Abstract
Background
Informed decision making is underlined by all tiers in the health system. Poor data record system coupled with under- (over)-reporting of malaria cases affects the country’s malaria elimination activities. Thus, malaria data at health facilities and health offices are important particularly to monitor and evaluate the elimination progresses. This study was intended to assess overall reported malaria cases, reporting quality, spatiotemporal trends and factors associated in Gedeo zone, South Ethiopia.
Methods
Past 8 years retrospective data stored in 17 health centers and 5 district health offices in Gedeo Zone, South Ethiopia were extracted. Malaria cases data at each health center with sociodemographic information, between January 2012 and December 2019, were included. Meteorological data were obtained from the national meteorology agency of Ethiopia. The data were analyzed using Stata 13.
Results
A total of 485,414 suspected cases were examined for malaria during the previous 8 years at health centers. Of these suspects, 57,228 (11.79%) were confirmed malaria cases with an overall decline during the 8-year period. We noted that 3758 suspected cases and 467 confirmed malaria cases were not captured at the health offices. Based on the health centers records, the proportions of Plasmodium falciparum (49.74%) and P. vivax (47.59%) infection were nearly equivalent (p = 0.795). The former was higher at low altitudes while the latter was higher at higher altitudes. The over 15 years of age group accounted for 11.47% of confirmed malaria cases (p < 0.001). There was high spatiotemporal variation: the highest case record was during Belg (12.52%) and in Dilla town (18,150, 13.17%, p < 0.001) which is located at low altitude. Monthly rainfall and minimum temperature exhibited strong associations with confirmed malaria cases.
Conclusion
A notable overall decline in malaria cases was observed during the eight-year period. Both P. falciparum and P. vivax were found at equivalent endemicity level; hence control measures should continue targeting both species. The noticed under reporting, the high malaria burden in urban settings, low altitudes and Belg season need spatiotemporal consideration by the elimination program.
Similar content being viewed by others
Background
In Ethiopia, Plasmodium falciparum (P. falciparum) and P. vivax are the predominant causative species of malaria. The two coexist in almost all malarious areas at different levels of co-endemicity. Overall, large proportion of infections reported is due to P. falciparum (~ 60%) followed by P. vivax (~ 40%) [1, 2] with micro-epidemiological and seasonal variation. Such co-endemicity makes malaria control and elimination more complicated in Ethiopia than in most other areas where the later species is absent or very low [2, 3].
Malaria transmission in Ethiopia is seasonal associated with precipitation and temperature changes; peaking from September to December following the large rainy season from June to August in most parts of the country [4]. This rainfall pattern doesn’t include the southern and south-eastern parts of the country, which have a bimodal rainfall periods with long rainy season from March to May and short period from September to October [5]. However, construction of dams and irrigation-based agricultural activities sometimes modify malaria seasonal trend in Ethiopia [6, 7].
Human mobility/displacement is another contributing factor to the resurgence/distribution of infectious diseases such as malaria [8]. As some other parts of the country, there are also internal displacements in the study area. Among the possible reasons for displacements in the area include seasonal agricultural work especially for coffee cultivation and inter-communal conflict along the borders. Such human activities might have implication on the malaria distribution or reporting system and influence on services rendered at health facilities (or on the health system) in the area.
The past decades witnessed a sharp decline in morbidity and mortality, putting Ethiopia among the few African countries on track to meet the global 2020 milestone of cutting incidence by 40% or more [9]. These successes, enabled the Ethiopian national malaria control program (NMCP) to stratify the country’s malaria transmission into four based on annual parasite incidence (API); malaria free (API ~ 0 cases/1000 population/year), low (API > 0 and < 5), moderate (API ≥ 5 and < 100) and high (API ≥ 100) [4], as a preparation to embark on nationwide malaria elimination. The policy and strategy shift to elimination requires data-driven decision making to tailor interventions [10, 11]. Thus, policy makers should be provided timely with quality and relevant data to inform national programs.
In Ethiopia, malaria data is captured through the Public Health Emergency Management (PHEM) at different tiers of the healthcare delivery systems. The hierarchy of data flow is from health posts (HPs) and health centers (HCs) to district health offices (HOs) which in turn channels to Zonal Health Departments, then to Regional Health Bureaus and finally to the Federal Ministry of Health [12]. As malaria in Ethiopia is a weekly reportable disease, HCs are expected to report to the district HO gets registered through the PHEM system. Therefore, HCs data that are organized and archived at respective district HOs are the ones that are analyzed to evaluate the spatial and temporal changes, local malaria dynamics and Plasmodia species distribution.
Both internal and external data quality control system were there at the HCs level in the study setting. The internal quality control system was done by using different readings and smear preparations. External microscopy quality assessment was employed through sending the randomly selected, both positive and negative, slides to the regional reference laboratory.
Although the overall malaria trend could help to evaluate the progress of elimination activities, under- (over)-reporting of cases could pause a serious repercussion on the country’s elimination efforts. Yet, validation studies comparing data from the different tiers of the health care delivery system hardly exist in most settings and at micro-epidemiological level. Although the six malarious districts of Gedeo Zone are stratified as elimination targeted low transmission districts by NMCP [4], little information is available to understand the overall trend of malaria and the above issues in the area. Thus, we assessed the overall trend of malaria, species composition, malaria data quality, spatiotemporal distribution and associated socio-demographic and climatic variables. Further, the accuracy of HO malaria records (PHEM data) was checked against the HC data (source document).
