Abstract
Background
Coronavirus disease 2019 (COVID-19) is an infectious disease characterized by cough, fever, and fatigue and 20% of cases will develop into severe conditions resulting from acute lung injury with the manifestation of the acute respiratory distress syndrome (ARDS) that accounts for more than 50% of mortality. Currently, it has been reported that some comorbidities are linked with an increased rate of severity and mortality among COVID-19 patients. To assess the role of comorbidity in COVID-19 progression, we performed a systematic review with a meta-analysis on the relationship of COVID-19 severity with 8 different underlying diseases.
Methods
PubMed, Web of Science, and CNKI were searched for articles investigating the prevalence of comorbidities in severe and non-severe COVID-19 patients. A total of 41 studies comprising 12,526 patients were included.
Results
Prevalence of some commodities was lower than that in general population such as hypertension (19% vs 23.2%), diabetes (9% vs 10.9%), chronic kidney disease (CKD) (2% vs 9.5%), chronic liver diseases (CLD) (3% vs 24.8%) and chronic obstructive pulmonary disease (COPD) (3% vs 8.6%), while some others including cancer (1% vs 0.6%), cardiovascular disease (6% vs 1.8%) and cerebrovascular disease (2% vs 0.9%) exhibited greater percentage in COVID-19. Cerebrovascular disease (OR = 3.70, 95%CI 2.51–5.45) was found to be the strongest risk factor in disease exacerbation, followed by CKD (OR = 3.60, 95%CI 2.18–5.94), COPD (OR = 3.14, 95% CI 2.35–4.19), cardiovascular disease (OR = 2.76, 95% CI 2.18–3.49), malignancy (OR = 2.63, 95% CI 1.75–3.95), diabetes (OR = 2.49, 95% CI 2.10–2.96) and hypertension (OR = 2.13, 95% CI 1.81–2.51). We found no correlation between CLD and increased disease severity (OR = 1.32, 95% CI 0.96–1.82).
Conclusion
The impact of all eight underlying diseases on COVID-19 deterioration seemed to be higher in patients outside Hubei. Based on different comorbidities, COVID-19 patients tend to be at risk of developing poor outcomes to a varying degree. Thus, tailored infection prevention and monitoring and treatment strategies targeting these high-risk subgroups might improve prognosis during the COVID-19 pandemic.
Similar content being viewed by others
Background
From December of 2019 to March of 2020, an outbreak of pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel type of coronavirus sharing 79.5% genome identity with SARS-CoV, was first found in Wuhan, Hubei province, and spread rapidly to other cities and provinces across China. The World Health Organization (WHO) has officially named this coronavirus as the 2019 novel coronavirus (2019-nCoV) and the corresponding disease as COVID-19 [1]. Compared to previously described acute respiratory infectious diseases, SARS in 2003 and the Middle East Respiratory Syndrome (MERS) in 2012, COVID-19 shows lower mortality ranging from 0.7 to 4%, but enhanced transmission due to more than ten times higher affinity to common target ACE2, causing a rapid spread worldwide [2]. COVID-19 patients manifest different degrees of clinical symptoms as mild, moderate, severe, and critical illness. Many patients with COVID-19 initially present flu-like symptoms as fever (91.3%), dry cough (67.7%), and fatigue (51.0%), followed by dyspnea (30.4%) [3]. Most patients with these symptoms have a good prognosis, while only a small portion of patients will convert into severe or critical cases, rapidly developing lethal complications (such as acute respiratory distress syndrome, septic shock, and irreversible metabolic acidosis) and even death, especially for the elderly and those with underlying diseases [4]. Moreover, it is found that many comorbidities correlate with the severity of COVID-19. Thus, it is critical to identify the sub-population that is more susceptible to the development of adverse outcomes of COVID-19 and prevent the deterioration from mild and moderate conditions to the severe ones and reduce mortality. Furthermore, the assessment of the specific risk factor underlying different comorbidities is conducive to the special care of the targeted population.
Thus far, common concerns presented from recent studies regarding the prevalence of comorbidity in severe COVID-19 patients include limited sample size, isolated data of the one-center study, and inconsistent conclusions, making it difficult to have an overall awareness. Thus, it is necessary to carry out a meta-analysis to evaluate convincing outcomes of this issue. This study aims to provide systematic evaluation and detailed estimation of prevalence rates of different kinds of common comorbidities in severe and non-severe COVID-19 patients and tries to illustrate the outcomes by combination with underlying pathogenesis to better understand how basic diseases contribute to the aggravation of SARS-CoV-2 infection.
