Abstract
Background
Ruxolitinib is a highly potent janus kinase inhibitor that places its users at risk for various bacterial infections and viral reactivation. However new reports are also emerging that suggest greater immunosuppression and risk for fungal disease.
Case presentation
We report the case of a 51 year-old veteran from Guam, treated with ruxolitinib for polycythemia vera, who developed disseminated histoplasmosis and concurrent cryptococcal meningitis.
Conclusion
This case draws attention to the degree of immunosuppression that may be seen with this drug and the need for heightened vigilance for opportunistic infections in those treated with inhibitors of janus kinase/signal transducers and activators of transcription (JAK/STAT) such as ruxolitinib.
Similar content being viewed by others
Background
Ruxolitinib is a highly potent JAK1 and JAK2 inhibitor, FDA approved for the treatment of myelofibrosis and polycythemia vera. The JAK/STAT pathway is the principal signaling mechanism for numerous cytokines and growth factors. It is responsible for cell proliferation, differentiation, cell migration and apoptosis, which in turn are critical for efficient and successful hematopoiesis and immune development [1]. Many studies have attempted to fully elucidate the mechanism by which ruxolitinib impacts the immune system. Stuebig et al. investigated in vivo and in vitro effects of ruxolitinib on T cells and found that the drug impairs T cells proliferation by inducing apoptosis through an up regulation of p53 [2]. Studies also suggest that ruxolitinib strongly affects dendritic cell (DC) function. Monocytes differentiated in the presence of ruxolitinib did not develop any morphologic DC features, thought to be due to inhibition of GM-CSF and IL-4 signaling pathways [3]. Heine et al. also demonstrated that ruxolitinib affected the function and phenotype in preexisting DCs. With this effect on both T cell and DC function, one may predict risk for multiple viral, fungal and other opportunistic pathogens.
The phase 3 study of ruxolitinib (COMFORT II trial) [4] reported that reactivation of herpes zoster virus and tuberculosis were the predominant infections seen with ruxolitinib, as well as a trend towards higher rates of bacterial infections. Since that time, case reports have emerged, documenting the development of Pneumocystis jiroveci pneumonitis, toxoplasmosis retinitis, progressive multifocal leukoencephalopathy (PML), as well as cryptococcal meningoencephalitis and pulmonary cryptococcus in patients treated with ruxolitinib [5,6,7,8,9].
We report a case of concurrent disseminated Histoplasmosis and cryptococcal meningitis in a young patient from Guam on ruxolitinib that developed within months of starting the medication. The case highlights the high degree of immunosuppression that can be seen with JAK/STAT inhibitors.
Case presentation
A 51 year-old male veteran presented with progressive lethargy, fevers and constant frontotemporal headache for past 3 weeks as well as 20 pound weight loss in past 6 months. Born in Guam, the patient had been stationed as part of the military in Texas, Arizona and Kansas. His medical history was notable for polycythemia vera (PCV) treated with ruxolitinib for 18 months. Three months before admission, he had recurrent mouth ulcers, followed by a dental root canal procedure complicated by ulcerative gingivitis, pulpitis and tooth erosions requiring antibiotics and multiple oral surgeries. All antimicrobials had been discontinued over a month prior to presentation.
On admission the patient was febrile to 103.5 °F, tachycardic, and saturating 95% on 2 l of oxygen by nasal cannula. Physical exam revealed somnolence, diminished breath sounds at the left lung base and diffuse abdominal tenderness. Neurologic exam identified no focal deficits. Initial laboratory studies (normal range) revealed hyponatremia to 125 (136–145) mmol/L and a creatinine elevation to 1.8 (0.67–1.17) mg/dL. He also had an elevated alkaline phosphatase of 208 (35–140) U/L and total bilirubin of 1.6 (< 1.2) mg/dL. White cell count was 8002 (4000-10,000) cells/mm3 with 74% polymorphonuclear cells and 13% lymphocytes. The C-reactive protein level was 3.89 (< 0.5) mg/dL and the erythrocyte sedimentation rate was 36 (< 30) mm/hr. Rapid HIV antibody testing, as well as HIV viral load, were negative.
