Abstract
Background
External and intraocular infections can lead to visual impairments, which is a major public health problem. Bacteria are the most frequent pathogens affecting ocular structures; the increasing rate of antimicrobial drug resistance is a worldwide concern. The aim of this study was to determine the occurrence of bacteria in ocular infections, their antimicrobial susceptibility patterns, and risk factors in bacterial ocular infection.
Methods
A hospital based cross-sectional study was conducted from September 2015 to December 2015 at Quiha Ophthalmic Hospital, Tigray, northern Ethiopia. Ocular specimens from blepharitis, blepharoconjunctivitis, conjunctivitis, keratitis, endophthalmitis, periorbital cellulitis and dacrocystitis were collected from 270 individuals with suspected ocular infection. Data on sociodemographic and risk factors were also collected using a structured questionnaire. Data analysis was performed using SPSS version 21 and 0.05 with a corresponding 95% confidence interval (CI) was considered statistically significant.
Results
Among 270 study subjects, 180 (66.7%) were culture positive for different bacterial isolates. The predominant bacterial isolates were Staphylococcus aureus (40, 22.2%), coagulase negative staphylococci (31, 17.2%) and Pseudomonas aeruginosa (21, 11.7%). Ocular surface disease, ocular trauma, hospitalization and cosmetic application practices were significantly associated with the occurrence of bacterial infection. Concerning antimicrobial susceptibility, most isolates were susceptible to amikacin (137, 93.2%), gentamicin (131, 89.1%) and ciprofloxacin (141, 89.2%). Overall, 40 (22.5%), 34 (19.1%) and 62 (34.8%) isolates were resistant to one, two, and three or more antimicrobials, respectively.
Conclusion
Bacteria were isolated from the majority of the study subjects. More than half of the bacterial isolates were resistant at least to one drug and a significant rate of multidrug resistance was detected. Therefore, identification of the etiologic agent and antimicrobial susceptibility testing should be practiced to select the appropriate antimicrobial agent to treat eye infections and prevent the emergence of drug resistant bacteria.
Similar content being viewed by others
Background
The eye, a functionally and structurally complex organ, experiences a variety of bacterial, viral, fungal and parasitic infections [1–3]. Bacterial infections (Both Gram-positive and Gram-negative) contribute to 32 to 74% of ocular infections globally [4–10]. Staphylococci are the leading ocular isolates worldwide among the Gram-positive bacteria, [9, 11–13]; while Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli are the major Gram-negative bacteria isolated from ocular infections [3, 14, 15].
Bacteria are the most common agents causing external ocular infections, including blepharitis, keratitis, dacryocystitis, and orbital cellulitis. Bacteria are responsible for 70–80% of conjunctival morbidity which poses a huge socioeconomic burdens to the general public [16–19]. Blepharitis, an inflammation of the eyelid, can cause complete loss of the eyelashes if not diagnosed early [20]. Bacterial keratitis is the leading cause of corneal blindness [18, 21, 22].
External ocular infections may remain localized, or progress to adjacent tissues. For instance, external and internal hordeolum can result from the spread of eyelid infection to the respective glands [23]. Dacryocystitis, inflammation of the nasolacrimal duct, is the result of nasolacrimal duct obstruction. The accumulation of fluid and edema in the eye secondary to dacryocystitis, is a potential danger to ocular tissues such as the cornea and conjunctiva [24, 25]. External ocular infections that result from systemic spread, surgery or are secondary to ocular trauma, can lead to sight threatening intraocular infections such as endophthalmitis [1, 22, 26, 27]. Left untreated, bacterial ocular infections, bacterial products and vigorous inflammation following infection, can irreversibly damage ocular structures [28, 29]. Inflammation and scarring, once present, may not be easily resolved and can result in visual impairment or permanent loss of vision [30, 31].
Additionally, bacterial ocular infections have been complicated by multidrug resistance;, a problem that is intensifying over time [32, 33]. This poses a challenge in the clinical management of bacterial ocular infections [34–36].
Eye infections are remarkably common in northern Ethiopia; however, no studies have been conducted on this topic in this region. Similarly, the antimicrobial susceptibility patterns of infecting bacteria in this region are not known. Therefore, we sought to determine the ocular bacterial infection, the risk factors and the antimicrobial susceptibility of bacterial isolates in ocular infections at Quiha Ophthalmic Hospital, Tigray, northern Ethiopia.
