean age, BMI, and time on ART (Table 1). In the CD4 count > 200 cells/μL group, 8 patients (47.1%) were male and 4 (23.5%) were of Han ethnicity; in the CD4 count < 200 cells/μL group, 7 (41.2%) were male and 4 (23.5%) were of Han ethnicity. In terms of HIV transmission routes, 3 (17.6%) and 12 (70.6%) individuals in the CD4 count < 200 cells/μL group were infected through intravenous drug use and sexual contact, respectively; in the CD4 count > 200 cells/μL group, the numbers were 1 (5.9%) and 14 (82.3%), respectively. For two patients (11.8%) in each group, the route of transmission was unknown. The demographic characteristics of the cohort are shown in Table 1.
Patients with CD4 count < 200 cells/μL had lower CD4+ T cell counts (P < 0.0001) nadir CD4+ T cell counts (P < 0.0001), and CD4/CD8 ratio (P = 0.0033) than those in the CD4 count > 200 cells/μL group. Additionally, the numbers of CD3+ T cells (P = 0.0518) and CD8+ T cells (P = 0.4902) were lower in the CD4 count < 200 cells/μL group, but not significant. CD4+ T cell counts were higher in the CD4 count > 200 cells/μL group than those in the CD4 count < 200 cells/μL group before the initiation of ART, although the difference between the two groups was nonsignificant (P = 0.1425).
Serum levels of pro-inflammatory cytokines are elevated in INRs with low CD4+ T cell counts
Chronic immune activation and inflammation persist in HIV-infected patients even when ART effectively inhibits viral replication. This was confirmed by our observation that serum levels of the pro-inflammatory cytokines TNF-α (P = 0.0070), IL-1α (P = 0.0141) and IP-10 (P = 0.0261) were higher in the CD4 count < 200 cells/μL group than in the CD4 count > 200 cells/μL group (Table 2). However, the latter patients had a higher CRP level, although this was not a statistically significant difference (P = 0.0834).
INRs with low CD4+ T cell counts have lower nutritional status
The results of the body composition analysis indicated that the levels of segmental water (left arm [LA]) (P = 0.0419), segmental water (trunk [TR]) (P = 0.0437), segmental lean (right arm [RA]) (P = 0.0496), segmental lean (LA) (P = 0.0496), and segmental lean (TR) (P = 0.0437) were lower in the CD4 count < 200 cells/μL group than in the CD4 > 200 cells/μL group (Table 3), suggesting that INRs with fewer CD4+ T cells are at higher risk for loss of muscle and edema. In terms of blood parameters, hemoglobin level (P = 0.0172) and hematocrit (HCT) (P = 0.0493) were markedly lower in INRs with CD4 count < 200 cells/μL (Table 3). The above results suggest that INRs in the CD4 count < 200 cells/μL group have a lower nutritional status.
Gut microbial diversity is altered with reduced CD4+ T cell counts
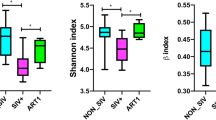
We compared the alpha diversity of intestinal microbiota between the two groups to determine whether differences in CD4 levels affected the stability of gut microbial communities. The abundance of OTUs differed between groups based on the Shannon index (P = 0.0287; Fig. 1A), although the Simpson index did not show a significant difference (P = 0.0987; Fig. 1B).
To determine how CD4+ T cell counts could affect microbial diversity in INRs, we examined beta diversity across samples. By PCoA and NMDS analysis based on unweighted UniFrac distances, we found that bacterial community structure differed to a certain extent between the two groups; ANOSIM analysis of the distance matrices showed that these differences were not statistically significant (PCoA: P = 0.111; Additional file 1: Fig. S1A; NMDS: P = 0.101, Additional file 1: Fig. S1B).
