Abstract
Background
Helicobacter pylori (H.pylori) infection is a common medical problem in resource limited areas. The treatment outcome after triple therapy has not been well studied in developing countries and preliminary data suggests a high rate of treatment failure. This study investigated the triple therapy treatment failure rate and associated factors among dyspeptic patients receiving H. pylori first line therapy at a tertiary hospital, Tanzania.
Methods
A prospective study in the Gastroenterology unit of the Bugando Medical Centre (BMC) was conducted between October 2015 and May 2017. All dyspeptic patients with stool antigen tests positive for H.pylori were given first line therapy, and stool antigen testing was repeated within 7 days and 5 weeks after completion of the treatment. Biopsies were taken before initiation of therapy and analysed for clarithromycin and quinolone resistance mutations using polymerise chain reaction (PCR) and sequencing. Adherence and other social-demographic characteristics were documented.
Results
A total of 210 patients were enrolled; the median age was 35 years (interquartile range, 27–48). First line treatment failure as defined by positive stool antigen 5 weeks post treatment was observed in 65/210 (31%) of patients. Independent predictors of first line treatment failure were presence of clarithromycin resistance mutations (OR: 23.12, 95% CI (9.38–56.98), P < 0.001) and poor adherence (OR: 7.39, 95% CI (3.25–16.77), P < 0.001). The sensitivity and specificity of stool antigen testing within 7 days after completion therapy in detecting treatment failure was 100 and 93.2%, respectively.
Conclusion
Nearly one-third of patients with clarithromycin resistance mutations and poor adherence develop first line treatment failure. Routine stool antigen testing within seven days after completion of therapy can be considered in order to initiate second line treatment early to prevent associated morbidities.
Similar content being viewed by others
Background
Helicobacter pylori is one of the most prevalent organism that can lead to erosions, ulcerations and cancers [1]. Overall the prevalence of H.pylori infection is about 44.3% worldwide and is more in developing countries than in developed countries [2]. The International Agency for Research on Cancer (IARC), which is part of the World Health Organization (WHO) declared H pylori as class I carcinogen [3].
H.pylori infected persons have a 10 to 20% lifetime risk of developing ulcer and a 1 to 2% lifetime risk of developing gastric cancer [4]. With increase resistance to clarithromycin worldwide there is an increased risk of first line H. pylori treatment failure [5, 6]. Treatment guidelines for the management of H. pylori infection differ among countries and depend on local susceptibility patterns [7]. The efficacy of standard 7 days therapy is decreasing globally [8, 9]. Eradication rates of H.pylori have been found to be less than 80% in some countries [10,11,12]. The H. pylori treatment failure has been linked to infections with antibiotic resistant strains [13,14,15,16], host genetic polymorphism in the cytochrome that may affect proton pump inhibitor pharmacokinetics (CYP2C19), poor adherence, short duration of therapy and smoking [17,18,19].
Previous efforts to improve the eradication of H. pylori have focused on increasing the numbers and types of drugs in the regimens and prolongation of the duration of standard triple therapy, none of which achieved higher eradication rates [20,21,22]. Other newer efforts include the implementation of concomitant (Proton pump inhibitor, amoxicillin, clarithromycin, and a nitroimidazole (tinidazole or metronidazole) given together for 3–10 days) and hybrid therapies (Proton pump inhibitor and amoxicillin for 7 days followed by another 7 days of Proton pump inhibitor, amoxicillin, clarithromycin, and a nitroimidazole) [23,24,25,26]. These therapies have resulted in eradication rates of 85 to 94%, and are currently advocated in places with greater than 15% clarithromycin resistance [27,28,29,30].
H. pylori treatment outcome and associated factors have not well been studied in East African countries. To-date there is the report of 10 patients who were investigated in 1999 from Ethiopia [12]. This study has provided the magnitude and factors associated with treatment failure in patients treated with first line regimen from developing country and has documented sensitivity and specificity of stool antigen testing within 7 days in detecting treatment failure. These information are very crucial in devising appropriate strategies to improve the outcome of H.pylori treatment.