Methods
Study design, data and specimens collection
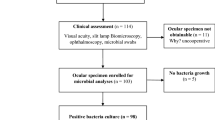
A hospital based cross-sectional study was conducted from September-December 2015 in Quiha Ophthalmic Hospital, Quiha, Tigray, Ethiopia. A total of 270 patients who presented with suspected ocular infection were consecutively included in the study. Sociodemographic, clinical and risk-factor related data were collected using a pretested structured questionnaire using interviewer-administered questionnaires. Study subjects were examined using a slit-lamp biomicroscope to screen for the presence of ocular infection. Ocular specimens were collected from periorbital cellulitis (N = 8), dacryocystitis (N = 13), blepharitis (N = 26), blepharoconjunctivitis (N = 16), conjunctivitis (N = 123), keratitis (N = 57) and endophthalmitis (N = 27) diagnoses. An ophthalmic surgeon collected specimens from all patients except those patients who had endophthalmitis who had their specimemens collected by an ophthalmologist [37–39].
Specimen transportation
The respective specimens were then transferred into 2 ml of brain-heart infusion broth (Oxoid, Hampshire, UK). Tubes were tightly capped, gently mixed, labeled and placed in cold chain. Finally, samples were transported to the Microbiology Laboratory, Mekelle University, for microbiological analysis [38, 39].
Cultivation and identification of isolates
The broth was gently mixed to homogeneity; 100 μl of inoculum was then dispensed and streaked onto blood agar, chocolate agar, MacConkey agar, Mannitol salt agar and modified Thayer-Martin agar (MTM) (Oxoid, Hampshire, UK). To maintain the presence of CO2, chocolate agar and MTM agar plates were placed in candle jars. All media were incubated overnight at 37 °C. After 24 h of incubation, each plate was inspected for any growth and negative plates were incubated for an additional 24 h. For eyelid and conjunctival swabs, culture positivity was determined based on a threshold criteria. Corneal specimens were considered as positive if there was a confluent growth at the site of inoculation [39]. All bacterial isolates were identified using standard clinical laboratory methods [40, 41].
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed using the standard Kirby-Bauer disk diffusion method [41]. For fastidious organisms, Muller-Hinton agar with 5% sheep blood was used and incubated in 5% CO2; GC agar and Haemophilus Test Medium were used for Neisseria gonorrhoeae and Haemophilus influenzae, respectively [42]. The following antibiotics were used: trimethoprim-sulfamethoxazole (SXT; 1.25/23.75 μg), erythromycin (15 μg), clarithromycin (15 μg), chloramphenicol (30 μg), clindamycin (2 μg), tetracycline (30 μg), doxycycline (30 μg), amikacin (30 μg), gentamicin (10 μg), ciprofloxacin (5 μg), ceftriaxone (30 μg) and cefoxitin (30 μg) (HIMEDIA). The zones of inhibition of the antimicrobial agents were measured using calipers; bacteria were described as susceptible or resistant based on CLSI guideline [42]. Multidrug resistance was defined as non-susceptible to ≥1 agent in ≥3 antimicrobial categories according to the definition of Magiorakos and colleagues [43].
Quality assurance
Standard operational procedures were strictly followed from the pre-analytical to the post-analytical phase. The questionnaire was pretested on 16 patients with ocular infection. Data collectors were trained on data collection procedures. Completed questionnaires were proofread immediately after completion by the research team to clarify any labelling errors or illegibility. Standard Operating procedures were followed during Specimen collection, handling, transportation, microbiological analysis and interpretation of results. Reagents, media and antimicrobial disks were checked for the expiry date, damage and storage problems. Media sterility was strictly monitored by random sampling and incubation to test for any growth. In addition, reference strains of Staphylococcus aureus ATCC25923, E. coli ATCC25922 and P. aeruginosa ATCC27853 were used to monitor the quality of this work [42].