Methods
Study setting
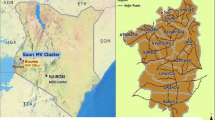
Gedeo zone (Fig. 1) is 360 km from Addis Ababa, it is one of the 14 zones in the Southern Nations, Nationalities and Peoples’ Region. It is located 5°53′N to 6°27′N latitude, and 38°8′ to 38°30′ east longitude. The altitude of the zone ranges from 1268 to 2993 m above sea level (masl). The mean annual temperature is between 12.6 °C and 30 °C and the mean annual rainfall ranges from 1001 to 1800 mm.
Map of the study districts; Gedeo Zone Southern Nationals Nationalities and Peoples Regional state (SNNPR), Ethiopia. Map produced by the authors using Arc-GIS Desktop v10.5 (Esri, Redlands CA, USA; https://www.redlands.edu/study/schools-and-centers/css/resources/arcgis-desktop/)
Based on the Gedeo Zone health department report, 36.31% (423,411/1,166,163) of the population is at risk of malaria. Malaria transmission in the zone is seasonal with peak from September to November. The API of the zone in 2019 was close to 2.0. The public health facilities in the area include; 1 referral hospital, 3 primary hospitals, 35 HCs and 146 HPs. The zone is sub-divide into six districts and two town administrations [13]. Districts, also known as “woredas” in Ethiopia, are the third level administrative divisions of the country, following regional states and zones and are further sub-divided into “kebeles” (the smallest administrative unit with its own jurisdiction). Six elimination-targeted settings, low transmission, by the NMCP; Dilla and Yirgacheffe towns, and Dilla zuria, Wonago, Kochore and Yirgacheffe rural districts were included [4]. Based on their altitudinal location, the study districts are within low altitude (< 1750 masl) and high altitude (1750–2500 masl) areas.
There are 3 categories of seasons in Ethiopia in general and Gedeo Zone in particular based on seasonal rainfall cycle. These are locally known as Belg (extends from February to May), Kiremt (June to September) and Bega (from October to January). Belg is the main rainy season for the south and southeast parts of Ethiopia including the current study area. But it is the minor rainy season in most parts of the country. Kiremt is the major rainy season across much of the nation except south and southeast of the country. It is the short rainy season in the area. Whereas, Bega (the dry-spell) is characterized by sunny, dry and cold weather condition with occasional falls prevailed over much of the country [5, 14]. As the present study area lies at the southern portions of Ethiopia, it benefited from a bimodal rainfall season with long periods during Belg and short rains from September to October [5].
Data collection
Retrospective record review of malaria data from district HOs and HCs over eight-year period (January 2012–December 2019) in Gedeo zone, South Ethiopia, were extracted. Malaria laboratory registration logbooks in the HCs and PHEM of the district HOs were used to extract the data. HCs found in malarious districts with at least 8 years of services and HOs in these districts were considered as eligible for the study. Accordingly, six district HOs and 17 public HCs were covered. HC records with missing information of cases, address (kebele/district), dates of HC visit, age, sex or results of malaria diagnosis were excluded from the main part analysis. These excluded data were again analyzed separately to address the data quality issues at HCs. Data on malaria diagnosis results (negative, or positive, and infecting Plasmodium species for positives), time of diagnoses (date/month/year) and socio-demographic data (kebele/district, age and sex) were collected. Microscopy was used as confirmatory diagnostic tests in all HCs. In addition, 8-year meteorological data; station level monthly and annual precipitation (in mm), maximum and minimum temperatures (in oC) and relative humidity (percent) was obtained from the national meteorology agency (NMA) of Ethiopia. The altitude records of each district were also collected using single hand-held Global Positioning System (GPS) (GPSMAP 64 s, 3BP329982). Data collectors attended adequate training to assure quality. Further, the consistency and completeness of the extracted data was checked for each HC and district. The completeness of data was checked by inspecting whether the completeness of variables for analysis, such as Plasmodia spp. case record, date, age, sex and address, from the captured data. The data is considered as complete when all variables needed for analysis are recorded. The consistency was again checked by whether the availability/matching of important variables and examination results at time (dates/months/years) and place (districts/HCs).
Data analysis
Microsoft office excel worksheet 2019 and the Stata data software 13 (College Station, Texas 77845 USA) were used for data entry and analysis. Descriptive statistics was used to show the distribution of malaria cases with respect to months, years, sex, age, Plasmodia species and district. The proportion of P. falciparum and P. vivax was expressed as the total number of each species per total number of confirmed malaria cases. Pearson’s chi-square (X2) test was used to assess the data quality issues in terms of missing data (data incompleteness), consistency and diagnostic performance issues. Multivariate logistic regression was also performed to assess the association of malaria cases with socio-demographic variables, altitude and districts. Odds ratio (OR) with the corresponding 95% confidence interval (CI) was used to assess the differences in malaria prevalence with selected predictors. The generalized linear model (GLM) was used to investigate the association of monthly malaria cases (as response variable) with seasonal and meteorological data (as predictor variables). P-value of less than 0.05 was taken as statistically significant.