Method
The protocol of this systematic review and meta-analysis was registered at the International Prospective Register of Systematic Reviews (PROSPERO) and the registration number is CRD42020178826. This meta-analysis was performed under the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (An additional file shows this following the checklist in more detail [see Additional file 1]).
Search strategy
To identify all the studies illustrating the prevalence of comorbidities of SARS-CoV-2 infection in China, the international databases, including PubMed and Web of Science, and the Chinese database CNKI were searched for articles published till 18 January 2021. The search items and corresponding synonyms were combined with Boolean operators “AND” and “OR” as follows:
-
(1).
“COVID-19” or “2019-nCoV” or “novel coronavirus” or “SARS-CoV-2” or “Coronavirus”.
-
(2).
“comorbidities” or “characteristics” or “clinical features” or “underlying diseases” or “basic diseases” or “condition”.
-
(3).
“China” or “Chinese”.
-
(4).
(1) AND (2) AND (3)
Inclusion criteria
(1) Designed studies published online. (2) Studies contained well-recorded clinical characteristics and epidemiological information of patients diagnosed with COVID-19, including age, gender, symptoms, date of hospitalization, and comorbidities. (3) Participants in the study must be stratified into different groups according to severity or disease progression, in the form of either mild/moderate/severe/critical illness based on clinical symptoms or ICU/non-ICU based on admission care. Additionally, the basis of classification should be clearly and officially defined.
Exclusion criteria
(1) Non-human studies, case report, systematic reviews or abstract only; (2) Those with unavailable data or incomplete information collected from study subjects; (3) Repeated studies; (4) Pre-print articles; (5) Cases from foreign countries outside China; (6) Cases merely classified on survival and death.
Literature selection and data extraction
Two reviewers (Y.T. and L.Y.) searched and selected the studies individually. First, titles and abstracts were screened. Second, full-text articles of potential interest were screened to decide whether the literature should be included according to the inclusion and exclusion criteria. The information extracted from the literature includes authors, duration of clinical observation, the specific hospital that cases come from, sample size, gender, age, respective numbers of severe and non-severe cases with and without any comorbidity, and the proportion of 4 common clinical symptoms among patients, including fever, cough, myalgia and dyspnea. The above information of included articles was encoded into an Excel Spreadsheets according to different basic diseases. (An additional file shows the data in more detail [see Additional file 2]) Then the numbers of specific comorbidities appearing in severe and non-severe patients, including hypertension, cardiovascular disease, diabetes, COPD, malignancy, CKD, CLD, and cerebrovascular disease, were extracted from the identified studies.
The primary outcome of this systematic review and meta-analysis was the prevalence of comorbidities among all included COVID-19 patients no matter the severity and was calculated as the proportion of those with one of 8 widespread basic diseases among all confirmed anticipants. The secondary outcome was identifying and estimating the risk comorbidities posed on the progression of COVID-19. The measure of the secondary outcomes was to compare the prevalence of 8 types of specific comorbidities in severe and non-severe cases (mild and moderate clinical types were regarded as non-severe, while severe and critically-ill were considered as severe). If patients were not divided into four standard clinical types, then we extracted the ICU and non-ICU cases as severe and non-severe instead, respectively. For cohort studies, after short-term following up, regarded those experiencing disease progression or poorer outcomes as severe, and those still in normal condition or better outcomes as non-severe.
Data analysis and assessment of risk bias
All data analyses were performed by STATA 15, and article deduplication was done with Note-Express. Forest plots were used to visually illustrate the distribution of the outcome and effect size obtained from each included study, and to demonstrate the pooled prevalence and the effect on the severity of respective comorbidities in COVID-19 from the selected studies. The pooled prevalence and 95% confidence intervals (CI) were calculated for each of the selected comorbidities. The odd risk (OR) and 95% CI were adopted to describe the effect size of different underlying diseases on the development of severe cases. Statistical significance of the difference (the P-value of Z zone from overall test effect) was set as P < 0.05 [5]. Statistical heterogeneity within and between studies was evaluated by Cochrane’s Q test for presence and I2 statistics for extent. To avoid false-positive results, the heterogeneity was indicated to be significant when P ≤ 0.1 or I2 ≥ 50% was performed [6]. Random-effects model was applied to all analyses, regardless of heterogeneity.
