Abstract
Background
Rwanda is a central African country with about 12 million inhabitants. The 1994 genocide against the Tutsi destroyed much of the infrastructure, including the health system. Although this has improved significantly, many challenges remain to be addressed. In this study, the prevalence of serological markers of past and ongoing hepatitis B virus (HBV) infection and HBV vaccine related immunity was investigated in samples from blood donors from all regions of Rwanda.
Methods
The results from hepatitis B surface antigen (HBsAg) analyses of all (45,061) blood donations collected countrywide in 2014 from 13,637 first time and 31,424 repeat blood donors were compiled. Samples from 581 HBsAg negative blood donors were selected for further analysis for antibodies against HBV, anti-HBs and anti-HBc. Additional 139 samples from HBsAg positive donors were analyzed for HBeAg/anti-HBe (132 samples) and for HBV DNA. The S-gene was amplified by PCR, products sequenced, and phylogenetic analysis was performed.
Results
HBsAg was found in 4.1% of first time donors with somewhat higher prevalence among those from the Central and Eastern regions than from other parts of the country. Indications of past infection was found in 21% of the HBsAg negative donors, 4.3% had only anti-HBs suggesting HBV vaccination. HBeAg was detected in 28 (21%), anti-HBe in 97 (73%), and both HBeAg and anti-HBe in 4 of 132 HBsAg positive donors. HBV DNA was found in 85 samples, and the complete S-gene was sequenced in 58 of those. Phylogenetic analysis of the sequences revealed that all HBV strains belonged to subgenotype A1, and formed one clade in the phylogenetic tree. In addition, 12 strains from first time donors had a unique 18 amino acid deletion in the N-terminal part of the pre-S2 region.
Conclusion
This study indicated that the prevalence of hepatitis B is intermediate in Rwanda and that the vaccination coverage is relatively low in young adults. All surveyed Rwandan blood donors were infected with similar subgenotype A1 strains, and a high frequency of those with anti-HBe had detectable HBV DNA. Several strains had in addition a unique pre-S2 deletion, the virulence of which needs to be further studied.
Similar content being viewed by others
Background
Hepatitis B virus (HBV) is a small DNA virus belonging to the Hepadnaviridae family [1]. Its DNA encodes for four overlapping open-reading frames, S, P, C and X [2]. The S-gene encodes for the surface proteins, preS1, preS2 and S (HBsAg); the C-gene for the nucleocapsid, and the pre-C/C region for the hepatitis B e antigen (HBeAg). The X protein is implicated in transactivation of the transcription of the HBV proteins and may play a role in carcinogenesis. The P-region encodes for a DNA-polymerase with reverse transcriptase and RNAse function, and the terminal protein, tp [1].
The viral pregenomic RNA is reverse transcribed to a partially double-stranded DNA by the viral polymerase, which by replication errors introduce mutations in the viral genome. Some of these mutations have become fixed and HBV has with time evolved into nine different genotypes (A-I) [1, 3]. Most of the genotypes are further genetically classified into subgenotypes. Genotypes A (subgenotypes A1, A3–A7), D (subgenotypes D1, D2, D4, and D6–D8) and E are prevalent in African countries [1, 4, 5].
Irrespective of genotype, early stages of HBV infection are usually asymptomatic and often unrecognized [6]. The risk for chronic infection increases inversely to the age of the patient at the moment of infection. Chronically infected patients have 15–60% lifetime risk for liver cirrhosis, liver failure and/or hepatocellular carcinoma [6–8]. Approximately 600,000 individuals die annually from complications of the infection [9, 10]. Currently, more than 2 billion people worldwide have been infected by HBV including almost 240 million with chronic infection. More than 50% of the chronically infected individuals live in Asia and in Sub-Saharan Africa where the prevalence among adults is estimated to 5–10% [4, 7].
In the natural history of chronic HBV infection, seroconversion from HBeAg to anti-HBe is a favorable sign usually accompanied by a decrease in HBV replication and remission of hepatitis [11]. However, for some patients the infecting virus strain mutate in the pre-core region to form the so-called pre-core mutant [12] or in the basal core promoter region [13, 14]. Patients infected with these mutants often have active liver disease with elevated HBV DNA levels in serum also after seroconversion to anti-HBe [15, 16]. The pre-core mutation occurs in almost all genotypes and subgenotypes of HBV, but rarely in subgenotypes A2, C1, F2, F3 and H [1, 16–18], and the basal pre-core mutations have been described from most genotypes [19, 20].
