Abstract
Background
Influenza vaccination is recognized as a primary public health intervention which prevents the illness of patients and relieves the societal burdens of influenza for medical community as well as the economy. To date, no effectiveness study of influenza vaccination has been conducted including a large population with a wide age span, in Japan. Here, we evaluated the clinical effectiveness of influenza vaccination in a large Japanese population.
Methods
We conducted a cohort study using a large-scale claims database for employee health care insurance plans. Vaccination status was identified using plan records for influenza vaccination subsidies. We excluded people aged 65 years or more because of the unavailability of vaccination records. Effectiveness of vaccination in preventing influenza and its complication was evaluated with doubly robust methods using inversed probability treatment weighting to adjust health conscious behaviours and other confounders.
Results
During the 2013/2014 influenza season, 369,425 subjects with age range from 1 to 64 years were eligible. Vaccination rate was 39.5% and an estimated odds ratio (OR) for influenza onset was 0.775 after doubly robust adjustment. Age-stratified ORs were significantly reduced in all age groups; lowest in subjects aged 1 to 4 years (0.600) and highest in those aged 13 to 19 (0.938). ORs for all the influenza complication outcomes were also statistically significant (0.403–0.709).
Conclusions
We confirmed the clinical effectiveness of influenza vaccination in people aged 1 to 64 years. Influenza vaccination significantly prevented influenza onset and was more effective in reducing secondary risks of influenza complications.
Similar content being viewed by others
Background
The heavy burden of influenza impacts not only patients – especially children, the elderly and those at high risk – such as in lives lost, but also medical institutions and the economy, in terms of lost productivity [1, 2]. Influenza vaccination is recognized worldwide as a primary public health intervention which reduces the healthcare, economic and social burden of influenza. Group influenza vaccination would protect the whole society from influenza and its aggravation [3, 4]. In Japan, a number of private employee health insurance plans and municipalities subsidize influenza vaccination.
Against this background, no large-scale effectiveness study of influenza vaccination covering a broad spectrum of generations has yet been reported in Japan. We previously conducted a vaccine effectiveness study in the largest pediatric population to date using a claims database in Japan [5]. In the present study we used the same database to extend the study population to cover generations, from children to workers.
In this study, we investigated the clinical effectiveness of influenza vaccination in a large population in Japan using a large-scale claims database.
Methods
Study design
The study was conducted under a retrospective cohort design using the same claims database and analytical methodology as in our previous pediatric study of influenza vaccination[5]. The claims database was provided by Japan Medical Data Center Co., Ltd. (Tokyo, Japan) and included more than 3 million enrollees of employee health care insurance plans, run mainly by large-scale private enterprises [6]. Deidentified claims data of employees and their dependents including diagnoses, procedures, and pharmacy prescriptions were collected from the health insurance plans. Study duration was the epidemic period, starting from 1 October 2013 to 31 May 2014 [7].
Ethical approval for the study protocol and waiver for informed consent to participate were provided by the Keio University Faculty of Pharmacy Ethics Committee for Research Involving Humans (No. 160118–1), in accordance with local ethical guidance for medical research involving human subjects.
Influenza vaccination
Seasonal trivalent inactivated and nonadjuvanted influenza vaccination is available for children aged 6 months or older and adults in Japan. The recommended dosage per season is two doses of 0.25 ml for those aged 6–24 months, two doses of 0.5 ml for those aged 3–12 years, and one dose of 0.5 ml for those over 12 years. Vaccines for the 2013/2014 season contained two influenza A strains (A/California/7/2009(X-179A)pdm09, A/Texas/50/2012(X-223)) and one influenza B strain (B/Massachusetts/2/2012(BX-51B)). Circulating viral subtypes of influenza in the 2013/2014 season were A/H1N1pdm09 (43%), A/H3N2 (21%), B/Yamagata (24%), B/Victoria (9%), and B/untyped (4%) [8].
The influenza vaccination period in Japan starts at the beginning of October and the vaccination schedule is managed by the Ministry of Health, Labour and Welfare [5]. The Japanese National insurance scheme, which provides basic universal coverage of all medical costs for all nationals, does not cover influenza vaccine. Japanese nationals have to pay the cost of vaccination out-of-pocket, except for the elderly aged 65 years or older, whose vaccination is provided at local government cost by public health service centers. Children and other adults receive influenza vaccination at community clinics or hospitals at their own cost. Several employment-based health insurance plans operated by private companies subsidize influenza vaccination and the scope of subsidy differs by the age group covered and fee. Accordingly, vaccination status and dates were identified from records in the health plans for the respective influenza vaccination subsidies.