The brain MRI revealed innumerable rim enhancing lesions at the gray-white junction consistent with pyogenic abscesses secondary to hematogenous infection (Fig. 1a). A lumbar puncture revealed 10 mononuclear cells and 9 polymorphonuclear cells/ml CSF. Glucose was 27 (40–70) mg/dL and protein was 72 (15–45) mg/dL. Vancomycin, ceftriaxone and metronidazole were initiated empirically.
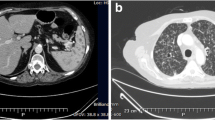
a T-1 weighted brain MRI with gadolinium showing innumerable rim enhancing lesions mostly at the gray-white junction. b and c CT thorax, abdomen and pelvis with contrast demonstrating a retrocardiac rim enhancing mass, measuring 2.7 cm and (d) masses infiltrating adrenal glands bilaterally, left larger than right measuring 8 cm
The patient subsequently underwent chest (Fig. 2b and c) and abdominal CT examinations (Fig. 2d) to evaluate diminished breath sounds and abdominal tenderness. A retrocardiac mass was seen measuring 2.7 cm as well as bilaterally enlarged adrenal glands consistent with infiltrative infection or neoplasm.
CSF cultures grew no bacteria; however, cryptococcal antigen was detected with titer of 1:> 256 in CSF and 1:128 in the serum. Fungal CSF cultures grew Cryptococcus neoformans. An adrenal biopsy performed by interventional radiology revealed numerous fungal organisms on histopathology. Gomorri methenamine silver (GMS) and periodic acid-Schiff (PAS) stains highlighted budding yeast forms within macrophages, most consistent with histoplasmosis. Histoplasma capsulatum subsequently grew in fungal blood cultures. Histoplasma antigen was 11.9 (< 0.5) ng/mL in urine, 8.46 (< 0.4) ng/mL in the serum and 1.86 (< 0.4) ng/mL in CSF. Cryptococcal susceptibilities ultimately returned with an MIC of < 0.03 μg/ml to isavuconazole and 0.25 μg/ml to posaconazole; Histoplasma susceptibilities were < 0.03 μg/ml to both isavuconazole and posaconazole. The patient was diagnosed with concurrent cryptococcal meningitis as well as disseminated histoplasmosis.
Amphotericin infusion as Ambisome at 5 mg/kg every 48 h with flucytosine 1 g q6hrs were initiated for treatment of both identified fungal organisms with improvement of symptoms. Given limited evidence of the successful use of the newer azoles, posaconazole [10,11,12,13] and isavuconazole [14,15,16,17] for CNS disease, the patient was continued on amphotericin infusions for three months and transitioned to 372 mg isavuconazole daily when renal toxicity was noted with Ambisome. A follow up MRI at that time demonstrated diminishing rim-enhancing lesions. The retrocardiac mass was smaller in size on repeat imaging; however, the appearance of the adrenal glands remained unchanged. Cryptococcal antigen titers were 1:16 in serum and 1:8 in CSF. Histoplasmosis antigen in the urine was 0.83 (< 0.5) ng/mL and was no longer detected in the serum. A biopsy of the brain lesions was not performed; however, we hypothesized that the brain lesions were caused by hematogenous spread of histoplasmosis to the gray-white junction with resulting granuloma formation. At the time this case report was written, the patient was still being treated with isavuconazole.
Of note, once the patient was diagnosed with the two fungal infections, ruxolitinib was discontinued. Given the severity of his presentation, his primary oncologist believed that a re-challenge with ruxolitinib was contraindicated.
After further discussion with the patient, he had recollected a 2 cm ulcerative, painful tongue mass (Fig. 2a) that first appeared three months after initiating ruxolitinib. This had been biopsied in the past and was not malignant, but had not been evaluated with fungal culture. Retrospective review of the pathology slides was suggestive of histoplasmosis, with small budding yeast forms noted within granulomas on GMS stain (Fig. 2b).
Discussion and conclusion
To the best of our knowledge this is the first case of both disseminated histoplasmosis and cryptococcal meningitis. This case draws attention to three key factors. The first is the degree of immunosuppression seen with ruxolitinib and the increasing number of reported fungal cases associated with the drug [5,6,7,8]. The second, which was noted on initial phase III trials of ruxolitinib, but must be emphasized, is that the adverse effects from immunosuppression may occur at various intervals after the initiation of ruxolitinib. And third, there is a scarcity of clinical trials and pharmacokinetic studies on the newer azoles that can support their use as step down therapy in severe CNS infections with these fungi.