Results
Annual suspected and confirmed malaria cases based on HCs versus HOs records
Overall, a notable decline in malaria, HCs (source data), was observed during the eight-year period except in 2016 and between 2018 and 2019. The number of confirmed malaria cases declined from 11,607 in 2012 to 2857 in 2019, an 8.34% reduction from the baseline. Maximum and minimum numbers of confirmed cases were documented during 2013 and 2018 respectively. The case burden due to P. falciparum (49.74%) was comparable to P. vivax (47.59%) (p = 0.795). Yet, P. vivax overtook P. falciparum in cases burden during 2013, 2014 and 2017. Mixed species infections, P. vivax and P. falciparum, accounted a low proportion (2.67%) (Table 1).
Over the 8-years period (2012–2019), there is an evidence of statistically significant inconsistency (p = 0.041) in both clinical and confirmed malaria case reports between HCs and HOs, reporting different figures. Higher suspected cases (485,414) were recorded at HCs compared to the HOs (481,656). Similarly, the corresponding confirmed malaria cases were 57,228 (11.79%) at the HCs and 56,761 (11.78%) at HOs although establishing which one is more accurate is rather not easy. With this, the number of suspected cases recorded by the HCs was higher by 3758. The data kept by the HOs was lower on average by about 470 each year (except 2012, 2018). This difference was pronounced in 2016. During 2012 and 2018 the numbers of suspected cases recorded at HCs were consistent with HOs data. In addition, the PHEM captured on average 58 less confirmed malaria cases each year except in 2012 and 2018. In 2012 and 2018 the numbers of confirmed cases were higher at HOs records than at HCs records (Table 1).
Year-based data with missing variables at HCs
In respective order 1852 (0.38%) and 249 (0.43%) of suspected cases and confirmed malaria cases were excluded from the downstream analysis due to incompleteness. Overall, there was a significant reduction in missing data between 2012 and 2019 (p = 0.001). During the first 2 years there were more numbers of both suspected and confirmed cases of missing variables. Specifically, the highest data with missing variables (413 suspected and 76 confirmed cases) occurred in 2012. Whereas, the lowest records of missing variables with 107 suspected and 10 confirmed cases were reported in 2019 and 2018 respectively. Generally, the number of suspected cases with missing variable declined from 413 in 2012 to 107 in 2019 by approximately 4-fold and confirmed cases from 76 in 2012 to 11 by 7-fold in 2019 (Table 2).
Diagnostic performance; data from HCs
Regarding the diagnostic performance, the yearly trend of suspected cases was directly proportional to the confirmed cases in each year. But the proportions of examined suspected cases against confirmed cases were significantly increased from 6.91 in 2012 to 16.32 in 2019 (p = 0.015). The highest proportion (16.32) was recorded in 2019, whilst the lowest (5.59) was during 2013. This shows the proportion of ‘non-malarial’ febrile cases were increasing from 2012 to 2019 except in 2016 (Table 3).
Malaria cases number by sex and age; data from HCs
Slightly more males (29,480 (11.34%)) were malaria positive (p > 0.05) than females (27,748 (12.30%)) (Fig. 2). The above 15 years age group was the most affected (30,406, 11.47%) than the other age groups, followed by under 5 children which accounted for 15,116 (13.83%) of the cases. The above 15 years age group was twice more likely (AOR = 2.00, 95% CI: 1.90, 2.11, p < 0.001) to have malaria compared to the under 5 (Table 4).
Spatiotemporal distribution of malaria cases
Overall confirmed malaria cases were significantly influenced by altitude, the higher in districts of < 1750 masl (53.71%). People residing in districts < 1750 masl were 1.2 times more likely to be infected with malaria as compared to those living in districts between 1750 and 2500 masl (AOR = 1.22, 95% CI: 1.12, 1.29). Plasmodium vivax (13656) was relatively higher at higher altitudes (1750–2500 masl) while P. falciparum (16013) was higher at the lower altitudes (< 1750 masl). But elevation and species distribution showed no statistically significant associations (Fig. 3a).
The peak confirmed case load (12.52%) was during Belg followed by Kiremt (11.28%) and Bega (11.55%). In Belg, the peak transmission season in the study area, the likelihood of having malaria is 1.28 times more than Bega (RR = 1.28, 95% CI: 1.09, 1.48). Similarly, the highest proportions of both P. falciparum (10,545 (50.14%)) and P. vivax (9924 (47.19%)) were noted during Belg particularly in April. On the other hand, in Bega the proportion of infection due to the two species, P. falciparum (8519, 49.45%) and P. vivax (8287, 48.11%) was relatively lowest. Although P. falciparum was higher than P. vivax in all seasons, the difference was the smallest (1.35%) during Bega, while in Belg it is 2.95%. The number of mixed infections also had same pattern throughout the three seasons (Fig. 3b).
Overall, based on the HCs records, the highest malaria case burden reported was from Dilla town (18,150 (13.17%)) from a total of 137,860 suspected cases followed by the adjacent district, Dilla zuria (12,588 (10.56%)) from 119,207 tested suspects. Both of the districts are < 1750 masl. The lowest was from Yirgacheffe rural district (5187 (10.44%), at 1750–2500 masl). Dilla town annual malaria cases remained the highest throughout the 8-year period except in 2013 and 2019 (AOR = 3.16, 95% CI: 2.11, 4.22, p < 0.001) (Table 4). The highest confirmed cases during these 2 years were reported by Kochore (4390, 19.78%) and Dilla zuria districts (1178, 8.04%) from 22,197 and 14,648 suspected cases respectively. Although there was an overall declining trend of confirmed malaria cases from 2012 to 2019, there were increases in Kochore during 2013, Dilla town and Dilla zuria in 2016 (Fig. 4). Plasmodia spp. distribution varied with district, the highest number of P. falciparum cases was seen in Yirgacheffe and Dilla towns and Dilla zuria district while P. vivax overtook in other districts (Supplementary Fig. 1).