Study population
Subjects were employees and their dependents aged 1 to 64 years as of October 1st 2013. Enrollees were eligible if their health plan provided subsidies for influenza vaccination. Because the Japan Pediatric Society does not recommend influenza vaccination for infants aged less than 1 year, potentially biasing pediatrician preferences for who should be vaccinated in this age group [9], we excluded infants aged ≤1 year from the evaluation of vaccine effectiveness. Some health plans do not provide a subsidiary program for influenza vaccination for particular age groups, such as adults aged 20 years or older. We therefore excluded subjects who were not eligible for subsidies or whose enrollment in the health plan started on or after October 2012 or ended before May 2014, to eliminate potential misclassification of vaccination status during the 2012/2013 and 2013/2014 seasons. Because seniors aged over 64 years were strongly recommended to receive vaccination and were often supported by subsidies from local governments rather than by health insurance plans, we also excluded subjects aged over 64 years to avoid misclassification of influenza vaccination status. We further excluded patients with prolonged hospitalization, on the basis that the extended period they spent in a managed environment may have affected their probability of being vaccinated or their risk of virus exposure. Patients with prolonged hospitalization were identified as those with a record of hospitalization for ≥24 days/month over ≥7 months.
The risk period for outcome events in vaccinees was from 14 days after the date of vaccination to 31 May 2014. Subjects who experienced an outcome event within 13 days after vaccination were excluded to ensure that the effects of vaccination were accurately assessed [10]. For non-vaccinees, the whole study period was the risk period for effectiveness. Subjects who got vaccinated after having experienced an outcome event were classified as non-vaccinees and censored at the time of outcome diagnosis.
Outcome definition
We used the same definitions for the incidences of influenza diagnosis and complications as the ones used for the preceding pediatric study, which were based on the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes [5]. A primary diagnosis of influenza was based on the ICD-10 codes J101, J110, J111, and J118. To test the robustness of the definition based on these codes in sensitivity analyses, three different definitions of influenza incidence were developed, as follows: 1) combination of the ICD-10 codes defined above with records of the use of a rapid-testing kit, identified by claim records for the influenza virus antigen (high-sensitive) test; 2) combination of these ICD-10 codes with a prescription for antiviral drugs, as determined using the Anatomical Therapeutic Chemical (ATC) J05B4 classification of the European Pharmaceutical Marketing Research Association; or 3) the combination of the J101 code “influenza due to identified seasonal influenza virus” and use of a rapid-testing kit. Secondary outcomes of influenza complications included pneumonia (J12-J18) and respiratory tract diseases (RTD: J00-J22, apart from the above influenza codes). There were both defined using ICD-10 codes only. Cases requiring hospitalization were additionally defined for secondary outcomes as subjects hospitalized within 3 days before or after the date of influenza diagnosis (hospitalization with influenza); subjects hospitalized within 7 days of the diagnosis date of RTD (hospitalization with RTD); and emergency hospitalization with influenza or pneumonia. We identified emergency hospitalization using claim codes of the national health insurance medical fee schedule for emergency hospitalization (A205 or A300) [11].
Confounding factors
Covariates considered for inclusion in adjustment of potential confounders in evaluating the effectiveness of vaccination included influenza vaccination status during the prior season (2012/2013 influenza season), age at October 1 2013, employment status, gender, number of other dependents aged 0 to 15 years covered under the same insurance number (number of children aged 0 to 15 in a family), preceding onset of influenza in family members during the current influenza season, history of high-risk medical conditions, emergency hospitalization, and number of outpatient visits during or outside office hours in the prior influenza off-season (June to September). High-risk medical conditions were defined using the definition of the US Centers for Disease Control and Prevention (CDC) [12]. Family members could be identified because employees and their dependents shared the same insurance number. “Preceding onset of influenza in family members” was considered to be the risk of second infection that subjects were exposed to when a family member received any influenza diagnosis code before his/her first influenza. This was considered in both the primary analysis and also in the secondary analysis for hospitalization using influenza as outcome.
Statistical analysis
Subject characteristics in the four age groups (1–4 years, 5–12 years, 13–19 years and 20–64 years) were summarized with descriptive statistics. Between-group comparisons were tested with the Mann-Whitney test for continuous variables and the chi-square test for categorical variables.