Ruminococcaceae abundance in the gut is reduced in INRs with low CD4+ T cell counts
To determine whether these changes in gut microbial community composition are associated with CD4+ T cell counts, we compared the abundance of bacterial taxa between the CD4 count > 200 cells/μL group and the CD4 count < 200 cells/μL group. The abundance of Ruminococcaceae, Succinivibrionaceae, and Bacteroidaceae were decreased whereas that of Enterobacteriaceae, Fusobacteriaceae, Veillonellaceae and Prevotellaceae were increased in the CD4 count < 200 cells/μL group compared to the CD4 count > 200 cells/μL group (Fig. 2A); of these, only Ruminococcaceae showed significantly different enrichment between the two groups (P = 0.0076; Fig. 2B), although the abundance of Bacteroidaceae was slightly higher (P = 0.3223; Additional file 1: Fig. S2A) and that of Prevotellaceae (P = 0.7339; Additional file 1: Fig. S2B) along with the Prevotellaceae/Bacteroidaceae ratio (P = 0.4023; Additional file 1: Fig. S2C) were lower in the CD4 > 200 group.
Ruminococcaceae abundance in INRs. A Relative distributions of families in the two groups are shown. Each bar represents the mean relative abundance of the microbial family. B Ruminococcaceae abundance was lower (P = 0.0076) in the CD4 count < 200 cells/µL group compared to the CD4 count > 200 cells/µL group. *P < 0.05 (Mann–Whitney test)
Pro-inflammatory cytokines levels are correlated with clinical parameters in INRs
We analyzed the relationship between clinical parameters and pro-inflammatory cytokines by constructing a heatmap to identify significant correlations (r > 0.3 or < − 0.3) between these variables (Fig. 3). Serum levels of TNF-α and IL-1α were negatively correlated with CD4+ T cell counts (r = − 0.400 to − 0.440, P < 0.05) and CD4/CD8 ratio (r = − 0.350 to 0.560, P < 0.05); MCP-1 level was negatively correlated with CD4+ T cell counts (r = − 0.470, P < 0.01); and sCD14 level was negatively associated with hemoglobin level as well as segmental water (LA), segmental water (TR), segmental lean (LA), segmental lean (RA) and segmental lean (TR) (r = − 0.300 to − 0.440, P < 0.05). D-dimer level was also negatively correlated with HCT and Hemoglobin level (r = − 0.400 to − 0.420, P < 0.05).
Abundance of specific families of gut bacteria is correlated with clinical variables in INRs
To determine the relationship between clinical variables and gut microbiota community composition and diversity at the family level, we constructed a heatmap and analyzed the correlations between variables (r > 0.3 or < − 0.3) (Fig. 4). CD4/CD8 ratio was negatively correlated with the relative abundance of Veillonellaceae (r = − 0.440, P < 0.05) and had a positive relationship with that of Succinivibrionaceae (r = 0.410, P < 0.05). The relative abundance of Ruminococcaceae was positively associated with CD4+ T cell counts (r = 0.400, P < 0.05) and negatively correlated with serum level of TNF-α (r = − 0.360, P < 0.05).
Discussion
The results of the present study demonstrated that INRs with lower CD4+ T cell numbers have increased inflammation as well as the lower relative abundance of the beneficial bacteria and reduced diversity of bacteria in the gut. Moreover, CD4+ T cell counts were closely associated with serum pro-inflammatory cytokines levels and the abundance of Ruminococcaceae in the gut.
The homeostasis of the immune system in HIV-infected individuals is partly affected by changes in cytokines levels. Dysregulation of both pro-inflammatory and anti-inflammatory cytokines production contributes to the immune impairment that is associated with the progression of HIV infection to AIDS [22]. Compared to patients with CD4 count > 200 cells/μL, serum levels of pro-inflammatory cytokines including TNF-α, IP-10 and IL-1α were increased whereas CRP level was only slightly decreased in patients with CD4 count < 200 cells/μL. These results suggest that chronic inflammation is exacerbated in HIV-infected INRs with fewer CD4+ T cells.
Malnutrition, as reflected by reduced levels of hemoglobin and vitamin D, was found to be associated with elevated levels of sCD14 and D-dimer in HIV/AIDS patients with immune reconstitution inflammatory syndrome [23]. Malnutrition skews immune cells toward an inflammatory state [24, 25]. We found here that parameters related to nutritional status were negatively correlated with sCD14 and D-dimer levels, which implies nutritional intervention is a potential strategy for immune reconstitution in INRs. Elevated plasma levels of inflammatory cytokines perturb T cell homeostasis in HIV-infected patients, resulting in the depletion of CD4+ T cells and expansion of CD8+ T cell pool [22, 26]. Accordingly, in the present study, there was a negative correlation between serum levels of pro-inflammatory cytokines such as TNF-α and IL-1α and CD4+ T cell counts or CD4/CD8 ratio. Our finding that MCP-1 level was negatively correlated with CD4+ T cell counts is consistent with a previous report [27].