Methods
Study population
This prospective study was done in the Gastroenterology and Hepatology unit of the Bugando Medical Centre a tertiary hospital located in the North-western Tanzania between October 2015 and May 2017. All dyspeptic patients who were planned to undergo esophagogastroduodenoscopy (EGD) aged 18 yrs. and above were invited in the study. Dyspepsia was defined using ROME criteria [31]. The study enrolled all patients who were H. pylori stool antigen positive with no history of antibiotic use in the past 30 days. The primary outcome was treatment failure which was defined as positive H.pylori stool antigen test 5 weeks after completion of the treatment [32].
Specimen collection and transportation
Participants who fulfilled inclusion criteria were serially enrolled. The stool antigen tests for H.pylori was performed using HpSA antigen test (SD- Bioline, H.pylori Ag Rapid test, Germany). In addition, on the same day the EGD was performed, two biopsies from antrum and fundus were taken. All patients who had positive stool antigen results were given a prescription for triple therapy according to standard treatment guidelines. A low cost regimen for limited-resource settings was prescribed to all patients (proton pump inhibitor, clarithromycin 500 mg and metronidazole500mg /Amoxicillin 1 g twice a day for 10 days), for those who failed the first line were given second line which included proton pump inhibitor, levofloxacin and amoxicillin [33]. Biopsies were taken followed by DNA extraction as described previously [34]. Amplification and sequencing of the clarithromycin resistance-determining regions to detect clarithromycin resistance mutations was done at the Department of Medical Microbiology, University of Gottingen, Germany.
Molecular testing
QIAamp DNA mini tissue extraction (Qiagen SA, Courtaboeuf, France) was used to extract DNA following the manufacturer instructions. H. pylori clarithromycin mutations were determined by amplification (Light Cycler- Roche) and sequencing of a 267 base pair (bp) fragment of the H. pylori 23S rRNA using oligonucleotides HPY-A: 5-CGCATGATATTCCCATTAGCAGT and HPY-S: 5-AGGTTAAGAGGATGCGTCAGTC [35]. The PCR was carried out in 40 cycles with denaturation process at 95 °C for 10 s, annealing at 60 °C for 10 s and extension at 72 °C for 20 s. In each cycle melting curve analysis was performed. Detection of mutations within the 23S gene was done by DNA sequencing followed with alignment with the wild type allele using the Geneious software package (Version 8.0.4 available from www.geneious.com (Biomatters, Ltd). DNA sequences were analysed for point mutations that are known to confer clarithromycin resistance at positions G2141, A2142, A2143 and A2144.
Follow up of patients
All enrolled patients had follow-up stool testing done twice: immediately (within seven days) after completion of medications and 5 weeks after completion of the treatment. Those with positive stool antigen 5 weeks after completion of treatment were declared as treatment failure. At the follow-up visits, the adherence was assessed by independent researcher as described by Gahi et al. [36]. In this scale the following questions were asked; in the past ten days, how often did you take your medications as the doctor prescribed?" Probable responses were; “All of the time” (100%), “Nearly all of the time” (90%), “Most of the time” (75%), “About half the time” (50%), or “Less than half the time” (< 50%). Poor adherence was defined as taking medications as prescribed 75% of the time or less.
All patients with treatment failure [30] were given a second-line treatment regimen according to the standard guidelines, tailored by resistance mutations identified.
Data analysis
Data were entered in the computer and analyzed using STATA version 13. The main outcome in this study was treatment failure. The categorical variables such as sex, were summarized as proportions while continuous variables such age were summarized as median with interquartile range (IQR). The univariate analysis was done which described the relationship between individual factors and treatment failure. Furthermore, multivariate analysis was done to eliminate confounders, odds ratios (OR) with respective 95% confidence intervals (CI) were computed. In all analyses, factors with a P < 0.05 were considered statistically significant. Furthermore, sensitivity and specificity of immediate testing of stool antigen using determination after five weeks post treatment as gold standard was done.