The primary analysis was to estimate the effectiveness of influenza vaccination in preventing the onset of influenza. The secondary analysis aimed to estimate the effect of influenza vaccination on influenza complications outcomes. First, we calculated odds ratios (ORs) of outcome events for influenza vaccination in the 2013/2014 season and other covariates using conventional multivariate logistic regression. To adjust confounding related to influenza vaccination for whole subjects and respective age groups, we next used a doubly robust method (DR) which combines a logistic regression model with inverse probability treatment weighting (IPTW) by propensity score (PS) to calculate the ORDRs [13]. PSs were calculated as those for the probability of being vaccinated in the 2013/2014 season by considering the covariates mentioned above and the presence of children aged 0 to 15 years in a family, but not considering preceding onset of influenza in family members or the number of children aged 0 to 15 years in a family. In the analysis for whole age subjects, age effect was modeled by linear tail-restricted cubic spline functions with 5 knots, based on percentiles on the basis that treating age effect as a linearity of age effect could not be assumed [14]. Vaccinees having a PS < 0.1 as well as non-vaccinees with PS > 0.9 were excluded because these subjects with opposite, extreme PS values were reported to have the potential to cause bias in the IPTW estimates by being excessively weighted with reciprocals of PSs for vaccinees and of (1-PS) for non-vaccinees [15]. C-statistics were calculated to ascertain the validity of PSs. When stratified by age group, actual age was not considered in calculating PSs or ORDR.
In addition to the DR method, Cox hazard regression analysis was also conducted as a sensitivity analysis, using the time-dependent covariates of current year vaccination and preceding onset of influenza among siblings. Some studies investigating the effects of influenza vaccine on elderly mortality using electronic health record databases have suggested a potential bias towards apparent greater effectiveness [16,17,18]. The presence of this bias was examined with a Cox hazard model by investigating vaccine effectiveness during the 2013 pre-epidemic period, with minimum influenza circulation between October 1st and December 16th, the latter date being the day when the National Statistics announced the beginning of the epidemic season [19].
All statistical analyses were conducted using SPSS version 25.0 (IBM Corp., Armonk, NY) or SAS version 9.4 (SAS Institute Inc., Cary, NC). Tests were 2-tailed and had a significance level of 0.05.
Results
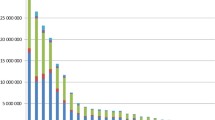
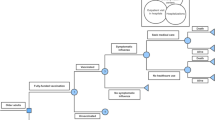
Of a total of 1,008,820 people entered in the study database, 638,654 subjects did not meet the inclusion criteria for effectiveness analyses (Fig. 1). After further excluding 741 subjects with extreme PSs or with the onset of an outcome within 13 days after their vaccination date, 369,425 subjects were eligible for the primary analysis. Approximately 50% of all subjects were children aged under 16 years. Vaccination rate was 39.5% in subjects aged 1 to 64 years in the 2013/2014 season, while a low rate was particularly observed in subjects aged 19 to 23 years (Fig. 2).
In every age group, vaccinees and non-vaccinees significantly differed in almost all characteristics (Table 1).
Vaccine effectiveness for influenza onset
Current-year vaccination was associated with a significant reduction in the risk of influenza onset after adjusting for covariates in conventional logistic regression, with an OR of 0.756 (95% confidence interval (CI): 0.738–0.776) for influenza onset (Table 2). Female sex significantly reduced the OR (0.960, 95%CI: 0.940–0.980). In contrast, vaccination in the preceding season (1.051, 95%CI: 1.024–1.079), high-risk condition (1.138, 95%CI: 1.110–1.167), number of outpatient visits during office hours (1.016, 95%CI: 1.014–1.018), number of other dependents aged 0 to 15 years with the same insurance number (1.114, 95%CI: 1.100–1.128) and preceding onset of influenza in family members (1.263, 95%CI: 1.234–1.294) significantly increased the risk of influenza.
Given that influenza was defined based on ICD-10 codes only, the ORDR was 0.775 (95%CI: 0.757–0.794) after doubly robust adjustment (Table 3). Sensitivity analysis using the outcome definition of J101 plus use of a rapid-testing kit gave similar ORDR (0.793, 95%CI: 0.769–0.817).
The age-stratified ORDRs for influenza onset outcomes were all significantly below one, and were lowest in younger children aged 1 to 4 years (0.600, 95%CI: 0.567–0.633) and highest in older children aged 13 to 19 years (0.938, 95%CI: 0.879–0.997).