To date, there are no anti-viral compounds that completely cure HBV infection [21]. An efficacious vaccine was developed in the 1980s. Since 1991, the WHO has recommended that HBV vaccination should be included in the child vaccination program globally. Countries that have implemented this policy more than two decades ago have a substantial decline of the HBV prevalence [22]. However, the infection remains a major health burden in highly endemic zones including sub-Saharan Africa [23].
Rwanda introduced universal vaccination of children against HBV in 2003, as part of its substantial reconstruction of the infrastructure of the health system after the 1994 genocide against Tutsis. The prevalence of HBV is still significant in the adult population, and has been estimated to be about 5% based on studies on small patient groups [24–26] and on data from neighboring countries [23, 27].
The aims of this study were to evaluate the prevalence of past and current HBV infection among blood donors in all five regions of Rwanda, and to determine the genetic variability and the geographical distribution of HBV strains circulating in the country.
Methods
Blood donors and serum samples
Data were aggregated on analyses for HBsAg in samples from all voluntary non-remunerated first time and repeat blood donors in Rwanda recruited county-wide from five different Regional Centers for Blood and Transfusion during 2014 (Fig. 1). In total, there were 45,061 blood donors including 13,637 first time donors and 31,424 repeat donors (Table 1). All samples were routinely analyzed within 24 h after collection at the National Center for Blood and Transfusion (NCBT)-Kigali for HBsAg, anti-HCV, HIV1/2 and syphilis. The analysis for HBsAg was performed by chemiluminescent microparticles immunoassay (CMIA) using HBsAg qualitative II assay by Abbott Laboratories (Abbott Park, IL, USA) on ARCHITECT i2000SR platform according to the instructions of the manufacturer. The results of the analyses were manually transferred to a register. This register was used for selection of the samples for this study.
All serum samples from blood donors are stored frozen at the NCBT-Kigali, and all of those with ≥ 2 mL serum from HBsAg positive first time and repeat blood donors and from about four times more HBsAg negative donors were obtained for further analyses. Since only 515 samples from donors sampled in 2014 were available (101 of those were HBsAg positive at NCBT-Kigali), an additional 205 samples from donors collected in January and February 2015 were included (35 of those were HBsAg positive at NCTB) A simplified schematic figure of the samples used for further analysis is given in Fig. 1. Only HIV negative blood donors were selected for the study.
All samples were stored at −70 °C until transportation to the Clinical Microbiology Laboratory (CML) at Sahlgrenska University Hospital, Gothenburg, Sweden, for analyses. The samples were transported on dry ice and arrived at CML within 16–18 h where they were kept at −70 °C until analyzed.
Analysis for HBsAg, anti-HBc, anti-HBs, HBeAg, anti-HBe, and anti-HDV at CML, Sweden
At the CML, all samples were re-analyzed by CMIA for HBsAg using ARCHITECT HBsAg (Abbott Laboratories, IL,USA) on ARCHITECT i4000SR platform according to the instructions of the manufacturer.
The samples were also analyzed with CMIA for anti-HBs and total anti-HBc using ARCHITECT anti-HBs and ARCHITECT anti-HBc II (Abbott Laboratories, IL, USA), respectively on the ARTCHITECT i4000SR machine following the manufacturer’s instructions. HBsAg positive samples were further analyzed for HBeAg and anti-HBe with the ARCHITECT HBeAg and ARCHITECT anti-HBe (Abbott Laboratories, IL, USA) assays. All HBsAg positive and isolated anti-HBc-positive samples were analyzed for antibodies against hepatitis delta virus (anti-HDV) with the ETI-AB-DELTA K-2 kits (Diasorin, Saluggia, Italy) according to the instructions by the manufacturer.