Results
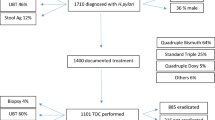
Total of 353 participants were invited and participated in the study. A total of 213 dyspeptic patients who tested positive for H.pylori stool antigen were included in the study. Out of 213 patients who were H. pylori stool antigen positive (Fig. 1), 3/213(1.4%) were lost to follow up (one was transferred to another region, two patients decided to use local herbs). A total of 210 participants were followed to completion, their median age was 35 yrs. (IQR 27–48). The endoscopy findings of 210 participants indicated that the majority had gastritis/duodenitis (Table 1).
H.pylori results on follow up
Immediately after completion of treatment (within seven days), all patients were tested for H.pylori infection, 75/210 (35.7%) were H. pylori stool antigen positive. Five weeks after completion of treatment, 65/210 (30.9, 95% CI; 24.6–37.2) were still stool antigen positive and were considered as treatment failure.
The sensitivity of immediately testing (within 7 days of completion treatment) in detecting treatment failure was 100% with the specificity of 93.2%, positive predictive value of 86.7% and negative predictive value of 100%. Sixty five (30.9%) patients who had treatment failure were treated with a second line or concomitant regimen [30] based on their mutation results. Among these 29/65(44.6%) failed the treatment and were given sorted drugs which achieved eradication. A total of 42/210(19.9%) reported side effects; some had more than one side effects. Side effects observed were abdominal discomfort 32/210(15.2%), nausea 6/210(2.8%), vomiting 2/210(0.9%) and dizziness 2/210(0.9%).
Factors associated with treatment failure
Among 210 patients, 152/210(72.3%) were tested for the clarithromycin resistance mutations (Table 2). Treatment failure was observed in 46/152(30.3%) patients with clarithromycin mutations while only 16(15.1%) patients in the group with no mutations had treatment failure (p < 0.001). Common mutations detected were A2143G 26/46(56.5%) and A2142G.
On the logistic regression analysis(Table 2), patients with isolates harbouring clarithromycin mutations were more likely to be H. pylori stool antigen positive 5 weeks post treatment (OR: 69.96, 95% CI; (19.61–249), P < 0.001) than those with no mutations. In addition, patients with poor adherence (OR: 7, 95% CI: 3.258–16.772, P < 0.001) were also more likely to be H.pylori stool antigen positive 5 weeks post treatment than those with good adherence. By multivariate logistic regression analysis, poor adherence (P < 0.001) and presence of clarithromycin resistance mutations (P < 0.001) significantly predicted treatment failure (Table 2).
Discussion
Standard H.pylori triple therapy has been a regimen of choice for many years. Unfortunately there is a decline in its efficacy hence clinicians have replaced standard triple therapy with sequential, concomitant and hybrid therapy (modified sequential) to increase cure rate [26, 37, 38]. It should be noted that the efficacy of standard triple therapies, which is still recommended in most guidelines, has decreased to < 80% in different countries [39,40,41,42,43,44].
As a general standard for the treatment of H.pylori, the anti- H.pylori therapeutic regimens should have an eradication rate of ≥90% [45]. Here, we report an alarming treatment failure rate of over 30% among patients with H. pylori infection seeking medical care in a tertiary hospital, Tanzania. This study for the first time in Tanzania has documented the H. pylori first line treatment failure among patients who were not previously on antibiotic. The treatment failure reported in the current study is significantly lower than what was reported in Egypt, this could be explained by the choice of patients. In current study, dyspeptic patients included were not on antibiotics within 30 days before the stool test was done while in Egyptian study the patients included were those who presented with dyspepsia without considering the prior use of antibiotics [46]. It should be noted that secondary clarithromycin resistance has been found to be significantly higher than primary resistance [47]. When compared to a study in Rwanda which reported treatment failure of 20% [44], no specific reason was elucidated to explain this variations pointing to geographical differences in the magnitude of resistance. High resistance to clarithromycin draws the urgent need to change treatment strategies in order to improve the treatment outcome in our setting. In addition, more studies are needed to explore drivers of the resistance.