The gut microbiome of HIV-infected individuals is characterized by enrichment of pro-inflammatory bacterial taxa such as Erysipelotrichaceae, Enterobacteriaceae, Desulfovibrionaceae, and Fusobacteria and depletion of anti-inflammatory taxa such as Lachnospiraceae, Ruminococceae, Bacteroides, and Rikenellaceae [6, 17, 20, 28, 29]. Ruminococcaceae family members are the main producers of short-chain fatty acids, which induce the differentiation of anti-inflammatory regulatory T cells [20, 29]. Our correlation analysis indicated that there was a negative correlation between Ruminococcaceae abundance and TNF-α level. The observed positive correlation between Ruminococcaceae abundance and CD4+ T cell counts is consistent with the previous findings that a higher abundance of the bacterial genera Faecalibacterium, Ruminococcus, and Akkermansia was associated with increased white blood cell counts and Ruminococcus enrichment was positively correlated with lymphocyte rates [30]. The study conducted in Uganda reported an altered abundance of Veillonellaceae in HIV-infected patients with CD4 count < 200 cells/μL and enrichment of Succinivibrionaceae in those with CD4 count > 200 cells/μL [1]. Members of the Succinivibrionaceae family with anti-inflammatory activity are associated with ART-related immune recovery, and the genus Succinivibrio was found to be more highly represented in elite controllers [21]. Conversely, the abundance of Veillonellaceae was increased in chronically ART-naïve individuals compared to those with HIV seronegative status [19]. Moreover, Veillonella abundance was correlated with elevated levels of pro-inflammatory cytokines [28]. This could explain our finding that CD4/CD8 ratio was negatively correlated with the relative abundance of Veillonellaceae and positively associated with that of Succinivibrionaceae. Thus, probiotics/prebiotics or fecal microbiota transplantation could potentially be used to restore intestinal homeostasis and promote immune reconstitution in HIV-infected INRs [6, 10].
Our study has some limitations. Firstly, it was a cross-sectional study based on a small number of Chinese subjects and there was no reliable and detailed information on AIDS events. Secondly, we did not recruit healthy control individuals with normal CD4+ T cell counts and immunological responders (IRs) with CD4+ T cell counts > 500 cells/μL for comparison. Thirdly, we did not exclude the effects of HIV transmission routes on gut microbiota community composition. The use of methamphetamine and cannabis was found to affect the gut microbiota profile of HIV/AIDS patients [28, 31, 32]. In addition, drug use increased HIV infection and viral replication in immune cells, which accelerated the progression of AIDS [33]. However, the patients in this study were successfully rehabilitated from drug abuse. Fourthly, the patient exclusion criteria did not include gender and sexual behavior; however, alterations in gut microbiota have been reported in HIV-infected patients regardless of sex and sexual activities [20]. Fifthly, Mutlu et al. found that the microbiota of mucosal samples in HIV-positive subjects has more profound changes [34]. However, considering the ethical issues and the difficulties in obtaining gut mucosal biopsies, we did not use gut mucosal specimens to study the microbiome. Finally, we did not examine differences in T cells phenotype/function between the CD4 count > 200 cells/μL group and the CD4 count < 200 cells/μL group because these data were unavailable. In the future, we intend to explore the microbiome of INRs from a functional standpoint, as restoring the function of gastrointestinal microbiota is a potential therapeutic strategy [10]. We will also measure metabolite levels in gut bacteria and plasma samples to identify potential biomarkers related to immune recovery [35, 36].