Yirgacheffe rural district had the highest variation (376, 80.51%) in terms of confirmed malaria records between HC and HO data (HC-HO) followed by Dilla town (164, 35.12%) and Dilla zuria district (− 138, 29.55%), data obtained by HCs-HOs. Whereas, Wonago district had the lowest (− 6, 1.28%) inconsistency in malaria data recordings between HCs and HOs (Fig. 4).
The monthly average confirmed malaria cases showed a strong association with monthly rainfall (RR = 11.47, 95% CI: 7.27, 15.68) and monthly minimum temperature (RR = 1.62, 95% CI: 1.05, 2.23) (Supplementary Table 1). Nevertheless, the maximum monthly temperature was negatively and weakly associated with the monthly confirmed malaria cases, as entire interval is below 1. Relative humidity (RH) seems also not to have a significant effect on malaria transmission in the study area. Apart from statistical association the lowest (60.8%) and highest (73.8%) records of RH were linked to monthly confirmed malaria cases. Relationship of monthly confirmed case records and climatic variables were displayed below (Fig. 5).
Discussion
There was an overall reduction of malaria case from 2012 to 2019. According to data from HCs (source data), a maximum of 16,037 and a minimum of 2546 of cases were observed during 2013 and 2018, respectively with 8.34% reduction. 2013 and 2016 were the exceptions to the declining trend as there were case increases in these periods in some parts of the Zone. The relatively lower malaria cases for the periods between 2014 and 2015 could be associated with rainfall variations and strong intervention activities in the zone. 2016 was an El Nino year that predominantly affected the normal rainfall frequency and distribution along the southern Ethiopia. Thus, this could be the possible contributing factor for the higher occurrence of malaria in the area during 2016. The overall malaria positivity rate (11.79%) in the current study was comparable with certain studies done in Ethiopia including from Batu town (12.43%) [15], Halaba special district (9.47%) [16] and north Shoa (8.39%) [17]. In contrast, higher overall malaria positivity rates were reported from related studies conducted in south-central Ethiopia [18], southern Ethiopia [19] and abroad in Dakar, Senegal [20] with 33.83, 21.79 and 19.68% respectively. On the other hand, the present figure was higher than records in other local studies [21, 22]. These differences might be due to the variation in quality of laboratory diagnoses, difference in intervention measures, micro-climatic/altitudinal differences, and presence of constructions responsible for occurrence of temporary and permanent dams and drug and insecticide resistances.
P. falciparum and P. vivax were detected where equivalent; congruent results were reported in some other parts of Ethiopia [18, 19]. While other local studies [15, 17, 23] documented that the dominant species was P. vivax. The proportion of mixed infection in this study was congruent with other studies [18, 22], whereas inconsistent with other reports [19, 23]. The higher number of P. vivax against the national figures could also be an implication for the ability of repeated relapse cases and early emergence of gametocytes during blood-stage infection. In addition, there could be heterogeneity of the Duffy phenotype and the high number of vulnerable Duffy-positive individuals that associated with population movement [24] in the study area. Environmental fluctuations that change target mosquito species abundance might have an impact on Plasmodia species occurrence [25]. Further research is required to clarify whether the equivalent proportion of the two species is associated with the current practice/uptake of primaquine for P. vivax in the study area. The Ethiopian Federal Ministry of Health states that radical cure primaquine combined with chloroquine should be used to treat vivax malaria cases without prior G6PD testing [4]. The possible reason for scarcity of mixed infections in this co-endemic area might be a competitive or an antagonistic effect of one Plasmodium species over the other within the human host during co-infection [18, 25]. In addition, since the area is low transmission setting, mixed infections with P. falciparum and P. vivax infection are less likely to be detected by microscopy. This is because, P. vivax has an inherently lower parasitemia than its counterpart species, and thus less likely to be detected in a co-infection with P. falciparum.
For the 8-years period, there was an overall notable difference in both total number of suspected cases and confirmed malaria cases recorded at HCs (source data) and HOs (PHEM data). During 2012 and 2018 the numbers of suspected cases recorded at HCs were consistent with HOs data. In these years the numbers of confirmed cases were higher at HOs records than HCs records. Yirgacheffe rural district had the highest deviation in terms of confirmed malaria records between HCs and HOs data followed by Dilla town and Dilla zuria district, whereas Wonago district was the least. This inconsistency could affect the quality control system of the facilities in the zone. Similar to our finding, a three-month facility-based study comprising of various settings conducted in southern Ethiopia showed that majority of facilities under-reported total malaria (both confirmed and clinical malaria) cases [12]. Contrary to the present study, a study employed in three provinces of Mozambique stated that under-reporting of suspected and confirmed cases is a bigger issue than over-reporting in the health facility records as compared to the values reported through the health management information system (HMIS). They reported that values of both suspected and confirmed malaria cases documented at health facility records were lower than those reported through the HMIS [26]. This deviation in malaria data between the two systems, HC and HO, could be due to errors during entering the data from the sources (HCs) into recording formats of PHEM, lack of cross-checking and proofing habits, training gaps on PHEM data use and unintentional/intentional false reports. In addition, limited computer access and skill, inadequate technical support [27], poor data management skills and limited functionality of electronic data management systems [28] might be the likely reasons for PHEM implementation challenges.