Statistical analysis
Data was entered into SPSS version 21, and descriptive statistics, bivariate and multivariate logistic regression analyses were performed. Bivariate logistic regression was employed to look for association between the outcome variable and independent variables. Variables with P-values <0.05 were reanalyzed in multivariate logistic regression to identify risk factors for bacterial occurrence. The corresponding P-value <0.05 and the confidence interval (CI) of 95% were considered statistically significant.
Results
Sociodemographic characteristics
A total of 270 study subjects with clinically diagnosed ocular infections were included in the study, of whom 151 (55.9%) females, 119 (44.1%) were males and 158 (58.5%) were rural dwellers (Table 1). The mean (SD) age of the study subjects was 37.8 (22.9) years.
Bacterial profile and clinical features
Conjunctivitis (123 cases of 270, 45.6%) was the leading clinical presentation, followed by keratitis (57, 21.1%), endophthalmitis (27, 10.0%), blepharitis (26, 9.6%), blepharoconjunctivitis (16, 5.9%), dacryocystitis (13, 4.8%), and periorbital cellulitis (8, 3.0%) (Table 2). Of the total 270 patients, 180 (66.7%) were culture positive for bacterial isolates. Single and mixed bacterial infection patients were seen in 174 (96.7%) and 6 (3.3%) cases, respectively (Table 2). The mixed isolates were detected from three patients with conjunctivitis, one patient with periorbital cellulitis, one with keratitis and one with endophthalmitis diagnosis.
Bacterial infection was significantly higher in patients with dacryocystitis 92.3% (COR = 20, 95% CI: 2.0–195, P = 0.01), periorbital cellulitis 87.5% (COR = 11.7, 95% CI: 1.1–183.8, P = 0.03), keratitis 80.7% (COR = 6.9, 95% CI: 2.0–23.3, P < 0.01) and endophthalmitis 74.1% (COR = 4.7, 95% CI: 1.2–17.9, P = 0.02) than for the other diagnoses (Table 2).
Identification of bacterial isolates
In this study, 113 (60.7%) bacterial isolates were Gram-positive, and 87 (39.3%) were Gram-negative. Overall, the dominant bacterial isolates were S. aureus (40, 21.5%) followed by coagulase-negative staphylococci (CoNS; 31, 16.7%), P. aeruginosa (21, 11.3%) and E. coli (15 8%) (Table 3).
Risk factors for bacterial occurrence in ocular infections
In this study, 11 independent variables were considered during the bivariate analysis of risk factors for bacterial infection. In multivariate analysis, ocular surface disease (AOR = 13.6, 95% CI: 3.8 49.3, P < 0.01), ocular trauma (AOR = 4.2, 95% CI: 1.4–13, P = 0.012), hospitalization (AOR = 3.3, 95% CI: 1.03–12.5, P = 0.04), and cosmetic application practices (AOR = 4.7, 95% CI: 1.6–13.9 P < 0.01) were significantly associated with bacterial occurrence (Table 4). Though not statistically significant, bacterial occurrence was higher in housewives 32 (74.4%) (COR = 2.3, 95% CI: 0.6–9.0, P = 0.06) and in those subjects with no formal education 90 (73.8%) (COR = 1.8, 95% CI: 0.6–5.0, P = 0.2) (Table 5).
Antimicrobial susceptibility pattern of bacterial isolates
In this study, the majority of bacterial isolate were susceptible to ciprofloxacin (141, 89.2%), amikacin (137, 93.2%), gentamicin (131, 89.1%), chloramphenicol (106, 70.2%), and doxycycline (100, 71.9%). However, bacterial isolates were less susceptible to tetracycline (79, 51.3%) and SXT (66, 48.7%). Among the Gram-negative bacteria, 47 bacterial isolates (83%) were susceptible to ceftriaxone. In addition, 79 (70.7%) of the Gram-positive isolates were susceptible to erythromycin. All isolates of S. pyogenes, S. pneumoniae, viridans streptococci and H. influenzae were susceptible to clarithromycin. Methicillin-resistance was observed in 7 (17.5%) S. aureus and 14 (45.2%) CoNS isolates (Table 6).