The efficacy of the first line treatment therapy for H. pylori is decreasing in different parts of the world [48, 49]. As observed in the current study, clarithromycin resistance has been implicated as the potential factor for the first line treatment failure in many studies [39, 42, 50,51,52]. A study from Rwanda [44] also observed that clarithromycin resistance was a significant factor that predicted treatment failure. It should be noted that clarithromycin mutations often predict treatment failure of clarithromycin-based regimens [53, 54]. This was the case in the current study, with big odd ratio and 95% CI being reported due to the fact that there was significant more participants in the group with no mutation than that with mutation.
Good adherence has been found to predict good outcome of medication [42, 55,56,57], this was also observed in the current study. Poor adherence always leads to the sub-therapeutic levels or sub-optimal dose resulting in poor clinical outcome [58]. Interestingly, a study looking at the role of adherence to medications found that close follow-up of patients did improve compliance and satisfaction for the patients but did not increase the H. pylori eradication rate [36, 59]. This might have been due to other factors like drug resistance which can affect the eradication of the infection. Despite age and sex being documented as factors associated with H. pylori treatment outcome, this was not the case in the present study [39] [60].
Standard practices for the management of H. pylori infection recommends that post treatment non-invasive tests to confirm the eradication of H. pylori must be performed 4 weeks or more after eradication therapy is completed. In current study, stool antigen test done within 7 days after treatment well predicted eradication and treatment failure (positive predictive value, 86.7%, with negative predictive value of 100%). This finding was also observed in the studies done in United States and Europe, whereby positive result on the stool antigen test 7 days after completion of therapy identified patients in whom eradication of H. pylori was unsuccessful [61]. These data suggest that earlier stool antigen follow-up testing would identify those who will fail therapy and allow early initiation of second-line treatment [62, 63].
Conclusion
About one third of patients with clarithromycin resistance mutations and poor adherence developed first line treatment failure. Stool antigen testing within seven days after completion of therapy can be considered in order to initiate second treatment early to prevent associated morbidities. Strengthening adherence counselling before and during treatment is crucial in ensuring treatment success. More effort is needed in developing countries to ensure individual tailored treatment basing on the susceptibility patterns is practised.
Abbreviations
- BMC:
-
Bugando Medical Centre
- Bp:
-
Base pair
- CI:
-
Confidence Interval
- CUHAS:
-
Catholic University of Health and Allied Health
- DNA:
-
Deoxyribonucleic acid
- EGD:
-
Esophagogastroduodenoscopy
- IQR:
-
Interquartile range
- OR:
-
Odds ratio
- PCR:
-
Polymerise chain reaction
References
Kuipers E, Thijs J, Festen H. The prevalence of helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 1995;9:59–69.
Zamani M, Ebrahimtabar F, Zamani V, Miller W, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan M. Systematic review with meta-analysis: the worldwide prevalence of helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–76.
Helicobacter pylori Working Group . 150 cours Albert Thomas Lyon Cedex 08 F: Helicobacter pylori eradication as a strategy for preventing gastric cancer, vol. 8. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports; 2014.
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–90.
Jaka H, Rhee JA, Östlundh L, Smart L, Peck R, Mueller A, Kasang C, Mshana SE. The magnitude of antibiotic resistance to helicobacter pylori in Africa and identified mutations which confer resistance to antibiotics: systematic review and meta-analysis. BMC Infect Dis. 2018;18(1):193.
Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in helicobacter pylori: a recent literature review. World journal of methodology. 2015;5(3):164.
Yang J-C, Lu C-W, Lin C-J. Treatment of helicobacter pylori infection: current status and future concepts. World J Gastroenterol: WJG. 2014;20(18):5283.
Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–53.
Gisbert J, Calvet X. The effectiveness of standard triple therapy for helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011;34(11–12):1255–68.
Chuah S-K, Tsay F-W, Hsu P-I, Wu D-C. A new look at anti-helicobacter pylori therapy. World J Gastroenterol: WJG. 2011;17(35):3971.
Heo J, Jeon SW. Optimal treatment strategy for helicobacter pylori: era of antibiotic resistance. World J Gastroenterol: WJG. 2014;20(19):5654.