The C-statistics of the PSs for influenza vaccination were 0.766 or more in whole subjects and 0.709 or more in the age-stratified groups.
In most age groups, except for children aged 13 to 19 years, Cox hazard regression also produced significantly reduced hazard ratios with similar but slightly smaller vaccine effectiveness overall (Appendix 1) as compared with the ORs derived from logistic regression (Table 3). No significant, positive vaccine efficacy was seen during the pre-epidemic period in any age group (Appendix 2).
Vaccine effectiveness for influenza complications
Significantly reduced ORDRs for all ages inclusive were observed for all secondary outcomes, ranging from 0.403 (emergency hospitalization with influenza or pneumonia) to 0.709 (hospitalization with influenza) (Table 4). In age group-stratified analyses, all ORDRs except for hospitalization with influenza and emergency hospitalization with influenza or pneumonia were significantly reduced. The ORDR of hospitalization with influenza was significant only in the 1–4 years age group (0.529).
Discussion
We conducted a large-scale effectiveness study of influenza vaccination in Japan using a health insurance claims database. Our study demonstrated that the effectiveness of influenza vaccination in preventing influenza onset was consistent across ages from 1 to 64 years old in the 2013/2014 season. Further, the risk of influenza complications, such as hospitalization with influenza, was significantly reduced in a real world setting, as previously reported [20, 21].
The incidence and ORDRs estimated in the present study likely reflect those for influenza-like illness, when the less stringent criterion of ICD10 codes only was applied. In contrast, the strictest criteria, namely the J101 code in combination with a rapid-testing kit, may provide the most conservative estimates of incidence and ORDR, albeit that this approach may lead to an increased number of false-negative cases [22]. However, given that a sensitivity analysis using a different definition of influenza onset and a different analysis technique with a Cox hazard model yielded similar risk estimates, the study results of influenza prevention appear to be robust. In calculating ORDRs for whole study subjects, the higher values of C-statistics for PSs using cubic spline function modeling of age-effect indicates better modeling than when age was treated as a linear effect (data not shown). Furthermore, given the high values of the C-statistics, the estimated PSs were considered to be valid.
The strongest effectiveness for the onset of influenza was observed in the vaccinated children aged 1 to 4 years. We speculate that this is probably due to careful, health-conscious behaviors of their families and high vaccination coverage. The US CDC recommends influenza vaccination for people at high risk of developing flu-related complications, including children aged under 5 years [12]. The Japanese Ministry of Health, Labour and Welfare recommends that, to decrease a child’s risk of influenza virus exposure, all family members remain apart from crowds, wash their hands after going outside and undergo vaccination as well [23]. In households with children, in general, family members are more likely to adopt health-conscious behaviors such as remaining apart from crowds and having vaccination every year for all family members, which may have resulted in greater effectiveness in aggregate [24]. The lowest effectiveness, observed in subjects aged 13 to 19 years, might be ascribable to lower influenza incidence and low immunization rates in this age group. Furthermore, because most of them are students and spend time with their peer group in school, they may have a higher chance of repeated exposure to various influenza viruses. However, significant increases in the OR estimates due to the number of siblings aged 0 to 15 years and preceding onset of influenza among family members may emphasize the importance of vaccination in household members; vaccine should be recommended regardless of age group.
Several risk factors for the onset of influenza were detected, including the number of children aged 0 to 15 years in a family. The possibility of introduction of influenza virus into a household would increase as the number of children increases [25]. Although employee insurance numbers helped identify familial infection, the possibility cannot be excluded that the dependents lived separately from the family. The present and our companion pediatric studies are the first to consider the risk of preceding onset of influenza in family members [5].
Influenza vaccination significantly reduced the risks of all outcomes of influenza complications in the 2013/2014 influenza season in the whole age population. Although some statistically insignificant ORDRs were observed in several age groups because of a small sample size and low incidences following age group stratification, vaccination effectively prevented most influenza complication events in most age groups.
Several cohort studies that used large-scale claims databases without laboratory-confirmed outcomes reported substantial bias in estimating vaccine effectiveness for elderly mortality [16, 18, 26]. Our study aimed to estimate the ORDR for the incidence of medically attended influenza onset and complications in Japanese subjects aged less than 65 years, and therefore differed from these elderly mortality studies in terms of both study subject and endpoint. The use of mortality as the primary endpoint in elderly subjects may generate immortal-time bias given that the endpoint, mortality, affects the status of vaccine exposure [27]. That is, the elderly vaccinees cannot have died to bide time until they got vaccinated (immortal time) and would consequently be expected to have reduced mortality compared with non-vaccinees. The HR estimates for influenza onset and pneumonia during the pre-epidemic period in this study, together with the results of our pediatric companion study (Appendix 2), clearly demonstrated the absence of such bias [5].