Overall, 40 (22.5%) bacterial isolates were resistant to at one antimicrobial agent; 96 (53.9%) were resistant to ≥2 antimicrobials (Table 7). A standard definition of MDR was applied for isolates of S. aureus, members of the Enterobacteriaceae and Acinetobacter spp. [43], by which 18 (45%) S. aureus, 17 (31.5%) isolates of Enterobacteriaceae, and one Acinetobacter isolate were found to be MDR. Among Enterobacteriaceae, Klebsiella spp. (6, 50%) and E. coli (7, 46.7%) were the commonest MDR isolates (Table 8).
Discussion
Our assessment revealed that a high proportion of ocular infections (66.7%) were due to bacterial infections elsewhere in Ethiopia, lower proportions have been observed (48.8–60.8%) [10, 11, 44]; however, higher proportions (74.7%) have been observed in Jimma, Ethiopia [6]. This variation could be attributed to the inclusion of only exra-ocular infection in other studies. Our observations were also higher than that seen in India (34.5%) [3], Japan (32.2%) [4], and Iran (37.5%) [45]; sociodemographic, geographical or climatic differences for the patient populations could partially explain this [38]. Similar to findings from India [46], Iran [9], and other studies in Ethiopia [6, 10, 11, 44], Gram-positive bacteria contributed to majority (60.7%) of the total bacterial isolates in our study. Overall, the predominant bacterial isolate was S. aureus (21.5%), as has been reported in Jimma (28.4%) [6], Gondar (21%) [10], Nigeria (23.7%) [23], and India (26.6%) [47]. Staphylococcal isolates were predominant among patients with conjunctivitis, blepharitis and blepharoconjunctivitis diagnoses, similar to that in patients from Jimma [6], Nigeria [23], and Columbia [48]. Conversely,, CoNS isolates were more frequent in keratitis (17%) and endophthalmitis (28.6%) diagnoses, as has been reported in Uganda [49], Mexico [50], Australia [8], Pakistan [51] and India [52].
As compared to published literature from other areas in Ethiopia, Nigeria, UK, Australia and India, most streptococcal isolates were from conjunctivitis diagnoses, with some isolates from blepharoconjunctivitis and keratitis. In the literature, Staphylococci and streptococci are known to be the main agents of post-traumatic periorbital cellulitis [54]; in our study, S. aureus (50%), S. pneumoniae (12.5%) and S. pyogenes (12.5%) were the main Gram-positive isolates from periorbital cellulitis. As has been seen in Gondar [19], Borumeda [44], and India [47], S. pneumoniae and S. pyogenes were the predominant species among patients with dacryocystitis.
The most common isolates among the Gram-negative bacteria were P. aeruginosa (11.3%), E. coli (8.1%) and Klebsiella spp. (6.4%) as has been seen in Hawassa; however, the proportions of P. aeruginosa (4.9%) and E. coli (4.9%) observed in Hawassa were lower than in our study [23]. This may be due to the additional isolates from endophthalmitis diagnoses we identified.
P. aeruginosa was the predominant isolate in keratitis (23.4% of diagnoses) similar to findings reported in Nigeria (23.8%) [23], the UK (24.3%) [7], Australia (21%) [8], and Iran (24.7%) [15]. Pseudomonal keratitis is a progressive infection with large infiltrate and scarring [22, 53]; this therefore means majority of patients in our population are at risk of blindness.
Our assessment illustrated that the presence of ocular surface disease, ocular trauma, hospitalization and cosmetic application practices were significantly associated with the occurrence of bacterial infection. Risk factors for bacterial ocular infections that have been described include a history of hospital stay in Columbia and Portugal [48, 55], ocular trauma in Australia, Florida, Iran, China and Mexicao [1, 8, 15, 22, 50] and ocular disease in Florida and Colombia [22, 48] Cosmetic application practices were considered only for female participants. Limited information exists on the relationship between cosmetic application practices and bacterial ocular infections.
Almost one quarter of the bacterial isolates in were susceptible to all of the tested antimicrobials. Resistance to two or more antimicrobials was seen in 53.9% of the isolates, which is lower than that reported in other regions in Ethiopia (69.9–87.1%) [10, 11]. S. pneumoniae (87.5%), CoNS (87%) and S. aureus (82.5%) were the most resistant among Gram-positive isolates. Acinetobacter spp. (100%), Klebsiella spp. (100%) and Enterobacter spp. (80.0%) were the most resistant among Gram-negative isolates. which is lower than that reported in other studies [56, 57].