HBV DNA extraction
Nucleic acids were extracted from 200 μL of serum from each HBsAg and isolated anti-HBc positive sample using the NucliSens EasyMag automated extractor and kit (BioMerieux, Marcy l’Etoile, France), according to the manufacturers’ instructions. The nucleic acids were eluted in 110-μL nuclease free sterile water.
qPCR for HBV DNA detection
The extracted nucleic acids were analyzed for HBV DNA by a TaqMan qPCR assay targeting the S region with primers HBV305S GCCAAAATTCGCAGTCCC, HBV460AS GATARTCCAGAAGAACCAAYAAGAAG and HBV-probe FAM-CGCGCGATGAGGCATAGCAGYAGGATRAARAACGCGCG-BHQ1. Each 25 μl reaction mix contained 5 μl of extracted DNA, 12.5 μl 2x universal master mix/MgCl2 and Platinum One-Step Quantitative RT-PCR System with ROX (Invitrogen, Carlsbad, CA, USA), and 0.5 μM of each primer and 0.4 μM probe. The amplification profile on Applied Biosystems 7300 platform (Carlsbad, CA, USA) was performed by initial denaturation at 95 °C for 10 min followed by 45 cycles at 95 °C for 15 s, 55 °C for 15 s and 72 °C for 30 s. RNA/DNAse-free water was used as negative control. Serial 10-fold dilutions (1/10 – 1/100 000) of two sera with 1.0 × 108 and 1.2 × 108 IU HBV DNA/mL, respectively, were used in each assay as positive controls and for approximate estimation of the HBV DNA concentrations of the samples investigated. The amount of HBV DNA in the positive control samples had previously been quantified by COBAS® AmpliPrep/COBAS® TaqMan® HBV Test, v2.0 (Roche AG, Basel, Switzerland). Patient samples with ct values above 42, corresponding to less than 4 IU HBV DNA/mL were considered as negative, and samples with ct values less than 23 were considered as having >5 × 108 IU/mL, due to limitations in the standardization of the assay, making extrapolations uncertain. The lowest Ct values of the control samples varied from ct 25 to ct28 in the assays.
PCR amplification of the S-gene for sequencing
A semi-nested PCR was performed to amplify the complete S-gene in a 50 μl reaction mix with 5 μl extracted nucleic acids as template. The PCR mix contained 31.9 μl of RNase-free H2O (Sigma), 1x Taq buffer (Applied Biosystems, Carlsbad, CA, USA), 3 mM MgCl2 (Applied Biosystems, Carlsbad, CA, USA), 0.2 mM dNTP, 0.3 mM of each primer, and 1 U of Taq polymerase. Pooled primers gtA1-2792S1 /gtA1-356AS1 were used for the first round PCR (Additional file 1 Table S1). The PCR reaction was performed with initial denaturation at 95 °C for 3 min followed by 40 cycles with 94 °C for 30 s, 60 °C for 60 s and 72 °C for 60 s, and one cycle at 72 °C for 10 min. Three μl of the first round product were used as template in the second amplification round with primers gtA1-2809S2 /gtA1-356AS2 (Additional file 1 Table S1) and 2.75 mM MgCl2. Deletions and rare mutations observed were confirmed by amplifying the complete S-gene with a semi-nested PCR using primer pools HBV-2730S and HBV-874R in the first PCR and semi-nest the product obtained in three reactions with primers HBV2730S and HBV-98R, HBV-3125S and HBV-548R, and HBV-464S and HBV-874R (Additional file 1 Table S1). The PCR reactions were performed as the PCR reaction described above, with modification of the annealing temperature to 59 °C.
Sequencing
All amplified PCR products were purified and extracted with QIAquick PCR Purification Kit (Qiagen; Hilden, Germany) according to the manufacturer’s description. The purified products were cycle sequenced in both directions by using 1.6 μM of the same primers as in the nested PCR in the BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems) according to the manufacturer’s instructions. The sequences were obtained by the 3130 × l Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA).
Phylogenetic analysis
The sequences obtained were analyzed in the SeqMan program in the DNAStar programme package version 10.1.2 (DNA Star Inc, Madison, WI 53705, USA). The sequences were aligned with the corresponding region of 641 sequences representing all HBV genotypes obtained from GenBank, including 252 genotype A strains from Africa. Phylogenetic analysis was carried out with the PHYLIP package version 3.65. Evolutionary distances were calculated using the F84 algorithm in the DNADIST program with a transition/transversion ratio of 1.53 with gamma correction with alpha 0.28. Phylogenetic trees were constructed using the unweight pair-group method using arithmetic averages (UPGMA) and the neighbor-joining method in the NEIGHBOR program in the PHYLIP package. Bootstrap analysis for 1,000 replicas was performed with the SEQBOOT and CONSENSE programs in the PHYLIP package.
HBsAg subtype determination
The HBsAg subtypes of the strains were determined from the deduced amino acid sequences based on the residues at positions 122, 127 and 160 of the small S-gene [28].