The limitations and strengths of the present study are in essence identical to those described for our pediatric study [5]. Since we used the outcome definitions based on diagnostic codes recorded in the claims reimbursement, which are likely to be less specific than PCR-confirmed influenza and may represent medically attended influenza-like illness, our estimates may be biased toward null, together with the study design [5, 28]. Confounding by unmeasured variables may have been present due to the observational nature of the study. We used application for an influenza vaccination subsidy as a substitute for vaccination records, possibly leading to the misclassification of vaccination status. In addition, the lack of information on the number of vaccinations each pediatric subject received prevented us from considering variation in the number of vaccinations in assessing vaccine effectiveness in a season. Moreover, ORs were potentially over- or under-estimated due to the exclusion of subjects who had an outcome within 13 days post-vaccination. Although patients who met the definition for prolonged hospitalization were excluded from analyses, some patients with repeated short-term hospitalization could not be excluded. The accuracy of the reason given for hospitalization in the claims databases was not verifiable, potentially leading to the misclassification of events requiring hospitalization. The present study population consisted of employees and their dependents enrolled in healthcare insurance plans operated by private, blue chip companies. The covered workers were therefore likely to be socially advantaged, and the healthy worker effect might accordingly have been prominent. This would in turn result in a better health condition and biased estimations [29]. Additionally, children aged under 15 years old accounted for over 40% of our study population, which is higher than in the general population, possibly leading to overestimated ORs [30]. Finally, the risk of secondary infection in schools or office areas was not considered. In contrast, the DR likely conferred robustness on the estimated ORDRs, together with the use of a large-scale claims database, since it seems to be the methodology best suited to addressing channeling bias regarding being vaccinated, such as health-conscious behaviors [24].
Conclusions
Our analysis confirmed that influenza vaccination in a large population of people aged 1 to 64 years significantly prevented the onset of influenza, and was similarly or more effective in reducing secondary risks due to influenza complications such as pneumonia in the 2013/2014 season. Since the study duration was limited to a single influenza season, annual study of each influenza season is required.
Availability of data and materials
All the data used in this study was provided by JMDC under the contract for the sole purpose of this study and not available for data sharing. Sharing the JMDC data set online is difficult owing to contractual agreements with JMDC. For inquiries about access to the data set used in this study, please contact JMDC (website, https://www.jmdc.co.jp).
Change history
12 August 2019
After publication of the original article [1], in Table 1, in the second and third column, “Vacnee” and “Non-vacnee” should be replaced with “Vaccinee” and “Non-vaccinee”.
Abbreviations
- DR:
-
Doubly robust method
- OR:
-
Odds ratio
- PS:
-
Propensity score
- RTD:
-
Respiratory tract diseases
References
Matias G, Haguinet F, Lustig RL, Edelman L, Chowell G, Taylor RJ. Model estimates of the burden of outpatient visits attributable to influenza in the United States. BMC Infect Dis. 2016;16(1):641.
Molinari N-AM, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96.
Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–96.
Sugaya N. A review of the indirect protection of younger children and the elderly through a mass influenza vaccination program in Japan. Expert Rev Vaccines. 2014;13(12):1563–70.
Shibata N, Kimura S, Hoshino T, Takeuchi M, Urushihara H. Effectiveness of influenza vaccination for children in Japan: Four-year observational study using a large-scale claims database. Vaccine. 2018;36(20):2809–15.
Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413–9.
Infectious Disease Weekly Report Japan [in Japanese], National Institute of Infectious Diseases. http://www.nih.go.jp/niid/ja/iasr-inf.html. Accessed 2 Dec 2018.
Infectious Disease Surveillance Center. In: National Institute of Infectious Diseases, editor. Report of Influenza Infection Surveillance in this winter (2014/2015 season). Tokyo: National Institute of Infectious Diseases; 2015.
Influenza vaccination for infants aged under 6 years [in Japanese]. https://www.jpeds.or.jp/modules/activity/index.php?content_id=198. Accessed 2 Dec 2018.