Here, relatively, amikacin (93.2%), gentamicin (89.1%) and ciprofloxacin (89.2%) were revealed high efficacy towards Gram-positive and Gram-negative isolates. Studies in Gondar have reported lower susceptibility to gentamicin (54.8%) and ciprofloxacin (74.2%) [10, 19]; differences in the variety of isolates tested may partly explain this. Outside Ethiopia, comparable susceptibility patterns to amikacin, ciprofloxacin and chloramphenicol were reported in Iran [15] and India [47]. However, the gentamicin susceptibility in this study is much higher than that in the study conducted in India (58.6%) [47]; regional variations in antibiotic prescription practices could explain this [38]. The resistance we observed to tetracycline and erythromycin was high, and this paralleled a study from India [3]. High resistance is usually due to the over use and empirical treatment of patients that ultimately leads to the emergence of drug resistant strains [58, 59].
Methicillin resistance was seen in 17.5% of the isolated S. aureus, which is in line with a study conducted in India (14%) [60], but lower than the rates in Taiwan (52.8%) [57] and Uganda (31.9%) [49]. In addition, 45.2% of CoNS were methicillin resistant, which was higher than detected in Uganda (27.6%) [49]. To date, other than a study conducted in Gondar, most studies in Ethiopia did not determine methicillin resistance rates for ocular staphylococcal isolates [19]. Our study was not without limitation; we did not isolate anaerobic bacteria or Chlamydia trachomatis due to unavailability of the prerequisite facilities. We also did not determine the antimicrobial susceptibility of Moraxella species or Aeromonas species due to a lack of antimicrobials for agar dilution.
Conclusions
In conclusion, a high prevalence of bacteria was found in patients with ocular infection. “Ocular surface disease, ocular trauma, hospitalisation and cosmetic application practices were significantly associated with the occurrence of bacterial infection. Overall, S. aureus, CoNS, and P. aeruginosa were the predominant isolates. The majority of the bacterial isolates were multidrug resistant. Methicillin resistance was higher in CoNS than in S. aureus. S. pneumoniae, CoNS of which S. aureus was the most resistant species among Gram-positive isolates; Klebsiella species and E. coli were most resistant among Gram-negative isolates. Identification of the specific etiologic agent and antimicrobial susceptibility testing should be practiced during the management of ocular infections to reduce the further emergence of multidrug-resistant bacteria”.
Abbreviations
- AOR:
-
Adjusted Odds Ratio
- ATCC:
-
American Type Culture Collection
- CAMP:
-
Christie, Atkins and Munch-Peterson
- CEO:
-
Chief Executive Officer
- CI:
-
Confidence interval
- CLSI:
-
Clinical and Laboratory Standards Institute
- CoNS:
-
Coagulase Negative Staphylococci
- COR:
-
Adjusted Odds Ratio
- ID:
-
Identification number
- MDR:
-
Multi-drug Resistance
- MRSA:
-
Methicillin resistant Staphylococcus aureus
- MRSE:
-
Methicillin Resistant Staphylococcus epidermidis
- MTM:
-
Modified Thayer-Martin
- PCR:
-
Polymerase Chain Reaction
- SPSS:
-
Statistical Package for Social Sciences
References
Long C, Liu B, Xu C, Jing Y, Yuan Z, Lin X. Causative organisms of post-traumatic Endophthalmitis: a 20-year retrospective study. BMC Ophthalmol. 2014;14:34.
Azari A, Barney P. Conjunctivitis: A Systematic Review of Diagnosis and Treatment. JAMA. 2013;310(16):1721–9.
Hemavathi, Sarmah P, Shenoy P. Profile of Microbial Isolates in Ophthalmic Infections and Antibiotic Susceptibility of the Bacterial Isolates: A Study in an Eye Care Hospital, Bangalore. J Clin Diagn Res. 2014;8(1):23–5.
Shimizu Y, Toshida H, Honda R, Ohta T, Asada Y, Murakami A. Prevalence of drug resistance and culture-positive rate among microorganisms isolated from patients with ocular infections over a 4-year period. Clin Ophthalmol. 2013;7:695–702.