Our case stresses the need for heightened vigilance for opportunistic infections complicating treatment with ruxolitinib or any JAK inhibitor, of which several appear to be in phase II trials at this time. Since ruxolitinib came to the market, multiple case reports have surfaced detailing its various infectious complications. A review published in 2018 by Dioverti et al., identified 32 cases of opportunistic infections in the setting of ruxolitinib [18]. While the majority of cases reported were either reactivations of tuberculosis (34%) or hepatitis B virus (9%), several fungal infections were also identified. Pulmonary as well as extrapulmonary cryptococcus was the most frequently reported fungus. Several cases of pneumocystis pneumonia and one case of rhino-orbital mucormycosis were also described. Histoplasmosis infections have not been reported previously.
It is also important to recognize that these infections can occur at any point in the course of taking the JAK inhibitor. In our patient, the first symptom, i.e. the tongue lesion, a likely oral manifestation of histoplasmosis, appeared within 3 months of initiating the drug. In reviewing the literature, immunosuppression associated with ruxolitinib was not time dependent occurring anywhere between 1 and over 100 weeks after initiation of the drug [4, 18]. Whether its effect is dose-dependent is still controversial. In the majority of cases ruxolitinib had been discontinued once a major infection was identified; however, for several cases not involving fungal infections, dose reductions were implemented [18]. In the past few years, there has been debate regarding whether the degree of immunosuppression seen with JAK inhibitors mandates initiation of prophylaxis [19, 20]. At this time, we do not believe that this is warranted given the relative infrequency of fungal complications recognized. However, a more proactive pre-emptive approach, that includes routine screening for those with high risk exposures may be prudent.
Although CNS histoplasmosis is still relatively rare [17, 21], other fungal infections involving the central nervous system are not, and azole resistance is on the rise [22]. In addition, azole toxicities and drug-drug interactions limit their use. Azoles are inhibitors and substrates of the CYP2C19, 2C9 and 3A4 enzymes and thus interact with other drugs that use these same CYP450 enzymes for metabolism (notably antiretrovirals, immunosuppressive drugs and cardiovascular medications) [23, 24]. Further clinical trials are needed to evaluate newer agents and their success in infections involving the nervous system.
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- DC:
-
Dendritic cell
- FDA:
-
Food and Drug Administration
- GMS:
-
Gomori methenamine-silver
- HIV:
-
Human immunodeficiency virus
- JAK/STAT:
-
Janus kinase/signal transducers and activators of transcription
- PAS:
-
Periodic acid-Schiff
- PCV:
-
Polycythemia vera
- PML:
-
Progressive multifocal leukoencephalopathy
References
Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3 [cited 2018 Nov 30]. Available from: http://jcs.biologists.org/content/joces/117/8/1281.full.pdf.
Stuebig T, Wolschke C, Alchalby H, Ayuk F, Heinzelmann M, Triviai I, et al. The Pan- JAK inhibitor Ruxolitinib impairs T-cell activation, cytokine production and proliferation in vivo and in vitro. Blood. 2013;122(2001) [cited 2018 Nov 30]. Available from: http://www.bloodjournal.org/content/122/21/2001.short
Heine A, Held SAE, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood. 2013;122(7):1192–202 [cited 2018 Nov 30]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23770777.
Harrison CN, Vannucchi AM, Kiladjian J-J, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–7 Available from: http://www.nature.com/doifinder/10.1038/leu.2016.148.
Hirano A, Yamasaki M, Saito N, Iwato K, Daido W, Funaishi K, et al. Pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis. Respir Med Case Reports. 2017;22:87–90 Available from: https://doi.org/10.1016/j.rmcr.2017.06.015.
Lee SC, Feenstra J, Georghiou PR. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. Case Reports. 2014;2014(jun02 1):bcr2014204950 Available from: http://casereports.bmj.com/cgi/doi/10.1136/bcr-2014-204950.
Chen CC, Chen YY, Huang CE. Cryptococcal meningoencephalitis associated with the long-term use of ruxolitinib. Ann Hematol. 2016;95:361–2.
Goldberg RA, Reichel E, Oshry LJ. Bilateral toxoplasmosis retinitis associated with Ruxolitinib. N Engl J Med. 2013;369(7):681–3 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23944322.