To preliminarily assess whether these basic diseases increase the susceptibility to SARS-CoV-2 infection, we compared the prevalence of these comorbidities between COVID-19 patients and overall Chinese population by u-test which is intended to infer whether the unknown total rate π represented by the sample is different from the known total rate π0. Generally, when np ≥ 5 and n (1-p) ≥ 5 (n: the sample size, p: the sample rate), the distribution of the sample rate is similar to normal distribution, and the hypothesis test of the difference between the sample rate and the overall population rate can be tested by using the principle of normal distribution. In this analysis, π0 was extracted from the latest published authoritative data of the prevalence of different comorbidities across China, p was obtained from the result of this meta-analysis on prevalence, and n refers to the number of included cases as 12,526. Set α = 0.05 as threshold, if π > 1.96, the difference between pooled prevalence from meta-analysis and the overall prevalence in China is statistically significant and thus not caused by sampling error but COVID-19. (The detailed computational procedures and formulas of u-test are available in additional file [see Additional file 2]).
To further test the correlation of comorbidity with adverse outcomes and assess whether the impact of infected areas was associated with comorbidities to disease progression, subgroup analysis based on patient source was conducted. Studies were stratified into two subgroups according to the region of patient source, one group contained cases only from Hubei province, and the other focused on regions out of Hubei. Besides, subgroup analysis was used to test sensitivity by exploring potential sources of heterogeneity from any of the two subgroups. The risk of publication bias was assessed by Begg rank correlation test and Egger linear regression test, and P < 0.05 indicated that significant publication bias existed in the pooled analysis. When results from two tests contradicted with each other, then resort to the result of Egger’s test for reference.
Results
Characteristics of included studies
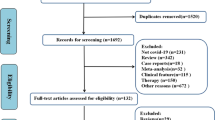
Initial searching retrieved a total of 7409 citations. After excluding duplicated and irrelevant studies, 399 potentially eligible studies left and were screened for full-text. Finally, we included 41 case series [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] for our literature review after excluding 318 records where necessary information was unavailable or no stratification based on severity among patients was displayed and 40 records which shared cases from the same hospitals with other included studies. The flow diagram of the search and study selection process is shown in Fig. 1. The general clinical characteristics of a total of 12,526 COVID-19 patients within 41 included studies are presented in Table 1. All 41 studies were focused on Chinese population and performed at different hospitals in China, among which 18 studies with a total of 6466 cases came from Wuhan and its adjacent cities within Hubei, the origin of COVID-19 outbreak in China, while others were conducted outside Hubei and covered 15 different provinces, including Beijing, Shanghai, Chongqing, Anhui, Hunan, Jiangsu, Jiangxi, Henan, Zhejiang, Guangxi, Guizhou, Guangdong, Liaoning, Jilin and Sichuan. All 41 included studies were further sorted by comorbidities, with 39 on hypertension, 39 on diabetes, 31 on COPD, 30 on cardiovascular diseases, 23 on CLD, 21 on CKD, 20 on malignancy and 16 on cerebrovascular diseases. Fever was the most prevalent symptom and cough the second, followed by myalgia or dyspnea. The age of infected patients was distributed around 50 years old, ranging from infants to very elderly people. The difference of COVID-19 infection between male and female patients was not distinct. As for comorbidity, the proportion of patients with one or more comorbidities in the severe group was noticeably higher than that in the non-severe.
Comorbidities in COVID-19 cases
Pooled estimation of prevalence of different comorbidities among COVID-19 patients are provided in Fig. 2 as forest plots and summarized in Table 2. The results showed that the comorbidities successively arrayed by proportion were hypertension (19, 95% CI 16–22%), diabetes (9, 95% CI 8–11%), cardiovascular diseases (6, 95% CI 4–7%), CLD (3, 95% CI 2–4%), COPD (3, 95% CI 2–4%), cerebrovascular diseases (2, 95% CI 1–2%), CKD (2, 95% CI 1–2%) and malignancy (1, 95% CI 1–2%). Based on Cochrane’s Q test, significant heterogeneity was observed in the estimates of all comorbidities, with I2 ranging from 37.8 to 93.9%. Results of u-test was listed in Table 3, the proportion of malignancy and cardiovascular and cerebrovascular diseases in COVID-19 patients was much higher than that in the general population [48, 49] (***, P < 0.001), while the prevalence of hypertension, diabetes, COPD, CLD and CKD were lower than that of the overall Chinese population [50,51,52,53] (***, P < 0.001).