In the present study there was a significant reduction in data incompleteness; with missing variables between 2012 and 2019 in terms of both suspected and confirmed cases. Apart from the statistical figures of declining trend of missing data (data incompleteness) from year-to-year, there are substantial proportions of missing data in recent years in the health facilities of the study area. When we ignored the missing data there could be over/under estimates of malaria data affecting the real figure of API [29] in the area. Since API estimates are vital for malaria stratification and further elimination activities. Thus, the declining trends of data incompleteness noted in this study needs to be sustained as the country’s NMCP has prioritized improving data quality issue in its strategic plan.
Concerning the diagnostic performance, the proportions of suspected cases against confirmed cases were increased from year to year. The decreased proportion of confirmed malaria cases detected could likely be due to decreasing malaria incidence in the study area. But the proportions of ‘non-malarial’ febrile cases were increasing from 2012 to 2019. This can be an indication of declining lab capacity of detecting malaria parasites. This declining lab capacity of detecting malaria might be due to the fact that the increased false negative reports associated with reduced sensitivity of microscopy with decreasing parasite densities [30], unable to detect sequestered P. falciparum parasites [31] and low competency of microscopists [32]. In the other way, such increased number of non-malarial febrile illnesses might be related to other febrile cases including yellow fever virus [33, 34] and typhoid fever [35, 36] infections, as per the studies conducted in southern and south-central Ethiopia. In addition, this high number of non-malarial febrile illness might be due to fevers among positive individuals with malaria where the fever is coexisted with but not caused by the Plasmodia infection [37]. Thus, non-malarial febrile illnesses of bacterial and viral (e.g. now covid-19) etiologies could complicate malaria diagnosis. This needs further investigation in the area. If laboratory performance percent confirmed declines it means; laboratory performance was decreasing over the years or something causing febrile illness in the area is increasing. Misdiagnosis and treating malaria clinically based only on fever with antimalarial drugs, which is still in remote settings, may contribute to the rapid emergence of antimalarial drug resistance [38, 39].
The slightly more infection among males in the present study was paralleled with other studies conducted in different parts of Ethiopia [15, 18, 19, 23]. However, this finding was not consistent with other reports in southern Ethiopia [17] and elsewhere in Mozambique [40] where higher malaria cases in females were documented. Individuals in the age group of 15 and above were also more significantly affected. This was in line with other local studies [15, 23]. In contrast, a finding in Metema, northwest Ethiopia by Ferede et al. [41] showed that 5–14 years old were more infected. Possible justifications for the higher occurrence of malaria among males and young adults and above age group could be their engagement in various outdoor activities and staying outdoors during the nights [42]. Apart from outdoor exposures, differences in treatment-seeking behavior, access to health facilities and travel history [43] might be the possible contributors for the sex- and age-based variations of malaria cases. In addition, a review report revealed that adult females are better protected from parasitic diseases than males due to genetic and biological (hormonal) factors [44].
Confirmed malaria cases were significantly influenced by altitude in the present study area. Higher malaria was in areas of low altitude (below 1750 masl) compared to in high altitudes (1750–2500 masl). Plasmodia spp. distribution also varied with elevation; P. vivax was relatively higher at higher altitudes while P. falciparum was higher at the lower altitudes. The likely reason for the slightly higher proportion of P. falciparum in lower altitudes and higher proportion of P. vivax in high elevations could be related to temperatures. That is, cooler environments are suitable for P. vivax while P. falciparum are adapted to a relatively higher temperature for the growth in human host and mosquito vectors [45, 46].
The peak number of confirmed malaria cases was recorded during Belg, following the long rainy season in the area, followed by Kiremt, short rainy season in the area, and Bega, the dry season in the area, with a statistically significant variation. The long rain season and peak malaria cases association seen in this finding is consistent with various studies in Ethiopia [17,18,19, 23] although the long rain season in these areas is from June to September. The high prevalence of malaria cases during the two seasons, following the rainfall pattern corroborates the two-season transmission pattern in the entire country. Following the long rainy season higher proportion of P. falciparum was recorded than P. vivax, while the difference was smallest during the dry season. The comparable proportion of P. vivax against P. falciparum in Bega might be explained by the fact that P. vivax has ability to relapse rather than new infections. Since such traits could affect the temporal patterns of P. vivax infections.