Statistical analysis
GraphPad Prism Version 6.0 g was used to compare the prevalence of HBV with origin and group of donors using the Chi-square test with Yates correction. Multivariate binary logistic regression was performed to compare the characteristics of donors with and without HBeAg adjusting for age, gender, origin, category of donors and viral load. This was also performed for donors with and without an 18 amino acid deletion in preS2. The Odds Ratio (OR) were used to express results with 95% confidence intervals (CI 95%), and p-values <0.05 were considered statistically significant. All analyses were performed using SPSS 22.0 (IBM, Chicago, USA).
Results
There were about three times more males than females among the 45,061blood donors sampled during 2014 in five different regions of Rwanda, and most, 43%, originated from Kigali city (Table 1). The majority (70%) of the donors were repeat donors.
HBsAg was identified in sera from 558 of 13,657 (4.1%) first time donors and in 33 out of 33,424 (0.1%) repeat donors (Table 1; Fig. 1). The highest HBsAg prevalence among the first time blood donors was observed in the Eastern Province (5.3%), Kigali City (5.3%) and the Northern Province (4.4%) compared to the Southern and Western Provinces, where the HBsAg prevalence was respectively 3.0 and 3.2% (p <0.001, OR: 1.69 [1.42-2.02]; Table 1).
The geographical distribution of the 720 blood donors from whom samples were obtained for further analyses did not mirror the geographical distribution of all blood donors from 2014 (Table 2). There were relatively more samples obtained from the Eastern Province (n = 182; 25%), from where only 9.6% of all donors originated during 2014. In addition, 43% of all persons donating blood in 2014 were from Kigali City, while only 9.3% of the 720 samples obtained were from blood donors from this region (Table 2). Furthermore, about half of the samples obtained (51%) were from first time donors, while this group was represented by only 30% of all donors in 2014.
Re-analysis for HBsAg
On re-analyzing 720 blood donor samples for HBsAg at CML in Sweden, nine of 136 HBsAg positive samples at NCBT in Rwanda were repeatedly non-reactive for HBsAg, and none of them were reactive for anti-HBc or anti-HBs, indicating that these samples were false positive in Rwanda. Also, there were 12 of 584 primarily HBsAg negative at NCBT that turned repeatedly reactive for HBsAg at CML. Nine of these samples were from first time donors, and all but one were positive for anti-HBc including one also positive for anti-HBs. Three of these were reactive for HBeAg and 7 for anti-HBe. The anti-HBc negative sample did not have any other marker for HBV infection, and may have had false reactivity for HBsAg at CML, which gives a false positive rate of 0.8% at CML and a 1.9% false negative rate of the test at NCBT-Kigali. The reason for this discrepancy may either be, as for two samples, a low HBsAg reactivity that was missed at NCBT in Rwanda, or a mistake in the manual transfer of the laboratory results into a register of the blood donors at NCBT.
In total there were thus 139 HBsAg positive samples and 581 HBsAg negative samples that were further analyzed for HBV markers (Table 3). Most of the HBsAg positive blood donors were first time donors and found in all age groups, however, 43.2% were younger than 26 years (Table 4).
HBeAg and anti-HBe
Of the 139 HBsAg positive samples, 131 (95%) could be analyzed for both HBeAg and anti-HBe, and one additional sample could only be analyzed for HBeAg (Table 3). HBeAg was found in 28 (21%) samples and anti-HBe in 97 (73%); four (3%) of the samples had both markers; and three (2.3%) lacked both HBeAg and anti-HBe (Table 3). There was a tendency of HBeAg-positive donors being younger (mean age of 25.2 +/− 6.2 years) than HBeAg-negative donors (mean age 29.8 +/− 7.6 years). This age difference was, however, not significant (OR = 0.920 [0.843-1.003], p = 0.059) (Additional file 1 Table S2). There was in addition no significant association between HBeAg reactivity and gender, category of donors or province of origin (Additional file 1 Table S2).
Anti-HBc and anti-HBs
All but one of the 139 HBsAg positive samples had detectable anti-HBc. Among the 581 HBsAg negative samples, 136 (23%) had detectable anti-HBc; 122 (90%) of these had also anti-HBs (Tables 3, 4). Fourteen blood donors, all male, had isolated anti-HBc, and 25 (4.3%) had isolated anti-HBs with mean anti-HBs titers of 355 mIU/mL (range 10 - >1000 mIU/mL). The donors with anti-HBs alone originated from all regions of Rwanda and were 21–45 years old.