The Meta-analysis of the prevalence of comorbidities in COVID-19 cases. Weight was calculated from the binary random-effects model analysis. The value represents the proportion of 8 diseases and 95% confidence intervals, respectively, together with heterogeneity analysis carried by Q-test and I2 index. Parts for each comorbidity are arrayed in the figure orderly as follows: hypertension, diabetes, cardiovascular disease, COPD, chronic kidney disease, chronic liver disease, cerebrovascular disease, malignancy
Evaluation for the impact of these comorbidities on COVID-19 severity was presented as forest plots in Fig. 3, showing that the proportion of all comorbidities in severe patients was significantly higher than that in non-severe patients, with the exception of CLD (OR = 1.32, 95% CI 0.96–1.82). Cerebrovascular disease had the highest OR value of 3.70 (95% CI 2.51–5.45), sequentially followed by CKD (OR = 3.60, 95% CI 2.18–5.94), COPD (OR = 3.14, 95% CI 2.35–4.19), cardiovascular disease (OR = 2.76, 95% CI 2.18–3.49), diabetes (OR = 2.49, 95% CI 2.10–2.96), malignancy (OR = 2.63, 95% CI 1.75–3.95) and hypertension (OR = 2.13, 95% CI 1.81–2.51). According to Cochran’s Q test result, apart from hypertension which had the P-value of 0.007 and CKD which had the P-value of 0.067 and thus was heterogeneous, no obvious heterogeneity (I2 ranged from 0 to 24.4%) existed among other comorbidities.
The risk of comorbidities in severe patients compared to Non-severe patients and subgroup analysis. Forest plots illustrate the comparison of the specific comorbidity’s prevalence in severe and non-severe patients, together with the heterogeneity analysis carried by the Q-test and I2 index. In these panels, the size of the diamonds reflects the sample size, and the whiskers extend to the lower and upper values of the 95% confidence interval (CI). Parts for each comorbidity are arrayed in the figure orderly as follows: hypertension, diabetes, cardiovascular disease, COPD, chronic kidney disease, chronic liver disease, cerebrovascular disease, malignancy.
The P values of Egger’s and Begg’s tests towards each comorbidity were above or approximate to 0.05, which appears to have little publication bias presented in each result. The detailed results of the tests for publication bias were summarized in Table 4 and the result interface from Stata15 can be found in the additional file [see Additional file 2]. Subgroup analysis based on patient source was conducted and presented within Fig. 3 as forest plots and the results were summarized in Table 5. In all comorbidities, the OR value of non-Hubei group was higher than that of Hubei group, and even doubled in some diseases, such as CKD (6.28 vs 2.95) and hypertension (3.36 vs 1.45).
Discussion
Compared with previous reports, the present systematic review and meta-analysis contained a relatively large sample of more than 12,000 cases covering 16 provinces in China and collected the most recent data (till January 2021) comparing subgroups of Hubei and non-Hubei to show the prevalence of 8 comorbidities and their impact on developing severity of COVID-19. On the whole, our results were in the line with previous studies [54,55,56]. Therefore, it is safe to draw that hypertension, diabetes, cardiovascular diseases are the most prevalent concomitant diseases in patients with COVID-19 [57].
When compared with the prevalence of these indicated morbidities in the general Chinese population, the pooled prevalence of cardio-cerebrovascular diseases and cancer were obviously higher in the hospitalized COVID-19 patients; on the contrary, that of COPD, CKD, and CLD were remarkably lower. Additionally, the estimated prevalence of hypertension and diabetes in the COVID-19 patients was not evidently different from that of the overall population, suggesting that such patients may not be more susceptible to SARS-CoV-2 infection. (Details see Table 3).
We have compared 4 recent meta-analyses [54, 55] [58, 59] on the correlation of various comorbidities with disease progression and all of them agreed that hypertension, cardiovascular disease, diabetes, and COPD are commonly associated with the poor outcomes in COVID-19. Three investigations have shown a significant association of CKD with severe COVID 19 cases [54, 55] [58]. According to a large multinational meta-analysis, CLD and CKD were predictors for severe COVID-19 with a similar power [55]. Besides, Singh A.K. et al. [54] reported that cancer (RR = 2.48, 95% CI 1.46–4.19) was significantly associated with a higher risk of severe COVID-19, compared to patients without comorbidities. However, Bolin Wang et al. [59] did not find the correlation between the above 3 comorbidities and aggravation of COVID-19. Few meta-analyses considered cerebrovascular disease independently, and some incorporated it with cardiovascular disease or overlooked it for a small sample size. Bolin Wang et al. [59] pointed that cerebrovascular disease was an important independent risk factor for COVID-19 (OR = 3.89), which is similar to the present meta-analysis. On the contrary, Singh A.K. et al. [54] indicated that cerebrovascular disease was not significantly associated with severe COVID-19 (RR = 1.73 95% CI 0.74–4.05).