Overall, high number of suspected cases and confirmed malaria cases were documented in Dilla town (urban) and Dilla zuria district (sub-urban) which are found at lower elevations. Except in 2013 and 2019, Dilla town annual malaria cases remained the highest all over the 8-year period. The highest confirmed cases during these 2 years were overtaken by Kochore in 2013 and Dilla zuria districts in 2019. While the lowest was from Yirgacheffe rural district. In general, though an overall declining trend of confirmed malaria cases from 2012 to 2019, increments were recorded in Kochore during 2013 and Dilla town in 2016. Although there is expectation of a better documentation, treatment-seeking behavior, access to health facilities, community knowledge and coverage of intervention activities in urban settings, the current data pointed to the contrary. Thus, in this study, high burden of urban and suburban malaria was noted. This could be because of massive construction activities (like road, house and small dams) and presence of coffee processing sites in Dilla town and its vicinity that could create suitable habitat for mosquito breeding. Travel history [43], differences in the competence and skills of the laboratory personnel and relatively good reporting system might also be the main responsible factors influencing the prevalence of malaria in Dilla town compared to rural districts. There have been healthy ongoing malaria control activities incorporating environmental management, indoor residual spraying (IRS), long-lasting insecticide-treated nets (LLINs) and artemisinin-based combination therapy in the area. These intervention activities could be attributed for the decreasing trends of malaria in other sites of the Zone. In addition, micro-environmental variations, micro-climatic situations [25] and changes in intervention (like IRS and LLINs) periods might have effect for these spatial differences of malaria cases. The higher prevalence of malaria in districts with low altitudes would indicate the association of malaria and elevation.
Monthly rainfall and minimum temperature demonstrated statistically significant correlation with malaria cases. Previous studies in Ethiopia [47, 48] and elsewhere [49, 50] documented similar findings. This association suggests an early warning system can be developed to forecast the start of the malaria season using rainfall and temperature forecasts in the area. However, the result of the current study on the association of rainfall and malaria cases was deviating from previous finding, stating higher rainfall does not necessarily influence the malaria changes [51]. This deviation may be due to high rainfall affected the breeding sites of mosquito vectors in other places apart from the current study area [52]. In addition, there could be prevention practice variations between the population of the current study area and other sites in taking preventive measures during and after the high rain seasons. In contrast to our finding, minimum temperature was weakly correlated with malaria cases in southwest Ethiopia [48]. Ideally, rainfall and minimum temperature play a vital role in breeding and survival of malaria vectors and the respective parasites. Moreover, average monthly maximum temperature and RH were weakly correlated with malaria cases. In disagreement to our finding, studies conducted in Jimma, Ethiopia by Alemu and others [47] and Sena and colleagues [48] in Gilgel-Gibe, southwest Ethiopia reported that inter-monthly RH was significantly associated with monthly malaria cases. Publications of Akinbobola and colleague showed humidity levels between 60 and 90% were favorable for breeding and multiplication of Plasmodia parasites [53]. Although RH in our study was occurred between these risky ranges, no strong correlation with malaria cases was seen. This could be explained by the fact that RH is affected by rainfall and temperature, these might confound the relationship. Yet, additional research is required to explain the relationship between malaria transmission and RH in the area.
The limitation of this study was incompleteness of patient data in the register with missed variables and only 8-year data were available during the data collection time at the HCs. This study was limited to collecting secondary data only at health facility level and district level PHEM data. This prevented the readers for getting a clear situation how there is mismanagement of data at regional and federal levels in the country. Since the age classification on the laboratory registration books of HCs were only < 1, 1–4, 5–14, 15+, it’s difficult to further subdividing of 15+ age groups. Malaria diagnosis in the urban areas (particularly in Dilla and Yirgacheffe towns) could also be done in other public health facilities and private clinics. But this study did not include data collected from these facilities and this might underestimate the actual trend of malaria in the area. Furthermore, clinically treated patients’ (without laboratory confirmation) data and malaria mortality data were not recorded in the laboratory registration logbooks. Hence, interpretation of the finding should be with caution.
Conclusion
Over the entire period, 2012 to 2019, the malaria control program performance improved; there was an overall drop in malaria burden and the proportion of data incompleteness declined significantly over the years. Yet, in the move for elimination data inconsistency between HCs and district HOs call for urgent correction. The equivalent P. falciparum and P. vivax co-endemicity level underline the need for interventions targeting both species. The high malaria prevalence in urban setting (Dilla town) and its vicinity requires consideration by the control campaign. The seasonal variation demands strengthening of interventions through short-term forecasting based on local meteorological factors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Pf :
-
Plasmodium falciparum
- Pv :
-
Plasmodium vivax
- NMCP:
-
National malaria control program
- API:
-
Annual parasite incidence
- PHEM:
-
Public Health Emergency Management
- HP:
-
Health post
- HC:
-
Health center
- HO:
-
Health office
- masl:
-
Meters above sea level
- NMA:
-
National meteorology agency
- COR:
-
Crude odds ratio
- AOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- GLM:
-
Generalized linear model
- RH:
-
Relative humidity
- HMIS:
-
Health management information system
- IRS:
-
Indoor residual spraying
- LLINs:
-
Long-lasting insecticide-treated nets
References
Ethiopian Public Health Institute. Ethiopia National Malaria Indicator Survey 2015. Addis Ababa: Ethiopian Public Health Institute; 2016.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee M, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103.
Gari T, Lindtjørn B. Reshaping the vector control strategy for malaria elimination in Ethiopia in the context of current evidence and new tools: opportunities and challenges. Malar J. 2018;17:454.
President’s Malaria Initiative Ethiopia. Malaria Operational Plan FY 2020; 2020. Retrieved from (www.pmi.gov).
National meteorological services agency agrometeorological bulletin. Seasonal agro-meteorological bulletin. BEGA, 2004/05. 2005;15:3.
Kibret S, Wilson GG, Ryder D, Tekie H, Petros B. Malaria impact of large dams at different eco-epidemiological settings in Ethiopia. Tropical Med Int Health. 2017;45:4.