Henriksen T-H, Nysæter G, Madebo T, Setegn D, Brorson Ø, Kebede T, Berstad A. Peptic ulcer disease in South Ethiopia is strongly associated with helicobacter pylori. Trans R Soc Trop Med Hyg. 1999;93(2):171–3.
Tanih N, Dube C, Green E, Mkwetshana N, Clarke A, Ndip L, Ndip R. An African perspective on helicobacter pylori: prevalence of human infection, drug resistance, and alternative approaches to treatment. Ann Trop Med Parasitol. 2009;103(3):189–204.
Hellmig S, Hampe J, Schreiber S. Helicobacter pylori infection in Africa and Europe: enigma of host genetics. Gut. 2003;52(12):–1799.
Asrat D, Kassa E, Mengistu Y, Nilsson I, Wadström T. Antimicrobial susceptibility pattern of helicobacter pylori strains isolated from adult dyspeptic patients in Tikur Anbassa University hospital, Addis Ababa. Ethiop Med J. 2004;42(2):79–85.
Seck A, Mbengue M, Gassama-Sow A, Diouf L, Ka MM, Boye CS-B. Antibiotic susceptibility of helicobacter pylori isolates in Dakar, Senegal. J Infect Dev Ctries. 2009;3(02):137–40.
Kuo C-H, Lu C-Y, Shih H-Y, Liu C-J, Wu M-C, Hu H-M, Hsu W-H, Yu F-J, Wu D-C, Kuo F-C. CYP2C19 polymorphism influences helicobacter pylori eradication. World J Gastroenterol: WJG. 2014;20(43):16029.
Graham DY, Lu H, Shiotani A. Failure of optimized dual proton pump inhibitor amoxicillin therapy: what now? Saudi J Gastroenterol. 2017;23(5):265.
Abadi ATB. Helicobacter pylori treatment: new perspectives using current experience. J Glob Antimicrob Resist. 2017;8:123–30.
De Francesco V, Zullo A, Hassan C, Della Valle N, Pietrini L, Minenna M, Winn S, Monno R, Stoppino V, Morini S. The prolongation of triple therapy for helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: a prospective, randomised study. Dig Liver Dis. 2004;36(5):322–6.
Graham DY, Shiotani A. Newer concepts regarding resistance in the treatment helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008;5(6):321.
Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148(12):923–31.
Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D’Ambrosio P, Castorani L, Bonfrate L, Vannella L, Hassan C, Portincasa P. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 2013;37(6):647–50.
De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. Sequential, concomitant and hybrid first-line therapies for helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 2014;63(5):748–52.
Treiber G, Ammon S, Schneider E, Klotz U. Amoxicillin/metronidazole/omeprazole/clarithromycin: a new, short quadruple therapy for helicobacter pylori eradication. Helicobacter. 1998;3(1):54–8.
Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16(2):139–45.
Treiber G, Wittig J, Ammon S, Walker S, van Doorn L-J, Klotz U. Clinical outcome and influencing factors of a new short-term quadruple therapy for helicobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162(2):153–60.
Wang B, Wang YH, Lv ZF, Xiong HF, Wang H, Yang Y, Xie Y. Efficacy and safety of hybrid therapy for helicobacter pylori infection: a systematic review and meta-analysis. Helicobacter. 2015;20(2):79–88.
Molina–Infante J, Romano M, Fernandez–Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia–Abadia E, Vinagre–Rodriguez G, Martinez–Alcala C, Hernandez–Alonso M. Optimized nonbismuth quadruple therapies cure most patients with helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145(1):121–128. e121.
Malfertheiner P, Megraud F, O'morain C, Gisbert J, Kuipers E, Axon A, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30.
Tack J, Talley NJ. Functional dyspepsia—symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10(3):134.
Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56(6):772–81.
Hunt R, Xiao S, Megraud F, Leon-Barua R, Bazzoli F, Van der Merwe S, Vaz Coelho L, Fock M, Fedail S, Cohen H. Helicobacter pylori in developing countries. World gastroenterology organisation global guideline. J Gastrointestin Liver Dis. 2011;20(3):299–304.