The present study revealed that with the exception of CLD, other 7 underlying diseases exhibited statistically significant correlation with severe COVID-19, of which cerebrovascular disease was the strongest risk factor, followed by CKD and COPD. Our results are in general in line with previous reports although variations and even discrepancy exist in some of individual studies. This may be due to differences in size and sources of the sample included, different statistical methods used, outcome criteria, and ethnic background.
According to the estimated prevalence and the odds risk of developing severe outcomes, 8 comorbidities can be stratified into 3 groups. Firstly, pre-existing cardio-cerebrovascular diseases and malignancy may both impose higher susceptibility to SARS-CoV-2 infection and develop to severe cases, which alerts both intensive health care and such patients themselves during the outbreak. The prevalence of cardia-cerebrovascular diseases in patients with COVID-19 was much higher than that of general population, which may be explained by the long-term use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs), which upregulate ACE2, leading to an increase in contracting SARS-CoV-2 [60, 61].
In keeping with Li et al. [62], our results showed that patients with hypertension, cardio-cerebrovascular diseases, and diabetes had more than 2-fold, 3-fold and 2-fold increased risk of developing severe COVID-19, respectively. These diseases share some common features, including vascular endothelial injury, dysfunctional hemostatic system, and pro-inflammatory state or chronic inflammation [63]. These conditions usually appear with the overload of cytokines induced by viral infection and are conducive to cytokine storms, leading to critical illness [64]. According to pathological analysis, most of the deaths are attributed to cytokine storm-triggered multiple Organ Failure (MOF) and Acute Respiratory Distress Syndrome (ARDS) [65]. On the other hand, COVID-19 patients with concurrent cardiovascular diseases such as arrhythmia and atherosclerosis can increase the likelihood of thromboembolic events, which may further contribute to fatal cerebral ischemia and acute stroke [60, 66]. And this trend could be accelerated by pre-existing compromised cerebral vasculature, hypercoagulability, and vessel inflammation in patients with cerebrovascular diseases. Other critical complications induced by the hypercoagulable state included systemic sepsis and micro-thrombosis formation in pulmonary blood vessels, which implies the possibility of life-threatening pulmonary embolism [60]. Although the pathophysiology behind may be overlapped, it is worth noting that the risk of developing severe outcomes where cerebrovascular and cardiovascular diseases pose on COVID-19 patients are mutually independent, and seem not affected by the presence of hypertension or diabetes [60].
Secondly, the overall pooled prevalence of cancer in COVID-19 infections was higher as compared to the overall population in China (0.6%), ranging from 1 to 3.9% [54, 56] [67, 68], within which our results fall (1, 95% CI 1–2%). This may be associated with the suppressed immunity that diminishes the ability of host surveillance. In addition, frequent hospital visits or hospitalization of cancer patients might increase the risk of contracting SARS-CoV-2. The study of Liang et al. [67] reported that patients with cancer had a higher risk of severity than non-cancer patients, possibly due to suppressed immune response as a result of chemo- and radio-therapies.
Thirdly, patients with COPD and CKD appear to have low risk of contracting SARS-CoV2 but tend to become severe once infected. Given the prominent pulmonary manifestations in COVID-19 patients and abundant ACE2 in the lung, it is reasonable to conceive severe outcomes of COVID-19 with the underlying COPD. However, published and this meta-analysis revealed the low prevalence of COPD in COVID-19 patients [69, 70]. One plausible explanation is that the long-term use of bronchodilators for the COPD patients, such as inhaled steroids, beta-agonists, or anticholinergics [71], could inhibit viral replication partly by lowering coronavirus receptor expression, suppressing the function of acidic endosomes, and modulating inflammation induced by infection in the airway, thus reducing the susceptibility to infection [72]. However, the patients with COPD are more vulnerable to the development of severe and critical conditions. A gene profile study has recently demonstrated that ACE-2 expression is significantly elevated on bronchial epithelial cells in the COPD patients, as compared to control subjects, which might contribute to exacerbated progression [73]. Additionally, it has been documented that COPD can lead to systemic hypoxia, which is sensitive to the induction of cytokine storms [74].
We did not find significant correlation between CLD and the risk of COVID-19 infection. It is well-known that there is a considerable burden of CLD (> 24.8%) in Chinese population. The reason for the low prevalence of CLD in Chinese COVID-19 patients is unclear. Two independent multi-national meta-analyses with 38,000 [55] and 24,299 [75] cases showed that that worldwide patients with CLD were more inclined to develop severe outcomes of COVID-19 than those without.