Samuel SO, Enock ME, Ekozien MI, Ehimen M, Nmorsi OPG, Omoti AE. Pattern of bacterial Conjunctivitis in Irrua Specialist Teaching Hospital. Nigeria J Microbiol Biotech Res. 2012;2(4):516–20.
Tesfaye T, Beyene G, Gelaw Y, Bekele S, Saravanan M. Bacterial Profile and Antimicrobial Susceptibility Pattern of External Ocular Infections in Jimma University Specialized Hospital. Southwest Ethiopia AJIDM. 2013;1(1):13–20.
Orlans HO, Hornby SJ, Bowler ICJW. In vitro antibiotic susceptibility patterns of bacterial Keratitis isolates in Oxford, UK: a 10-year review. Eye. 2011;25:489–93.
Ly NC, Pham N, Badenoch R, Bell M, Hawkins G, Rafferty L. Bacteria commonly isolated from Keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin. Clin Exp Ophthalmol. 2006;34:44–50.
Khosravi AD, Mehdinejad M, Heidari M. Bacteriological findings in patients with ocular infection and antibiotic susceptibility patterns of isolated pathogens. Singapore Med J. 2007;48(8):741–3.
Muluye D, Wondimeneh Y, Moges F, Nega T, Ferede G. Types and drug susceptibility patterns of bacterial isolates from eye discharge samples at Gondar University Hospital, Northwest Ethiopia. BMC Res notes. 2014;7:292.
Amsalu A, Abebe T, Mihre A, Delelegn D, Tadess E. Potential bacterial pathogens of external ocular infections and their antibiotic susceptibility pattern at Hawassa University teaching and referral Hospital, Southern Ethiopia. Afr J Microbiol Res. 2015;9(14):1012–119.
Summaiya M, Neeta K, Sangita R. Ocular infections: rational approach to antibiotic therapy. Natl J Med Res. 2012;2(1):22–4.
Kaliamurthy J, Kalavathy MC, Parmar P, Jesudasan N, Philip A, Thomas. Spectrum of Bacterial Keratitis at a Tertiary Eye Care Centre in India. Biomed Res Int. 2013;2013:1–8.
Idu FK, Odjimogho SE. Susceptibility of conjunctival bacterial pathogens to fluoroquinolones.A comparative study of ciprofloxacin, norfloxacin and ofloxacin. Online J Health Allied Scs. 2003;2(3):1–5.
Rahimi F, Hashemian MN, Khosravi A, Moradi G, Bamdad S. Bacterial Keratitis in a Tertiary Eye Centre in Iran: A Retrospective Study. Middle East Afr J Ophthalmol. 2015;22(2):238–44.
Epling J. Bacterial conjunctivitis. Clin Evid. 2012;2:704.
Buznach N, Dagan R, Greenberg D. Clinical and bacterial characteristics of acute bacterial conjunctivitis in children in the antibiotic resistance era. Pediatr Infect Dis J. 2015;24:823–8.
Cao J, Yang Y, Yang W, Wu R, Xiao X, Yuan J, Xing Y, Tan X. Prevalence of infectious keratitis in central China. BMC Ophthalmol. 2014;14:43.
Assefa Y, Moges F, Endris M, Zereay B, Amare B, Bekele D. Bacteriological profile and drug susceptibility patterns in Dacryocystitis patients attending Gondar University Teaching Hospital, Northwest Ethiopia. BMC Ophthalmol. 2015;15:34.
Kowalski RP, Dhaliwal DK. Ocular bacterial infections: current and future treatment options. Expert Rev Anti Infect Ther. 2005;3(1):131–9.
Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear. Optom Vis Sci. 2007;84(4):273–8.
Henry CR, Flynn W, Miller D, Forster RK, Alfonso EC. Infectious Keratitis Progressing to Endophthalmitis: A 15-Year-Study of Microbiology, Associated Factors, and Clinical Outcomes. Ophthalmology. 2012;119(12):2443–9.
Ubani, Ahanna U. Common bacterial isolates from infected eye. JNOA. 2009;15:40–7.