QIAamp D: Mini and blood mini handbook. Qiagen 2012.
Ménard A, Santos A, Mégraud F, Oleastro M. PCR-restriction fragment length polymorphism can also detect point mutation A2142C in the 23S rRNA gene, associated with helicobacter pylori resistance to clarithromycin. Antimicrob Agents Chemother. 2002;46(4):1156–7.
Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167(16):1798–803.
Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56(10):1353–7.
Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for helicobacter pylori eradication. Helicobacter. 2009;14(2):109–18.
Kim SE, Park MI, Park SJ, Moon W, Choi YJ, Cheon JH, Kwon HJ, Ku KH, Yoo CH, Kim JH. Trends in helicobacter pylori eradication rates by first-line triple therapy and related factors in eradication therapy. Korean J Intern Med. 2015;30(6):801.
Feng L, Wen M-Y, Zhu Y-J, Men R-T, Yang L. Sequential therapy or standard triple therapy for helicobacter pylori infection: an updated systematic review. Am J Ther. 2016;23(3):e880–93.
Nyssen OP, McNicholl AG, Megraud F, Savarino V, Oderda G, Fallone CA, Fischbach L, Bazzoli F, Gisbert JP. Sequential versus standard triple first-line therapy for helicobacter pylori eradication. Cochrane Database Syst Rev. 2016;(6).
Li H, Liang X, Chen Q, Zhang W, Lu H. Inappropriate treatment in helicobacter pylori eradication failure: a retrospective study. Scand J Gastroenterol. 2018;53(2):130–3.
Lee SJ, Lim YJ, Hong SB, Nam JH, Jang DK, Kang HW, Kim JH, Lee JK, Koh M-S, Lee JH. Standard first-line triple therapy for helicobacter pylori infection: a comparison of eradication rates based on timing of Administration of Proton Pump Inhibitors. Korean J Helicobacter Upper Gastrointest Res. 2018;18(2):115–9.
Kabakambira JD, Hategeka C, Page C, Ntirenganya C, Dusabejambo V, Ndoli J, Ngabonziza F, Hale D, Bayingana C, Walker T. Efficacy of helicobacter pylori eradication regimens in Rwanda: a randomized controlled trial. BMC Gastroenterol. 2018;18(1):134.
Rimbara E, Fischbach LA, Graham DY. Optimal therapy for helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8(2):79.
Abd-Elsalam S, El Nawasany S, Elkhalawany W, Awny S, Mansour L, Ali LA, Soliman S. Increasing rates of treatment failures with the standard triple therapy for helicobacter pylori: a unique and alternative treatment option is urgent. Indian J Med Res Pharm Sci. 2016;3(4):13–6.
Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155(5):1372–1382. e1317.
Shin WG, Lee SW, Baik GH, Huh KC, Lee SI, Chung JW, Jung WT, Park MI. Jung Hk, Kim HU: eradication rates of helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: nationwide survey. Helicobacter. 2016;21(4):266–78.
Chang JY, Shim K-N, Tae CH, Lee KE, Lee J, Lee KH, Moon CM, Kim S-E, Jung H-K, Jung S-A. Triple therapy versus sequential therapy for the first-line helicobacter pylori eradication. BMC Gastroenterol. 2017;17(1):16.
Lee JY, Kim N, Kim MS, Choi YJ, Lee JW, Yoon H, Shin CM, Park YS, Lee DH, Jung HC. Factors affecting first-line triple therapy of helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59(6):1235–43.
Hsu P-I, Kao S-S, Wu D-C, Chen W-C, Peng N-J, Yu H-C, Wang H-M, Lai K-H, Cheng J-S, Chen A. A randomized controlled study comparing reverse hybrid therapy and standard triple therapy for helicobacter pylori infection. Medicine. 2015;94(48):e2104.
Park JY, Dunbar KB, Mitui M, Arnold CA, Lam-Himlin DM, Valasek MA, Thung I, Okwara C, Coss E, Cryer B. Helicobacter pylori clarithromycin resistance and treatment failure are common in the USA. Dig Dis Sci. 2016;61(8):2373–80.