Conclusion
Currently, the reliability of results from most clinical studies is limited by small sample size and isolated source of cases. Few systemic reviews shed light on the comprehensive perspective of various underlying diseases. In this meta-analysis, we analyzed eight different common comorbidities about their prevalence among COVID-19 patients and found that COPD, cardio-cerebrovascular diseases, diabetes, hypertension, and CKD were significant risk factors in development to severe and critical cases. As the global population with comorbidity is rapidly growing and respiratory infections like COVID-19 seriously threaten public health, our results alert both patients and medical workers about the impact of various comorbidities on SARS-CoV-2 infection and deterioration of pneumonia and highlight the necessity of tailored therapy and monitoring those with basic diseases to avoid further cost and deaths.
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript and its supplementary information files.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- MERS:
-
Middle East Respiratory Syndrome
- COPD:
-
Chronic obstructive pulmonary disease
- CLD:
-
Chronic liver disease
- CKD:
-
Chronic kidney disease
- MOF:
-
Multiple organ failure
- ARDS:
-
Acute respiratory distress syndrome
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ARBs:
-
Angiotensin receptor blockers
References
Guo YR, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- An update on the status. Mil Med Res. 2020;7(1):11.
Wrapp D, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.
Yang J, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5.
Guan WJ, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547.
Danat IM, et al. Impacts of overweight and obesity in older age on the risk of dementia: a systematic literature review and a meta-analysis. J Alzheimers Dis. 2019;70:s87–99.
Liang Y, et al. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocr J. 2019;66:51–63.
Cheng K, Wei M, Shen H. Clinical characteristics of 463 patients with common and severe type coronavirus disease 2019. Shanghai Med J. 2020;1:1–15.
Fang XW, et al. Clinical characteristics and treatment strategies of 79 patients with COVID-19. Chin Pharmacol Bull. 2020;36:453–9.
Li D, Long Y, Huang P, Guo W, Wu S, Zhou Q. Clinical characteristics of 80 patients with COVID-19 in Zhuzhou City. Chin J Infect Control. 2020;19:227–33.
Li K, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig Radiol. 2020;55:327–31.
Sun L, Shen L, Fan J, Gu F, Hu M, An Y, Zhou Q, Fan H, Bi J. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing. China J Med Virol. 2020;92(10):2055–66.
Xiao KH, et al. The clinical features of the 143 patients with COVID-19 in north-east of Chongqing. J Third Mil Med Univ. 2020;42(06):549–54.
Yuan J, et al. Analysis of clinical characteristics of 223 patients with new coronavirus pneumonia in Chongqing (Chinese). J Southwest Univ (Natural Science Edition). 2020;42(03):17–24.
Zhang G, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan. China J Clin Virol. 2020;127:104364.
Zhang JJ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–41.
Zhao C, et al. Comparison of CT imaging and clinical features between common and severe/critical type of COVID-19 patients (Chinese). Inter J Med Radiol. 2020;43(03):257–61.
Zhao XY, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:311.
Zheng Y, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020;127:104366.
Zhang S, Liu L, Yang B, Li R, Luo J, Huang J, Long Y, Huang Y, Zhou J, Zha Y, Zhang X. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir Res. 2020;21(1):314.
Wei Y, Zeng W, Huang X, Li J, Qiu X, Li H, Liu D, He Z, Yao W, Huang P, Li C, Zhu M, Zhong C, Zhu X, Liu J. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis. 2020;20(1):549.
Fang H, Liu Q, Xi M, Xiong D, He J, Luo P, Li Z. Impact of comorbidities on clinical prognosis in 1280 patients with different types of COVID-19. J Investig Med. 2021;69(1):75–85.
Yang XH, Xiong R, Hu SL, et al. Clinical characteristics, antibody and nucleic acid test results in COVID-19 patients with different clinical type. Pract J Cardiac Cerebral Pneumal Vasc Dis. 2020;28(9):10–5.
Ren MX, et al. Analysis of clinical characteristics and prognostic factors of severe COVID-19 Beijing. Med J. 2020;10:920–4.
Analysis of clinical and epidemiological characteristics for 92 cases of COVID-19. Prog in Microbiol Immunol. 2020;48(05):39–44.
Chunyong Y, Yangfan L, Yuanju J. Clinical characteristics and chest computed tomographic findings: analysis of 66 novel coronavirus pneumonia cases. J Capital Med Univ. 2020;41(06):997–1001.
LvYaodong et al. Gansu Medical Journal. 2020; 39(03): 247250.
fang C, et al. Clinical features of ordinary and severe COVID-19 cases. Prev Med. 2020;32(09):886–94.