HDV coinfection or superinfection
Analysis for anti-HDV was performed in 134 of the 139 HBsAg positive samples and in 11 of the 14 samples with isolated anti-HBc. All samples were negative for this marker.
HBV DNA detection
HBV DNA was detected by qPCR in 85 (61%) of the 139 HBsAg positive samples, and in none of the 14 samples with isolated anti-HBc (Table 3). Almost all of the HBeAg positive samples (27/28; 96%) and 48/97 (49%) anti-HBe positive samples had detectable HBV DNA (Table 3). HBV DNA was also detected in all four samples with both HBeAg and anti-HBe and in none of the three samples lacking both these HBe-markers.
The HBV DNA levels, given as IU/mL based on the serial dilutions of the two positive sera were higher in HBeAg than anti-HBe positive sera with median 3.7 × 106 vs. 500 IU HBV DNA /mL (p <0.0001). The four samples with both HBe-markers had a median value of 4.3 × 103 IU HBV DNA /ml. There were 31 samples with a viral load of ≥104 IU HBV DNA /ml (Additional file 1 Table S2); 22 of these samples had detectable HBeAg (Additional file 1 Table S2).
Sequencing the S-gene
The complete S-gene could be amplified and sequenced in 58 of the 85 HBV DNA-positive samples. Based on the deduced amino acid sequence of the small S-gene, the subtype was inferred to adw2 for 56 strains (97%), since they expressed Lys122, Pro127 and Lys160. Two samples, RW14-173 and RW14-203, expressed Arg122 and were ayw1 strains. Both these two later strains were from first time donors, one from the Eastern and the other from the Western Province. None of the sequenced strains had the vaccine escape mutation Gly145/Arg/Ala.
Several mutations were observed when the pre-S regions were analyzed. The most common mutations were deletion in the pre-S1 and pre-S2 regions. Two larger deletions in pre-S1 was identified in two strains, RW14-219 with a deletion between amino acids 30 and 84, and RW14-226 with a 20 amino acid deletion between residues 67 and 86. There were in addition an 18 amino acid deletions in the N-terminal region of pre-S2, between residues 5 and 22 in twelve strains (RW14-03, − 05, −10, −14, −40, −41, −43, −118, −136, −173, −190, and −216). Other deletions (9, 11 and 14 amino acids) in the same region were observed for another three strains (RW14-22, −198 and −222). The18 amino acid deletion in pre-S2 was not independently associated with age or gender of the donors, presence of HBeAg/anti-HBe, or viral load (Additional file 1 Table S3).
Phylogenetic analysis
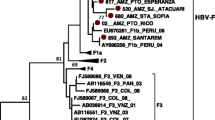
Based on phylogenetic analysis of the complete S-gene, all 58 sequenced strains in this study were found to belong to subgenotype A1 (Fig. 2). All strains belonging to this subgenotype formed three subclades in the phylogenetic tree. All but five of the strains in this study formed one of these subclades together with previously published strains from Kigali, Rwanda, and one strain each from Somalia and the Democratic Republic of the Congo (Fig. 2). The formation of this clade was supported by 68% bootstrap value. Four of the strains from the blood donors in Rwanda were not found in this A1 subclade, and were more closely related to Indian, Chinese and Japanese subgenotype A1 isolates. Another strain, RW14-41 from a first time donor in the Eastern Province, was divergent and formed a separate branch within genotype A. This strain had in addition to the large 18 amino acid deletion in pre-S2 also several amino acid substitutions in pre-S2, and was also divergent from the genotype A strains when the small S-gene was analyzed.
Phylogenetic tree based on 1212 nucleotides of the large S-gene (pre-S1/pre-S2/S) of 699 strains representing all HBV genotypes. All 252 available genotype A sequences of the large S-gene of strains from different African countries were included in the analysis. The genotypes are indicated on the branches. The branch with 53 of the 58 A1 strains from this study and additional 7 strains from Rwanda and 21 strains from other African countries is enlarged. The strains sequenced in this study are shown in red. Strains obtained from GenBank are given with accession number and country of origin at the nodes. Strains with the 18 amino acid deletion in pre-S2 are marked with a red arrowhead at the node
The subgenotype A1 strains also formed separate clades when the small S-gene was analyzed. In the clade formed by the small S-genes of most strains from Rwanda, there was one branch formed mainly by strains from Rwanda together with 6 strains from Kenya. The other strains from Rwanda were intermixed with A1 strains from other African countries in this clade (Additional file 2 Figure S1).