Maheshwari R, Maheshwari S, Shah T. Acute dacryocystitis causing orbital cellulitis and abscess. Orbit. 2009;28:196–9.
Amin RM, Hussein FA, Idris HF, Hanafy F, Abdallah DM. Pathological, immunohistochemical and microbiological analysis of lacrimal sac biopsies in patients with chronic dacrocystitis. Int J Ophthalmol. 2013;6(6):817–26.
Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial Endophthalmitis: an East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107(8):1483–91.
Wallin O, Abdullah M, Lundstrom M, Montan P. Endophthalmitis and severe blebitis following trabeculectomy. Epidemiology and risk factors; a single-center retrospective study. Acta Ophthalmol. 2014;92(5):426–31.
Pandey KR, Yu F, Kumar A. Targeting Toll-like receptor signaling as a novel approach to prevent ocular infectious disease. Indian J Med Res. 2013;138(5):609–19.
Zaidi T, Yoong P, Pier GB. Staphylococcus aureus Corneal Infections: Effect of the Panton-Valentine Leukocidin (PVL) and Antibody to PVL on Virulence and Pathology. IOVS. 2013;54(7):4430–8.
Forrester JV, Xu H. Goodnews badnews: the Yin and Yang of immune privilege in the eye. Front Immunol. 2012;3(338):1–18.
Wiskur BJ, Hunt JJ, Callegan MC. Hypermucoviscosity as a Virulence Factor in Experimental Klebsiella pneumoniae Endophthalmitis. Invest Ophthalmol Vis Sci. 2008;49(11):4931–8.
Lee K, Lee H, Kim M. Two Cases of Corneal Ulcer due to Methicillin-Resistant Staphylococcus aureus in High Risk Groups. Korean J Ophthalmol. 2010;24:240–4.
Bertino JS. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol. 2009;3:507–21.
Sharma S. Antibiotic resistance in ocular bacterial pathogens. Indian J Med Microbiol. 2011;29:218–22.
Rodriguez-Gonzalez F, Marrero-Saavedra D, Rutllan-Civit J, Cabrera-Vargas E, Martinez-Quintana E. Ocular necrotizing fasciitis due to Pseudomonas aeruginosa in a non-neutropenic patient. Saudi J Ophthalmol. 2013;27:281–2.
Brown L. Resistance to ocular antibiotics: an overview. Clin Exp Optom. 2007;90:258–62.
Baron J, Miller JM, Weinstein P, Richter S, Gilligan P, Thomson B, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2013 Recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57(4):22–121.
Sharma S. Diagnosis of Infectious disease of the eye. Eye. 2012;26:177–84.
Therese KL, Madhavan HN. Microbiological Procedures for Diagnosis of Ocular Infections. www.ijmm.org/documents/ocular/pdf. Accessed 5 Aug 2015.
Parija SC. Text Book of Practical Microbiology. 1st ed. Banglore: Ahuja publishers; 2006.
Cheesbrough M. District laboratory practice in tropical countries. Part 2. 2nd ed. New York: Cambridge University Press; 2006.
Clinical and Laboratory standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests, vol. 34. Wayne: Twenty-Fourth Informational Supplement; 2014. p. 1.
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81.
Shiferaw B, Gelaw B, Assefa A, Assefa Y, Addis Z. Bacterial isolates and their antimicrobial susceptibility pattern among patients with external ocular infections at Borumeda Hospital, Northeast Ethiopia. BMC Ophthalmol. 2015;15:103.
Aghadoost D, Khorshidi A. Antibiotic Resistance Patterns of Ocular Surface Bacterial Flora. J Infect Dis Antimicrob Agents. 2005;22(2):53–7.
Namitha BN, Mahalakshmi, Rao A. Aerobic Bacteriological Profile in Cases of Ocular Infections in a Tertiary Care Hospital (Navodaya Medical College and Research Centre, Raichur). IOSR-JDMS. 2014;13(11):14–21.
Bharathi JM, Ramakrishan R, Shivakumar C, Meenakshi R, Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care Hospital in south India. Indian J Ophthalmol. 2010;58(6):497–507.
Blanco C, Nunez MX. Antibiotic susceptibility of Staphylococci isolates from patients with chronic conjunctivitis: including risk factors and clinical evaluation. J Ocul Pharmacol TH. 2013;29(9):803–8.