Chung B. Impact of irrigation extension on malaria transmission in Simret, Tigray, Ethiopia. Korean J Parasitol. 2016;54(4):399–405.
Mukhtar AYA, Munyakazi JB, Ouifki R. Assessing the role of human mobility on malaria transmission. Math Biosci. 2020;320:108304.
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Moonen B, Cohen JM, Tatem AJ, Cohen J, Hay SI, Sabot O, et al. A framework for assessing the feasibility of malaria elimination. Malar J. 2010;9:322.
WHO. From malaria control to malaria elimination: a manual for elimination scenario planning. Geneva: World Health Organization; 2014.
Endriyas M, Alano A, Mekonnen E, Ayele S, Kelaye T, Shiferaw M, et al. Understanding performance data: health management information system data accuracy in southern nations nationalities and People’s Region, Ethiopia. BMC Health Serv Res. 2019;19:175.
Gedeo zone health department. Gedeo zone health department annual report 2019. Dilla; 2019.
Alhamshry A, Fenta AA, Yasuda H, Kimura R, Shimizu K. Seasonal rainfall variability in Ethiopia and its long-term link to global sea surface temperatures. Water. 2020;12:55.
Hassen J, Dinka H. Retrospective analysis of urban malaria cases due to Plasmodium falciparum and Plasmodium vivax: the case of Batu town, Oromia, Ethiopia. Heliyon. 2020;6:e03616.
Shamebo T, Petros B. Trend analysis of malaria prevalence in Halaba special district, Southern Ethiopia. BMC Res Notes. 2019;12(1):190.
Feleke DG, Gebretsadik D, Gebreweld A. Analysis of the trend of malaria prevalence in Ataye, north Shoa, Ethiopia between 2013 and 2017. Malar J. 2018;17:323.
Yimer F, Animut A, Erko B, Mamo H. Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar J. 2015;14:230.
Dabaro D, Birhanu Z, Yewhalaw D. Analysis of trends of malaria from 2010 to 2017 in Boricha district, southern Ethiopia. Malar J. 2020;19:88.
Diallo MA, Badiane AS, Diongue K, Sakandé L, Ndiaye M, Seck MC, et al. A twenty-eight-year laboratory-based retrospective trend analysis of malaria in Dakar, Senegal. PLoS ONE. 2020;15(5):e0231587.
Derbie A, Alemu M. Five years malaria trend analysis in Woreta health center, Northwest Ethiopia. J Health Sci. 2017;27(5):465.
Yimer M, Hailu T, Mulu W, Abera B, Ayalew W. A 5 year trend analysis of malaria prevalence with in the catchment areas of Felegehiwot referral hospital, Bahir Dar city, Northwest-Ethiopia: a retrospective study. BMC Res Notes. 2017;10:239.
Solomon A, Kahase D, Alemayehu M. Trend of malaria prevalence in Wolkite health center: an implication towards the elimination of malaria in Ethiopia by 2030. Malar J. 2020;19:112.
Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, et al. Global epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95(Suppl 6):15–34.
Phimpraphi W, Paul RE, Yimsamran S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, et al. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar J. 2008;7:99.
Colborn JM, Zulliger R, Da Silva M, Mathe G, Chico AR, Castel-Branco AC, et al. Quality of malaria data in public health facilities in three provinces of Mozambique. PLoS One. 2020;15(4):e0231358.
Kiberu VM, Matovu JK, Makumbi F, Kyozira C, Mukooyo E, Wanyenze RK. Strengthening district-based health reporting through the district health management information software system: the Ugandan experience. BMC Med Inform Decis Mak. 2014;14:40.
Ledikwe JH, Grignon J, Lebelonyane R, Ludick S, Matshediso E, Sento BW, et al. Improving the quality of health information: a qualitative assessment of data management and reporting systems in Botswana. Health Res Policy Syst. 2014;12:7.
Wimberly MC, Davis JK, Nekorchuk DM. Surveillance data for national malaria stratification in Ethiopia: analysis of malaria incidence from 2014 to 2019 at the woreda scale. Technical report; 2020.
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). Am J Trop Med Hyg. 2007;77(Suppl 6):119–27.
Leke RF, Djokam RR, Mbu R, Leke RJ, Fogako J, Megnekou R, et al. Detection of the Plasmodium falciparum antigen histidinerich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J Clin Microbiol. 1999;37:2992–6.
Nega D, Abebe A, Abera A, Gidey B, G/Tsadik A, Tasew G. Comprehensive competency assessment of malaria microscopists and laboratory diagnostic service capacity in districts stratified for malaria elimination in Ethiopia. PLoS ONE. 2020;15(6):e0235151.
Lilay A, Asamene N, Bekele A, Mengesha M, Wendabeku M, Tareke I, et al. Reemergence of yellow fever in Ethiopia after 50 years, 2013: epidemiological and entomological investigations. BMC Infect Dis. 2017;17:343.
Nigussie E, Shimelis T, Eshetu D, Shumie G, Chali W, Aseffa A, et al. Seropositivity of yellow fever virus among acute febrile patients attending selected health facilities in Borena district, southern Ethiopia. Ethiop Med J. 2020;58:57–62.
Habte L, Tadesse E, Ferede G, Amsalu A. Typhoid fever: clinical presentation and associated factors in febrile patients visiting Shashemene Referral Hospital, southern Ethiopia. BMC Res Notes. 2018;11:605.