Pebody R, Warburton F, Ellis J, Andrews N, Potts A, Cottrell S, Johnston J, Reynolds A, Gunson R, Thompson C. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Eurosurveillance. 2016;21(38). https://doi.org/10.2807/1560-7917.ES.2016.21.38.30348. Accessed 2 Dec 2018.
Various Information of Medical Fee [in Japanese]. http://www.iryohoken.go.jp/shinryohoshu/. Accessed 2 Dec 2018.
People at high risk for developing flu-related complications. http://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed 2 Dec 2018.
Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly Robust Estimation of Causal Effects. Am J Epidemiol. 2011;173(7):761–7.
Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP. Influenza vaccine effectiveness against 2009 pandemic influenza A (H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213(10):1546–56.
Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570–80.
Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–44.
Nichol KL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine. 2009;27(45):6305–11.
Campitelli MA, Rosella LC, Stukel TA, Kwong JC. Influenza vaccination and all-cause mortality in community-dwelling elderly in Ontario, Canada, a cohort study. Vaccine. 2010;29(2):240–6.
Infectious Disease Surveillance Center. In: National Institute of Infectious Diseases, editor. Report of Influenza Infection Surveillance in this winter (2013/2014 season). Tokyo: National Institute of Infectious Diseases; 2014.
Cheng AC, Kotsimbos T, Kelly PM, Investigators F. Influenza vaccine effectiveness against hospitalisation with influenza in adults in Australia in 2014. Vaccine. 2015;33(51):7352–6.
Song JY, Lee JS, Wie S-H, Kim HY, Lee J, Seo YB, Jeong HW, Kim SW, Lee SH, Park K-H. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2015;22(2):229–34.
Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156(7):500–11.
Influenza Q&A Q.9 Not to catch the flu [in Japanese]. https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou01/qa.html. Accessed 2 Mar 2019.
Shrank WH, Patrick AR, Alan Brookhart M. Healthy User and Related Biases in Observational Studies of Preventive Interventions: A Primer for Physicians. J Gen Intern Med. 2011;26(5):546–50.
Brown CR, McCaw JM, Fairmaid EJ, Brown LE, Leder K, Sinclair M, McVernon J. Factors associated with transmission of influenza-like illness in a cohort of households containing multiple children. Influenza Other Respi Viruses. 2015;9(5):247–54.
Baxter R, Lee J, Fireman B. Evidence of bias in studies of influenza vaccine effectiveness in elderly patients. J Infect Dis. 2010;201(2):186–9.
Suissa S. Immortal Time Bias in Pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–9.
Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, Orenstein WA. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36(3):623–31.
Fukasawa T, Tanemura N, Kimura S, Urushihara H. Utility of a Specific Health Checkup Database Containing Lifestyle Behaviors and Lifestyle Diseases for Employee Health Insurance in Japan. J Epidemiol. 2019. https://doi.org/10.2188/jea.JE20180192. Accessed 2 Mar 2019.
The estimated national population as of October 1, 2013 [in Japanese]. https://www.stat.go.jp/data/jinsui/2013np/. Accessed 2 Mar 2019.
Acknowledgements
We thank Dr. Guy Harris for assisting with the drafting of the manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
HU and NS designed the study, analyzed and interpreted the data and drafted the manuscript. SK provided the data and advised on the manuscript. TH provided the analysis program and advised on the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study protocol was provided by the Keio University Faculty of Pharmacy Ethics Committee for Research Involving Humans (No. 160118–1), in accordance with local ethical guidance for medical research involving human subjects. Informed consent of each patient was waived by the committees, because this study used anonymized data which was retrospectively collected from medical records at the responded medical institutes.
Consent for publication
Not applicable.
Competing interests
Kimura. S is a representative director of Japan Medical Data Center Co., Ltd., which has provided data for this study. Urushihara. H is a part-time consultncy for Eisai Co., Ltd. and Nippon Boehringer Ingelheim Co., Ltd. and received the research fund from CAC Croit Corporation. All other authors declare no potential competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: in Table 1, in the second and third column, “Vacnee” and "Non-vacnee” should be replaced with “Vaccinee” and “Non-vaccinee”.
Appendices
Appendix 1
Appendix 2
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shibata, N., Kimura, S., Hoshino, T. et al. Influenza vaccination effectiveness for people aged under 65 years in Japan, 2013/2014 season: application of a doubly robust method to a large-scale, real-world dataset. BMC Infect Dis 19, 586 (2019). https://doi.org/10.1186/s12879-019-4186-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4186-x