Mshangila B, Paddy M, Kajumbula H, Ateenyi-Agaba C, Kahwa B, Seni J. External ocular surface bacterial isolates and their antimicrobial susceptibility patterns among pre-operative cataract patients at Mulago National Hospital in Kampala, Uganda. BMC Ophthalmol. 2013;13:71.
Chirinos-Saldana P, Lucio B, Hernandez-Camarena JC, Navas A, Ramirez-Miranda A, Vizuet-Garcia L. Clinical and microbiological profile of infectious Keratitis in children. BMC Ophthalmol. 2013;13(54):1471–2415.
Abdullah FE, Khan MI, Waheed S. Current pattern of antibiotic resistance of clinical isolates among conjunctival swabs. Pak J Med Sci. 2013;29(1):81–4.
Jayasudha R, Narendran V, Manikandan P, Prabagarana SR. Identification of Polybacterial Communities in Patients with Postoperative, Posttraumatic, and 39 Endogenous Endophthalmitis through 16S rRNA Gene Libraries. J Clin Microbiol. 2014;52(5):1459–66.
Karsten E, Watson L, Foster R. Diversity of Microbial Species Implicated in Keratitis: A Review. Open Ophthalmol J. 2012;6:110–24.
Givner LB. Periorbital versus Orbital cellulitis. Pediatr Infect Dis J. 2002;21:1157–8.
Dias C, Gonçalves M, Joao A. Epidemiological Study of Hospital-Acquired Bacterial Conjunctivitis in a Level III Neonatal Unit. Sci World J. 2013;2013:1–5.
Das S, Constantinou M, Daniell M, Taylor HR. Moraxella keratitis: predisposing factors and Clinical review of 95 cases. Br J Opthalmol. 2006;90:1236–12368.
Chuang C-C, Hsiao C-H, Tan H-Y, Ma DH-K, Lin K-K, et al. Staphylococcus aureus Ocular Infection: Methicillin-Resistance, Clinical Features, and Antibiotic Susceptibilities. PLoS One. 2012;8(8):e42437.
Gaynor BD, Chidambaram JD, Cevallos V, Miao Y, Miller K, Jha HC, et al. Topical ocular antibiotics induce bacterial resistance at extraocular sites. Br J Ophthalmol. 2005;89:1097–9.
Lieberman JM. Appropriate antibiotic use and why it is important: the challenges of bacterial resistance. Pediatr Infect Dis J. 2003;22:1143–51.
Biradar S, Chandrashekhar DK, Gangane R, Chandrakanth C, Biradar KG, VinodKumar CS. Spectrum of microbial keratitis and antimicrobial susceptibility at tertiary care teaching Hospital in north Karnataka. Int J Pharm Biomed Res. 2012;3(2):117–20.
Acknowledgement
The authors would like to acknowledge the Mekelle University and Tigray Health Research and Regional Laboratory for financial support and providing laboratory space and facilities to conduct their experiments. Quiha Ophthalmic Hospital and all study participants are acknowledged for their cooperation during sample collection.
Funding
This work was supported by Mekelle University, College of Health sciences, Post graduate Students Research fund.
Availability of data and materials
Data supporting the conclusions of this article are available by request to M. Teweldemedhin. The relevant raw data will be made available to researchers wishing to use them for non-commercial purposes.
Authors’ contributions
Conceived and designed the experiments: MT, MS, DG. Performed the experiments: MT, MS, DG. Analyzed the data: MT, MS, DG, AG. Contributed reagents/materials/analysis tools: ARH, THRL. Wrote the manuscript: MT, DG, MS, AG. All authors provided critical review of the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical clearance was obtained from Mekelle University, College of Health sciences, Research Ethics Review Committee (ERC0670/2015). Permission was also obtained from Tigray Regional Health Bureau and Quiha Hospital. Written informed consent and assent was obtained from the study subjects before data collection.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Teweldemedhin, M., Saravanan, M., Gebreyesus, A. et al. Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: an evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect Dis 17, 207 (2017). https://doi.org/10.1186/s12879-017-2304-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-017-2304-1