Discussion
This study on HBV infections in blood donors from all five different provinces of Rwanda confirms that this country has intermediate endemicity for this infection, despite its geographical location in a region of Africa with high HBV prevalence. The prevalence of HBsAg positivity was equal in the five regions of Rwanda, and was 4.1% among first time blood donors. The actual HBsAg prevalence may differ between the regions, since the number of first time blood donors investigated from each region did not mirror the population size in the regions, especially not from Kigali City. In addition, fist time donors may not represent the general population, especially since all HIV positive blood donors were excluded in the study. However, the HBsAg prevalence found in this study is comparable to that previously described from different groups of female patients and health care workers in Kigali City [23–26], which indicates that the results obtained from the first time blood donors may be regarded as indicative of the HBV prevalence in the general population.
All Rwandan children are vaccinated against HBV, starting from six-weeks of age, since 2003, when the pentavalent vaccine was introduced by the WHO-sponsored Expanded Program of Immunization through Gavi, the Vaccine Alliance. Thus, most HBV infections are now occurring among adults. In this study, about one quarter (23%) of the HBsAg negative blood donors, who may be representative of the wider adult population in the country, have been exposed to HBV and cleared the infection. This is a relatively lower prevalence of anti-HBc and anti-HBs compared to many other Sub-Saharan African countries especially those in West Africa [29], which suggests that a large part of the Rwandan adult population is susceptible to hepatitis B. In addition, the vaccination coverage seems to be low among adults in Rwanda, with 4.3% of the blood donors in this study having only anti-HBs, indicating immunity obtained by HBV vaccination. This is in accordance with a study on 378 health care workers in Rwanda, among who 4.5% reported having received the HBV vaccine [24]. There is thus a need to implement a vaccination policy for adults in Rwanda to reduce the number of new infections in this predominantly sexually active population.
In Africa, HBV genotypes A, D and E are the most prevalent [30]. The findings in this study were consistent with this, with all Rwandan strains belonging to genotype A. The strains formed a separate clade among the subgenotype A1 strains in the phylogenetic tree, and strains from the five different regions of the country were intermixed with each other within this clade. This may indicate that once there was an introduction of this strain which has spread throughout the country, and is now the dominant HBV strain. The absence of strains of other HBV genotypes in samples from the blood donors is surprising, and may indicate that hepatitis B was rather recently introduced into the country. This finding is consistent with the identified intermediate to low prevalence of HBV among blood donors, and the single major clade with a common ancestor formed by the strains from all regions of the country. Genotype D has been reported from neighboring countries and from pregnant HIV positive women in Kigali City, however the country of origin of these women was not reported [31, 32]. In this study there were few serum samples from HBsAg positive blood donors from Kigali City, therefore other HBV genotypes may be present in the capital of Rwanda, even if they were not identified in this study. However, other previously described HBV strain from Kigali were of subgenotype A1 [31] and was found intermixed with strains from this study in the A1 clade that was formed only by Rwandan strains in the phylogenetic tree. A comprehensive study on liver disease patients recruited from different regions of the country including more patients from Kigali City may be able to determine if A1, and especially this strain forming the A1 clade, is predominant in all HBV carriers of Rwandan nationality.
Several of the HBV strains sequenced in this study had a large deletion in the N-terminal region of pre-S2. These strains were interspread in the A1 clade, which indicates that the deletion had occurred independently in the strains. There was no difference between the carriers of strains with and without this deletion with regard to age, gender, origin, viral load, or HBeAg. The liver disease progression of the blood donors infected with strains with and without the deletion mutant could not be determined in this study. The impact on disease progression of these strains may be investigated by comparing their prevalence in chronic HBV carriers and patients with HBV-induced HCC and/or liver cirrhosis in Rwanda, since such deletions have been found in patients infected by other genotypes and have developed cirrhosis or HCC [33–36].