Increased α-diversity is generally considered to be a marker of health and while decreased diversity has been associated with a variety of disease states [32]. Our results show that if the CD4+ T cell counts of INRs are > 200 cells/μL, they are healthier. Although the previous study found that nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) regimen does reduce α-diversity [37], we excluded four patients receiving the combination of NRTIs and PIs in the patients with CD4 count < 200 cells/μL and found that difference in α-diversity was still observed between the two groups (unpublished data). In addidtion, the immune situation of INRs with CD4 count < 200 cells/μL may be worse than that of INRs with CD4 count > 200 cells/μL, but the previous study revealed that immunological responders (IRs) with CD4 count > 350 cells/μL had a higher CRP level yet not statistically significant than INRs with CD4 count < 350 cells/μL before the prebiotic intervention. More seriously, INRs with CD4 count < 350 cells/μL had a significantly higher CRP level after the prebiotic intervention [38]. Our results also indicated that INRs with CD4 count > 200 cells/μL had a higher CRP level yet not statistically significant. We proposed that the increased CRP level results from the excessive activated T cells [39]. Therefore, it is likely that there exists a positive correlation yet not significant between CRP and CD4+ T cell counts and CD4/CD8 ratio. Finally, We found that compared with subjects with CD4 count < 200 cells/μL, INRs with CD4 count > 200 cells/μL had less Enterobacteriaceae or Fusobacteriaceae. It is reasonable that Enterobacteriaceae and Fusobacteriaceae were positively related to TNF-α in HIV-infected INRs. However, in correlation analysis, TNF-α seems to be negatively correlated with Enterobacteriaceae and Fusobacteriaceae. Therefore, we speculate that there exists a false correlation between TNF-α and Enterobacteriaceae or Fusobacteriaceae in our study.
Conclusions
Our results indicate that compared to INRs with CD4 count > 200 cells/μL, patients with CD4 count < 200 cells/μL had a lower CD4/CD8 ratio, lower nutritional status, and higher levels of pro-inflammatory cytokines including TNF-α, IP-10, and IL-1α. Alpha diversity of gut microbiota differed significantly between the two groups, and the relative abundance of Ruminococcaceae was markedly reduced in patients with lower CD4+ T cell counts. The correlation analysis indicated that CD4+ T cell counts were negatively associated with serum TNF-α and IL-1α levels and positively correlated with the relative abundance of Ruminococcaceae. Our findings provide a rationale and guidance for the subsequent nutritional intervention targeting gut microbiota to promote immune reconstitution in HIV-infected INRs.
Availability of data and materials
The dataset used in the manuscript is available from the corresponding author on reasonable request.
References
Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, Lankowski A, Baldridge MT, Wilen CB, Flagg M, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19(3):311–22.
Zhou Y, Ou Z, Tang X, Xu H, Wang X, Li K, He J, Du Y, Wang H, Chen Y, et al. Alterations in the gut microbiota of patients with acquired immune deficiency syndrome. J Cell Mol Med. 2018;22(4):2263–71.
Rb-Silva R, Goios A, Kelly C, Teixeira P, Joao C, Horta A, Correia-Neves M. Definition of immunological nonresponse to antiretroviral therapy: a systematic review. J Acquir Immune Defic Syndr. 2019;82(5):452–61.
Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612.
Dirajlal-Fargo S, Yu J, Albar Z, Sattar A, Mahtab S, Jao J, Myer L, Zar HJ, McComsey GA. Monocyte activation and gut barrier dysfunction in South African youth on art and their associations with endothelial dysfunction. AIDS 2020;34(11):1615-23.
Geng S, Zhang Z, Wang Y, Lu D, Yu J, Zhang J, Kuang Y, Wang K. Regulation of gut microbiota on immune reconstitution in patients with acquired immunodeficiency syndrome. Front Microbiol. 2020;11:594820.
Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18.
Seang S, Somasunderam A, Nigalye M, Somsouk M, Schacker TW, Sanchez JL, Hunt PW, Utay NS, Lake JE. Circulating LOXL2 levels reflect severity of intestinal fibrosis and GALT CD4(+) T lymphocyte depletion in treated HIV infection. Pathog Immun. 2017;2(2):239–52.
Tincati C, Merlini E, Braidotti P, Ancona G, Savi F, Tosi D, Borghi E, Callegari ML, Mangiavillano B, Barassi A, et al. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically suppressive combination antiretroviral therapy. AIDS. 2016;30(7):991–1003.
Tincati C, Douek DC, Marchetti G. Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther. 2016;13:19.
Wang Y, Lifshitz L, Gellatly K, Vinton CL, Busman-Sahay K, McCauley S, Vangala P, Kim K, Derr A, Jaiswal S, et al. HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat Immunol. 2020;21(3):274–86.
d’Ettorre G, Rossi G, Scagnolari C, Andreotti M, Giustini N, Serafino S, Schietroma I, Scheri GC, Fard SN, Trinchieri V, et al. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis. 2017;5(3):244–60.
Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, Bandera A, Capetti A, Rizzardini G, Clerici M. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24(13):1991–2000.
Tincati C, Merlini E, d’Arminio Monforte A, Marchetti G. Is weak CD4+ gain in the course of suppressive combination antiretroviral therapy for HIV infection a current clinical challenge? A case report and brief review of the literature. BMC Infect Dis. 2018;18(1):8.
Zicari S, Sessa L, Cotugno N, Ruggiero A, Morrocchi E, Concato C, Rocca S, Zangari P, Manno EC, Palma P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses. 2019;11(3):200–18.
Lu W, Feng Y, Jing F, Han Y, Lyu N, Liu F, Li J, Song X, Xie J, Qiu Z, et al. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front Microbiol. 2018;9:1451.
Cook RR, Fulcher JA, Tobin NH, Li F, Lee D, Javanbakht M, Brookmeyer R, Shoptaw S, Bolan R, Aldrovandi GM, et al. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. AIDS. 2019;33(5):793–804.
Armstrong AJS, Shaffer M, Nusbacher NM, Griesmer C, Fiorillo S, Schneider JM, Preston Neff C, Li SX, Fontenot AP, Campbell T, et al. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome. 2018;6(1):198.
Zilberman-Schapira G, Zmora N, Itav S, Bashiardes S, Elinav H, Elinav E. The gut microbiome in human immunodeficiency virus infection. BMC Med. 2016;14(1):83.
Vujkovic-Cvijin I, Sortino O, Verheij E, Sklar J, Wit FW, Kootstra NA, Sellers B, Brenchley JM, Ananworanich J, Loeff MSV, et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun. 2020;11(1):2448.
Vesterbacka J, Rivera J, Noyan K, Parera M, Neogi U, Calle M, Paredes R, Sonnerborg A, Noguera-Julian M, Nowak P. Richer gut microbiota with distinct metabolic profile in HIV infected elite controllers. Sci Rep. 2017;7(1):6269.
Osuji FN, Onyenekwe CC, Ahaneku JE, Ukibe NR. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J Biomed Sci. 2018;25(1):88.
Musselwhite LW, Andrade BB, Ellenberg SS, Tierney A, Belaunzaran-Zamudio PF, Rupert A, Lederman MM, Sanne I, Sierra Madero JG, Sereti I. Vitamin D, D-dimer, Interferon gamma, and sCD14 levels are independently associated with immune reconstitution inflammatory syndrome: a prospective, International Study. EBioMedicine. 2016;4:115–23.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055.
Koethe JR, Heimburger DC, PrayGod G, Filteau S. From wasting to obesity: the contribution of nutritional status to immune activation in HIV infection. J Infect Dis. 2016;214(Suppl 2):S75-82.
Freeman ML, Shive CL, Nguyen TP, Younes SA, Panigrahi S, Lederman MM. Cytokines and T-Cell homeostasis in HIV infection. J Infect Dis. 2016;214(Suppl 2):S51-57.
Pastor L, Urrea V, Carrillo J, Parker E, Fuente-Soro L, Jairoce C, Mandomando I, Naniche D, Blanco J. Dynamics of CD4 and CD8 T-cell subsets and inflammatory biomarkers during early and chronic HIV infection in mozambican adults. Front Immunol. 2017;8:1928.
Cook RR, Fulcher JA, Tobin NH, Li F, Lee DJ, Woodward C, Javanbakht M, Brookmeyer R, Shoptaw S, Bolan R, et al. Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Sci Rep. 2019;9(1):14840.
Vujkovic-Cvijin I, Somsouk M. HIV and the gut microbiota: composition, consequences, and avenues for amelioration. Curr HIV/AIDS Rep. 2019;16(3):204–13.
Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;25(10):020–2971.
Fulcher JA, Hussain SK, Cook R, Li F, Tobin NH, Ragsdale A, Shoptaw S, Gorbach PM, Aldrovandi GM. Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J Infect Dis. 2018;218(10):1560–70.
Tuddenham S, Koay WL, Sears C. HIV, sexual orientation, and gut microbiome interactions. Dig Dis Sci. 2020;65(3):800–17.
Lu D, Sun H, Yu J, Kuang YQ, Wang KH. Chemsex drugs regulate HIV infection and replication in immune cells: a vicious circle. AIDS. 2021;35(1):147–50.
Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):1003829.
Serrano-Villar S, Rojo D, Martinez-Martinez M, Deusch S, Vazquez-Castellanos JF, Bargiela R, Sainz T, Vera M, Moreno S, Estrada V, et al. Gut bacteria metabolism impacts immune recovery in HIV-infected individuals. EBioMedicine. 2016;8:203–16.
Serrano-Villar S, Rojo D, Martinez-Martinez M, Deusch S, Vazquez-Castellanos JF, Sainz T, Vera M, Moreno S, Estrada V, Gosalbes MJ, et al. HIV infection results in metabolic alterations in the gut microbiota different from those induced by other diseases. Sci Rep. 2016;6:26192.
Villanueva-Millan MJ, Perez-Matute P, Recio-Fernandez E, Lezana Rosales JM, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc. 2017;20(1):21526.
Serrano-Villar S, Vazquez-Castellanos JF, Vallejo A, Latorre A, Sainz T, Ferrando-Martinez S, Rojo D, Martinez-Botas J, Del Romero J, Madrid N, et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017;10(5):1279–93.
Camargo JF, Pallikkuth S, Moroz I, Natori Y, Alcaide ML, Rodriguez A, Guerra G, Burke GW, Pahwa S. Pretransplant levels of C-reactive protein, soluble TNF receptor-1, and CD38+HLADR+ CD8 T cells predict risk of allograft rejection in HIV+ kidney transplant recipients. Kidney Int Rep. 2019;4(12):1705–16.
Acknowledgements
The authors gratefully acknowledge all patients who participated in this work. We thank Dr. Hui Luo and Dr. Yi-Man Dong at Second People’s Hospital of Dali City for their assistance in patient recruitment and sample collection. We also gratefully acknowledge Dr. Qing-Yan Peng, Mrs. Li-Mei Cao, and Mr. Yong-Jin Zhang at NHC Key Laboratory of Drug Addiction Medicine, First Affiliated Hospital of Kunming Medical University for their excellent technical assistance. We are grateful to Yunnan Province Postdoctoral Management Committee Office for their support in research work; LC-Bio Technologies Co., Ltd (Hangzhou, China) for 16S data analysis.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81660094 and 32060177); Fund of Yunling Scholar (No. YLXL20170002); Project for Innovation Team of Department of Science and Technology of Yunnan Province (No. 2018HC005); Fund of Yunnan Provincial Clinical Medical Center for General Surgical Diseases (No. ZX2019-03-03) from the Health Commission of Yunnan Province; Yunnan Provincial Clinical Research Center for Skin Immune Diseases (No. 2019ZF012) from Department of Science and Technology of Yunnan Province; and China Postdoctoral Science Foundation (No. 2019M663580).
Author information
Authors and Affiliations
Contributions
DL participated in study design, patient recruitment, statistical analysis, data interpretation, and manuscript drafting. JBZ, YX, YXW, TGS, ZZ, and SYL participated in patient recruitment and sample collection. YXW and STG performed genomic DNA extractions; KHW and YQK conceived the study and participated in study design, data interpretation, and manuscript revision. All authors read and approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This was approved by the Human Clinical Research Ethics Committee of the First Affiliated Hospital of Kunming Medical University (No. 2018L43). All participants included in this study provided written informed consent to participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Fig. S1. Beta diversity of gut microbiota in INRs. (A, B) Ordination of unweighted UniFrac distance between samples by PCoA (A) or NMDS (B). R andP values were derived from ANOSIM analyses of distance metrics. Fig. S2. Rectal microbial community composition of study participants. (A–C) A slight decrease in Bacteroidaceae (P = 0.3223) (A) and increase in Prevotellaceae (P = 0.7339) (B) and Prevotellaceae/Bacteroidaceae ratio (P = 0.4023) (C) were observed in the CD4 count < 200 cells/μL group compared to the CD4 count > 200 cells/μL group. The differences were statistically significant at a cut-off value of P < 0.05 (Mann-Whitney test).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, D., Zhang, JB., Wang, YX. et al. Association between CD4+ T cell counts and gut microbiota and serum cytokines levels in HIV-infected immunological non-responders. BMC Infect Dis 21, 742 (2021). https://doi.org/10.1186/s12879-021-06491-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06491-z