Wathes R, Moule S, Milojkovic D. Progressive Multifocal Leukoencephalopathy Associated with Ruxolitinib. N Engl J Med. 2013;369(2):197–8 [cited 2019 Feb 9]. Available from: http://www.nejm.org/doi/10.1056/NEJMc1302135.
Kim JH, Williams K. Posaconazole salvage treatment for invasive fungal infection. Mycopathologia. 2014;178(3–4):259–65 [cited 2017 Oct 15]. Available from: http://link.springer.com/10.1007/s11046-014-9792-y.
Li Y, Theuretzbacher U, Clancy CJ, Nguyen MH, Derendorf H. Pharmacokinetic/Pharmacodynamic Profile of Posaconazole. Clin Pharmacokinet. 2010;49(6):379–96 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20481649.
Lipp H-P. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: an overview. Br J Clin Pharmacol. 2010;70(4):471–80 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20840439.
Ruping MJGT, Albermann N, Ebinger F, Burckhardt I, Beisel C, Muller C, et al. Posaconazole concentrations in the central nervous system. J Antimicrob Chemother. 2008;62(6):1468–70 [cited 2017 Oct 15]. Available from: https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dkn409.
Thompson GR, Rendon A, Ribeiro Dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Clin Infect Dis. 2016;63(3):356–62.
Falci DR, Pasqualotto AC. Profile of isavuconazole and its potential in the treatment of severe invasive fungal infections. Infect Drug Resist. 2013;6:163–74 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24187505.
Wiederhold NP, Kovanda L, Najvar LK, Bocanegra R, Olivo M, Kirkpatrick WR, et al. Isavuconazole is effective for the treatment of experimental cryptococcal meningitis. Antimicrob Agents Chemother. 2016;60(9):5600–3 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27324761.
Nyalakonda H, Albuerne M, Suazo Hernandez LP, Sarria JC. Central nervous system histoplasmosis in acquired immunodeficiency syndrome. Am J Med Sci. 2016;351(2):177–86 [cited 2017 Oct 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26897273.
Dioverti MV, Abu Saleh OM, Tande AJ. Infectious complications in patients on treatment with Ruxolitinib: case report and review of the literature. Infect Dis (Auckl). 2018;50(5):381–7.
Heine A, Brossart P, Wolf D. Ruxolitinib is a potent immunosuppressive compound: is it time for anti-infective prophylaxis? Blood. 2013;122(23):3843–4 [cited 2019 Feb 9]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24288410.
McLornan DP, Khan AA, Harrison CN. Immunological consequences of JAK inhibition: friend or foe? Curr Hematol Malig Rep. 2015;10(4):370–9 [cited 2019 Feb 9]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26292803.
Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin N Am. 2016;30(1):207–27.
Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis. 2008;46(1):120–8 [cited 2017 Oct 15]. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1086/524071.
Lempers VJ, Martial LC, Schreuder MF, Blijlevens NM, Burger DM, Aarnoutse RE, et al. Drug-interactions of azole antifungals with selected immunosuppressants in transplant patients: strategies for optimal management in clinical practice. Curr Opin Pharmacol. 2015;24:38–44 [cited 2019 Feb 16]. Available from: https://www.sciencedirect.com/science/article/pii/S147148921500079X?via%3Dihub.
Allen D, Wilson D, Drew R, Perfect J. Expert review of anti-infective therapy azole antifungals: 35 years of invasive fungal infection management. 2015 [cited 2019 Feb 16]; Available from: https://www.tandfonline.com/doi/full/10.1586/14787210.2015.1032939
Acknowledgements
We are grateful to all those who cared for our patient during his lengthy hospitalization and the Infectious Diseases department in assisting with the overall management of this patient’s unique presentation.
Funding
No funding was required for this case report.
Availability of data and materials
N/A
Author information
Authors and Affiliations
Contributions
KP: Conceptualization, Data curation, Writing – original draft, revision. DR: Writing – original draft, revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
Written consent was obtained from patient.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Prakash, K., Richman, D. A case report of disseminated histoplasmosis and concurrent cryptococcal meningitis in a patient treated with ruxolitinib. BMC Infect Dis 19, 287 (2019). https://doi.org/10.1186/s12879-019-3922-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-019-3922-6