Zhang SY, Lian JS, Hu JH, Zhang XL, Lu YF, Cai H, Gu JQ, Ye CY, Jin CL, Yu GD, Jia HY, Zhang YM, Sheng JF, Li LJ, Yang YD. Clinical characteristics of different subtypes and risk factors for the severity of illness in patients with COVID-19 in Zhejiang. China Infect Dis Poverty. 2020;9(1):85.
Lv Z, Lv S. Clinical characteristics and analysis of risk factors for disease progression of COVID-19: a retrospective cohort study. Int J Biol Sci. 2021;17(1):1–7.
Feng Z, Li J, Yao S, Yu Q, Zhou W, Mao X, Li H, Kang W, Ouyang X, Mei J, Zeng Q, Liu J, Ma X, Rong P, Wang W. Clinical factors associated with progression and prolonged viral shedding in COVID-19 patients: a multicenter study. Aging Dis. 2020;11(5):1069–81.
Zhao C, Bai Y, Wang C, Zhong Y, Lu N, Tian L, Cai F, Jin R. Risk factors related to the severity of COVID-19 in Wuhan. Int J Med Sci. 2021;18(1):120–7.
Sheng L, Wang X, Tang N, Meng F, Huang L, Li D. Clinical characteristics of moderate and severe cases with COVID-19 in Wuhan, China: a retrospective study. Clin Exp Med. 2020;1:1–5. https://doi.org/10.1007/s10238-020-00662-z.
Nie Y, Li J, Huang X, Guo W, Zhang X, Ma Y, Wang H, Qi M, Tang X, Shen X, Dai X. Epidemiological and clinical characteristics of 671 COVID-19 patients in Henan Province, China. Int J Epidemiol. 2020;49(4):1085–95.
Chen SL, Feng HY, Xu H, Huang SS, Sun JF, Zhou L, He JL, Song WL, Wang RJ, Li X, Fang M. Patterns of Deterioration in Moderate Patients With COVID-19 From Jan 2020 to Mar 2020: A Multi-Center, Retrospective Cohort Study in China. Front Med (Lausanne). 2020;7:567296.
Cheng B, Hu J, Zuo X, Chen J, Li X, Chen Y, Yang G, Shi X, Deng A. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26(10):1400–5.
Liu S, Luo H, Wang Y, Cuevas LE, Wang D, Ju S, Yang Y. Clinical characteristics and risk factors of patients with severe COVID-19 in Jiangsu province, China: a retrospective multicentre cohort study. BMC Infect Dis. 2020;20(1):584.
Yu X, Sun X, Cui P, Pan H, Lin S, Han R, Jiang C, Fang Q, Kong D, Zhu Y, Zheng Y, Gong X, Xiao W, Mao S, Jin B, Wu H, Fu C. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai. China Transbound Emerg Dis. 2020;67(4):1697–707.
Yanpei ZhangZhang Y, Xiao LS, Li P, Zhu H, Hu C, Zhang WF, Sun QC, Shen MY, Liu SS, Zhang WL, Zeng HY, Gong M, Liu L, He YL, Zhu H. Clinical Characteristics of Patients With Progressive and Non-progressive Coronavirus Disease 2019: Evidence From 365 Hospitalised Patients in Honghu and Nanchang, China. Front Med (Lausanne). 2020;7:556818.
Yuan CenCen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, Jiang WR, Xu HT, Chen Y, Zhu J, Zhang LL, Liu YH. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-Centre observational study. Clin Microbiol Infect. 2020;26(9):1242–7.
Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, Wei J, Gong Z, Zhou C, Yu H, Yu M, Lei H, Cheng F, Zhang B, Xu Y, Wang G, Dong W. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan. China Clin Microbiol Infect. 2020;26(6):767–72.
Zhang Q, Xu Q, Chen YY, Lou LX, Che LH, Li XH, Sun LY, Bao WG, Du N. Clinical characteristics of 41 patients with pneumonia due to 2019 novel coronavirus disease (COVID-19) in Jilin. China BMC Infect Dis. 2020;20(1):961.
Wang JB, Wang HT, Wang LS, Li LP, Xv J, Xv C, Li XH, Wu YH, Liu HY, Li BJ, Yu H, Tian X, Zhang ZY, Wang Y, Zhao R, Liu JY, Wang W, Gu Y. Epidemiological and clinical characteristics of fifty-six cases of COVID-19 in Liaoning Province, China. World J Clin Cases. 2020;8(21):5188–202.