Boyanova L, Markovska R, Yordanov D, Gergova G, Mitov I. Clarithromycin resistance mutations in helicobacter pylori in association with virulence factors and antibiotic susceptibility of the strains. Microb Drug Resist. 2016;22(3):227–32.
Yakoob J, Jafri W, Abbas Z, Abid S, Naz S, Khan R, Khalid A. Risk factors associated with helicobacter pylori infection treatment failure in a high prevalence area. Epidemiology & Infection. 2011;139(4):581–90.
Kotilea K, Mekhael J, Salame A, Mahler T, Miendje-Deyi VY, Cadranel S, Bontems P. Eradication rate of helicobacter pylori infection is directly influenced by adherence to therapy in children. Helicobacter. 2017;22(4):e12383.
Graham DY, Lew GM, Malaty HM, Evans DG, Evans DJ, Klein PD, Alpert LC, Genta RM. Factors influencing the eradication of helicobacter pylori with triple therapy. Gastroenterology. 1992;102(2):493–6.
Wermeille J, Cunningham M, Dederding J-P, Girard L, Baumann R, Zelger G, Buri P, Metry J-M, Sitavanc R, Gallaz L. Failure of helicobacter pylori eradication: is poor compliance the main cause? 2002.
Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 2014;12(7):465.
O'connor A, Lamarque D, Gisbert JP, O'morain C. Treatment of helicobacter pylori infection 2017. Helicobacter. 2017;22:e12410.
Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of helicobacter pylori therapy—results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17(1):99–109.
Vaira D, Vakil N, Menegatti M, van't Hoff B, Ricci C, Gatta L, Gasbarrini G, Quina M, Garcia JMP, van der Ende A. The stool antigen test for detection of helicobacter pylori after eradication therapy. Ann Intern Med. 2002;136(4):280–7.
Chen M-J, Chen C-C, Chen Y-N, Chen C-C, Fang Y-J, Lin J-T, Wu M-S, Liou J-M. Systematic review with meta-analysis: concomitant therapy vs. triple therapy for the first-line treatment of helicobacter pylori infection. Am J Gastroenterol. 2018, 113:1444–57.
Hsu P-I, Wu D-C, Chen W-C, Tseng H-H, Yu H-C, Wang H-M, Kao S-S, Lai K-H, Chen A, Tsay F-W. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58(10):5936–42.
Acknowledgements
We would like to thank Dr. Sarah W. Matuja and Pancras Kahise for follow up of patients. I also acknowledge the support from Department of Medical Microbiology, University of Gottingen, Germany by allowing us to do molecular work in their laboratory.
Funding
This study was funded by triangular partnership between, Mission Institute Würzburg in German and Stellenbosch South Africa and the Catholic University of Health and Allied Sciences-Bugando Tanzania. The funding body had no role in the design of the study, collection, analysis, interpretation of data and in writing the manuscript.
Availability of data and materials
All data have been summarized and included in this manuscript. The raw data is available upon request and the request should be made to the Director of research and Innovation Catholic University of Health and allied Sciences.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions, HJ and SEM did the conception and design of the study, analysis and interpretation of data, drafting the manuscript. HJ did the acquisition of data, laboratory work and clinical work. HJ, CK and SEM did the analysis of data and drafting of article. SEM, CK and AM revised the manuscript critically for important intellectual content. All authors approved the final version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the National Institute for Medical Research Tanzania, Research number NIMR/HQ/R.8a/Vol.IX/2047 and Ethics and Research Committee of CUHAS/BMC with clearance number CREC/066b/2015. Written consent has been obtained from the participants. All information collected after obtaining consent from patients and was kept strictly confidential.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jaka, H., Mueller, A., Kasang, C. et al. Predictors of triple therapy treatment failure among H. pylori infected patients attending at a tertiary hospital in Northwest Tanzania: a prospective study. BMC Infect Dis 19, 447 (2019). https://doi.org/10.1186/s12879-019-4085-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-4085-1