Teferi M, Desta M, Yeshitela B, Beyene T, Espinoza LM, Im J, et al. Acute febrile illness among children in Butajira, south-central Ethiopia during the typhoid fever surveillance in Africa Program. Clin Infect Dis. 2019;69(Suppl 6):S483–91.
Dalrymple U, Cameron E, Arambepola R, Battle KE, Chestnutt EG, Keddie SH, et al. The contribution of non-malarial febrile illness co-infections to Plasmodium falciparum case counts in health facilities in sub-Saharan Africa. Malar J. 2019;18:195.
Onchiri FM, Pavlinac PB, Singa BO, Naulikha JM, Odundo EA, Farquhar C, et al. Frequency and correlates of malaria over-treatment in areas of differing malaria transmission: a cross-sectional study in rural Western Kenya. Malar J. 2015;14:97.
Nyaoke BA, Mureithi MW, Beynon C. Factors associated with treatment type of non-malarial febrile illnesses in under-fives at Kenyatta National Hospital in Nairobi, Kenya. PLoS ONE. 2019;14(6):e0217980.
Temu A, Coleman M, Abilio P, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;7:e31409.
Ferede G, Worku A, Getaneh A, Ahmed A, Haile T, Abdu Y, et al. Prevalence of malaria from blood smears examination: a seven-year retrospective study from Metema hospital, northwest Ethiopia. Malar Res Treat. 2013;2013:704–30 5.
Kigozi SP, Kigozi RN, Epstein A, Mpimbaza A, Sserwanga A, Yeka A, et al. Rapid shifts in the age-specific burden of malaria following successful control interventions in four regions of Uganda. Malar J. 2020;19:128.
Mathanga DP, Tembo AK, Mzilahowa T, Bauleni A, Mtimaukenena K, Taylor TE, et al. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar J. 2016;15:590.
Krogstad DJ. Malaria as a re-emerging disease. Epidemiol Rev. 1996;18:77–89.
Bodker E. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. Med Entomol. 2003;40:706–17.
BradfieldLyon E. Temperature suitability for malaria climbing the Ethiopian highlands. Environ Res Lett. 2017;12:064015.
Alemu A, Abebe G, Tsegaye W, Golassa L. Climatic variables and malaria transmission dynamics in Jimma town, Southwest Ethiopia. Parasit Vectors. 2011;4:30.
Sena L, Deressa W, Ali A. Correlation of climate variability and malaria: a retrospective comparative study, Southwest Ethiopia. Ethiop J Health Sci. 2015;25:2.
Gunda R, Chimbari MJ, Shamu S, Sartorius B, Mukaratirwa S. Malaria incidence trends and their association with climatic variables in rural Gwanda, Zimbabwe, 2005-2015. Malar J. 2017;16:393.
Nanvyat N, Mulambalah CS, Barshep Y, Dakul DA, Tsingalia HM. Retrospective analysis of malaria transmission patterns and its association with meteorological variables in lowland areas of plateau state, Nigeria. Int J Mosq Res. 2017;4(4):101–6.
Haque U, Hashizume M, Glass GE, Dewan AM, Overgaard HJ, Yamamoto T. The role of climate variability in the spread of malaria in Bangladeshi highlands. PLoS ONE. 2010;5(12):1–9:e14341.
Tompkins AM, Ermert V. A regional-scale, high resolution dynamical malaria model that accounts for population density, climate and surface hydrology. Malar J. 2013;12:65.
Akinbobola A, Omotosho JB. Predicting malaria occurrence in southwest and north-Central Nigeria using meteorological parameters. Int J Biometeorol. 2013;57(5):721–8.
Acknowledgments
We would like to thank heads of HOs and HCs for their willingness to provide the retrospective malaria data and the NMA of Ethiopia for providing meteorological data of the study area. We are also grateful to Mr. Eyuel Asemahegn for constructing the study area map.
Funding
This study was partially funded by Dilla and Addis Ababa Universities, Ethiopia. But the funders have no role in the study design, data collection, analysis, interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
EM designed the study, involved in data collection, analyzed and interpreted the data, and drafted the manuscript. SWB contributed in data analysis works. FGT initiated the idea, involved in the data entry template development and gave feedback on the data collection protocol. SD was responsible for revising the draft manuscript. EG initiated the idea, guided the process of data collection and substantively revised the manuscript. HM participated in guidance of the data collection progresses and critically commented the whole section of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from Department of Microbial, Cellular and Molecular Biology and College of Natural and Computational Sciences Ethical Review Committee, Addis Ababa University (CNSDO/318/11/2019). After discussing the objective and method of the study, written consent was sought from zone health department office. Verbal consent was then obtained from the respective heads of district health offices and health centers before starting the data collection. All data was anonymized before use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Figure 1. District level Plasmodia species distribution in Gedeo zone, South Ethiopia, 2012–2019. Supplementary Table 1. Association of HC-level monthly confirmed malaria cases and meteorological variables using GLM regression, Gedeo zone, 2012–2019.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Molla, E., Behaksra, S.W., Tadesse, F.G. et al. Past eight-year malaria data in Gedeo zone, southern Ethiopia: trend, reporting-quality, spatiotemporal distribution, and association with socio-demographic and meteorological variables. BMC Infect Dis 21, 91 (2021). https://doi.org/10.1186/s12879-021-05783-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-05783-8