Zhang N, Zhang H, Tang Y, Zhang H, Ma A, Xu F, Sun Y, Jiang L, Shan F. Risk factors for illness severity in patients with COVID-19 pneumonia: a prospective cohort study. Int J Med Sci. 2021;18(4):921–8.
Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, Bai X, Ding M, Liu W, Liu K, Chu Y. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390–9.
Shu Z, Zhou Y, Chang K, Liu J, Min X, Zhang Q, Sun J, Xiong Y, Zou Q, Zheng Q, Ji J, Poon J, Liu B, Zhou X, Li X. Clinical features and the traditional Chinese medicine therapeutic characteristics of 293 COVID-19 inpatient cases. Front Med. 2020;14(6):760–75.
Huang H, Song B, Xu Z, Jiao Y, Huang L, Zhao P, Huang J, Zhou Z, Zhao Z, Tian J, Zhou Y, Wang FS, Jiang T. Predictors of coronavirus disease 2019 severity: a retrospective study of 64 cases. Jpn J Infect Dis. 2021;74(1):54–60.
Wang CZ, Hu SL, Wang L, Li M, Li HT. Early risk factors of the exacerbation of coronavirus disease 2019 pneumonia. J Med Virol. 2020;92(11):2593–9.
Ma L-Y, et al. China cardiovascular diseases report 2018: an updated summary. J Geriatr Cardiol. 2020;17:1–8.
World Health Organization. Globocan 2018. https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. Accessed 18 Nov 2020.
Wang L, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA - J. Am Med Assoc. 2017;317(24):2515–23.
Wang C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–17.
Xiao J, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71:212–21.
Bikbov B, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–33.
Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–24.
Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191.
Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35.
Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J, Altaf M. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clin Med. 2020;1:1–8.
Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, Sharma M, Jain A, Warikoo V. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetol Metab Syndr. 2020;14(5):1017–25.
Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(7):6049–57.
Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems: what do we know so far? J Am Heart Assoc. 2020;9(13):e016793.
Tadic M, Cuspidi C, Grassi G, Mancia G. COVID-19 and arterial hypertension: hypothesis or evidence? J Clin Hypertens (Greenwich). 2020;22(7):1120–6.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8.
Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–33.
Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheum. 2017;69(6):1135–43.
Xu Z, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Goldberg MF, Goldberg MF, Cerejo R, Tayal AH. Cerebrovascular disease in COVID-19. AJNR Am J Neuroradiol. 2020;41(7):1170–2.
Liang W, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7.
Salunke AA, Nandy K, Pathak SK, Shah J, Kamani M, Kottakota V, Thivari P, Pandey A, Patel K, Rathod P, Bhatt S, Dave P, Pandya S. Impact of COVID −19 in cancer patients on severity of disease and fatal outcomes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2020;14(5):1431–7.
Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, Quaderi S, Mandal S, Hurst JR. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147.
Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941.
Gómez Antúnez M, Muiño Míguez A, Bendala Estrada AD, de la Calle G M, Monge Monge D, Boixeda R, Ena J, Mella Pérez C, Anton Santos JM, Lumbreras Bermejo C. SEMI-COVID-19 Network. Clinical Characteristics and Prognosis of COPD Patients Hospitalized with SARS-CoV-2. Int J Chron Obstruct Pulmon Dis. 2021;15:3433–45.
Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, Shimotai Y, Momma H, Ichinose M, Kawase T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–68.
Leung JM, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688.
Guo X, et al. Nuclear translocation of HIF-1α induced by influenza a (H1N1) infection is critical to the production of proinflammatory cytokines. Emerg Microb Infect. 2017;6(5):e39.
Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):612–20.
Acknowledgments
We are thankful to Professor Peng Huang for his constructive suggestions.
Funding
This work was supported by the National Natural Science Foundation of China (81572753 to ZL, 81560299 to YY) and Innovation and Entrepreneurship grant from Jiangxi Province Bureau of Foreign Experts (to ZL).
Author information
Authors and Affiliations
Contributions
Tingxuan Yin, performed the literature search, study design, data collection and analysis, prepared figures and tables, and wrote the draft; Yuanjun Li, participated in writing and editing; Ying Ying, participated in writing, formatting references; Zhijun Luo, performed study design and data interpretation and wrote the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Study design and performance according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Additional file 2.
Primary data extracted from included studies and sorted based on different comorbidities, computational process of u-test and the result pages of begg’s test and egger’s test in Stata15.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yin, T., Li, Y., Ying, Y. et al. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis 21, 200 (2021). https://doi.org/10.1186/s12879-021-05915-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-05915-0