Complete genomes of A1 strains with and without the deletion will reveal if this mutation predisposes for other mutations in the HBV genome as in the promoter regions. Sequencing strains from HBsAg positive liver disease patients from different regions of Rwanda will also reveal if this strain is more virulent or more easily transmissible than the other A1 strains found in this study. In addition, more than half of the blood donors infected with anti-HBe positive strains also had detectable HBV DNA, indicating a high prevalence of the pre-core mutant or basal core promoter mutants [37, 38]. The HBV pre-core mutant strains have been shown to cause fulminant hepatitis in the recipient when horizontally transmitted [39–41] and found in both chronic active and inactive hepatitis HBsAg carriers [42]. In addition, the basal core promoter mutants have been shown to be involved in the development of cirrhosis and HCC [43–46]. The mutations in the Rwandan strains and their involvement in liver disease progression in HBV carriers could not be evaluated in this study, and need to be investigated. Further studies are therefore needed on HBV strains from patients with different stages of liver disease in Rwanda.
Conclusions
This is the first study to show an extensive prevalence of HBV serum markers among adults in Rwanda. Furthermore, the study showed that all blood donor strains belonged to subgenotype A1, and that there is a high prevalence of HBV strains with several pre-S mutations whose clinical implications need to be explored. These data are of paramount importance for healthcare planning for management, vaccination policy, and surveillance of HBV infections in Rwanda.
Abbreviations
- Anti-HBc:
-
Hepatitis B viral core antibodies
- Anti-HBe:
-
Hepatitis B viral e antibodies
- Anti-HBs:
-
Hepatitis B viral surface antibodies
- CI:
-
Confidence interval
- CMIA:
-
Chemiluminescent microparticles immunoassay
- CML:
-
Clinical microbiology laboratory, gothenburg, Sweden
- DNA:
-
Deoxyribonucleic acid
- HBeAg:
-
Hepatitis B viral e antigen
- HBsAg:
-
Hepatitis B viral surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- NCBT:
-
National center for blood transfusion
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- RBC:
-
Rwanda biomedical center
- RNA:
-
Ribonucleic acid
- WHO:
-
World Health Organization
References
Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol. 2016;64 Suppl 1:S4–s16.
Wain-Hobson S, Pourcel C, Brechot C, Charnay P, Dubois MF, Fritsch A, et al. Structure and expression of the hepatitis B virus genome. Dev Biol Stand. 1981;50:293–300.
Norder H, Courouce AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47(6):289–309.
Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res: Off J Jpn Soc Hepatol. 2007;37 Suppl 1:S9–S19.
Schaefer S. Hepatitis B virus: significance of genotypes. J Viral Hepat. 2005;12(2):111–24.
Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4(12):a024935.
Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–63.
Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med. 2011;61(8):531–40.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–128.
Basnayake SK, Easterbrook PJ. Wide variation in estimates of global prevalence and burden of chronic hepatitis B and C infection cited in published literature. J Viral Hepat. 2016;23(7):545–59.
Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med. 1981;94(6):744–8.
Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2(8663):588–91.
Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002;9(1):52–61.
Baqai SF, Proudfoot J, Yi DH, Mangahas M, Gish RG. High rate of core promoter and precore mutations in patients with chronic hepatitis B. Hepatol Int. 2015;9:209–17.
Bonino F, Rosina F, Rizzetto M, Rizzi R, Chiaberge E, Tardanico R, et al. Chronic hepatitis in HBsAg carriers with serum HBV-DNA and anti-HBe. Gastroenterology. 1986;90(5):1268–73.
Hagiwara S, Kudo M, Minami Y, Chung H, Nakatani T, Fukunaga T, et al. Clinical significance of the genotype and core promoter/pre-core mutations in hepatitis B virus carriers. Intervirology. 2006;49(4):200–6.
Norder H, Arauz-Ruiz P, Blitz L, Pujol FH, Echevarria JM, Magnius LO. The T(1858) variant predisposing to the precore stop mutation correlates with one of two major genotype F hepatitis B virus clades. J Gen Virol. 2003;84(8):2083–7.
Inoue J, Kondo Y, Umetsu T, Yamamoto T, Miura M, Mano Y, et al. Shifting hepatitis B virus genotypes of acute hepatitis B patients in northeast Japan. J Med Virol. 2016;88(1):69–78.
Lindh M, Hannoun C, Dhillon AP, Norkrans G, Horal P. Core promoter mutations and genotypes in relation to viral replication and liver damage in East Asian hepatitis B virus carriers. J Infect Dis. 1999;179(4):775–82.
Sato S, Suzuki K, Akahane Y, Akamatsu K, Akiyama K, Yunomura K, et al. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann Intern Med. 1995;122(4):241–8.
Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62(6):1893–908.
Su WJ, Liu CC, Liu DP, Chen SF, Huang JJ, Chan TC, et al. Effect of age on the incidence of acute hepatitis B after 25 years of a universal newborn hepatitis B immunization program in Taiwan. J Infect Dis. 2012;205(5):757–62.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–9.
Kateera F, Walker TD, Mutesa L, Mutabazi V, Musabeyesu E, Mukabatsinda C, et al. Hepatitis B and C seroprevalence among health care workers in a tertiary hospital in Rwanda. Trans R Soc Trop Med Hyg. 2015;109(3):203–8.
Pirillo MF, Bassani L, Germinario EA, Mancini MG, Vyankandondera J, Okong P, et al. Seroprevalence of hepatitis B and C viruses among HIV-infected pregnant women in Uganda and Rwanda. J Med Virol. 2007;79(12):1797–801.
Rusine J, Ondoa P, Asiimwe-Kateera B, Boer KR, Uwimana JM, Mukabayire O, et al. High seroprevalence of HBV and HCV infection in HIV-infected adults in Kigali, Rwanda. PLoS One. 2013;8(5):e63303.
Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74–80.
Norder H, Hammas B, Lee SD, Bile K, Courouce AM, Mushahwar IK, et al. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J Gen Virol. 1993;74(7):1341–8.
Amponsah-Dacosta E, Lebelo RL, Rakgole JN, Selabe SG, Gededzha MP, Mayaphi SH, et al. Hepatitis B virus infection in post-vaccination South Africa: occult HBV infection and circulating surface gene variants. J Clin Virol. 2015;63:12–7.
Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol. 2014;20(18):5427–34.
Hubschen JM, Mugabo J, Peltier CA, Karasi JC, Sausy A, Kirpach P, et al. Exceptional genetic variability of hepatitis B virus indicates that Rwanda is east of an emerging African genotype E/A1 divide. J Med Virol. 2009;81(3):435–40.
Ochwoto M, Chauhan R, Gopalakrishnan D, Chen CY, Ng’ang’a Z, Okoth F, et al. Genotyping and molecular characterization of hepatitis B virus in liver disease patients in Kenya. Infect Genet Evol. 2013;20:103–10.
Chen CH, Changchien CS, Lee CM, Hung CH, Hu TH, Wang JH, et al. Combined mutations in pre-s/surface and core promoter/precore regions of hepatitis B virus increase the risk of hepatocellular carcinoma: a case-control study. J Infect Dis. 2008;198(11):1634–42.
Preikschat P, Gunther S, Reinhold S, Will H, Budde K, Neumayer HH, et al. Complex HBV populations with mutations in core promoter, C gene, and pre-S region are associated with development of cirrhosis in long-term renal transplant recipients. Hepatology. 2002;35(2):466–77.
Fang ZL, Sabin CA, Dong BQ, Wei SC, Chen QY, Fang KX, et al. Hepatitis B virus pre-S deletion mutations are a risk factor for hepatocellular carcinoma: a matched nested case-control study. J Gen Virol. 2008;89(11):2882–90.
Huy TT, Ushijima H, Win KM, Luengrojanakul P, Shrestha PK, Zhong ZH, et al. High prevalence of hepatitis B virus pre-s mutant in countries where it is endemic and its relationship with genotype and chronicity. J Clin Microbiol. 2003;41(12):5449–55.
Grandjacques C, Pradat P, Stuyver L, Chevallier M, Chevallier P, Pichoud C, et al. Rapid detection of genotypes and mutations in the pre-core promoter and the pre-core region of hepatitis B virus genome: correlation with viral persistence and disease severity. J Hepatol. 2000;33(3):430–9.
Dupinay T, Restorp K, Leutscher P, Rousset D, Chemin I, Migliani R, et al. High prevalence of hepatitis B virus genotype E in Northern Madagascar indicates a West-African lineage. J Med Virol. 2010;82(9):1515–26.
Fagan EA, Smith PM, Davison F, Williams R. Fulminant hepatitis B in successive female sexual partners of two anti-HBe-positive males. Lancet (Lond Engl). 1986;2(8506):538–40.
Oren I, Hershow RC, Ben-Porath E, Krivoy N, Goldstein N, Rishpon S, et al. A common-source outbreak of fulminant hepatitis B in a hospital. Ann Intern Med. 1989;110(9):691–8.
Arankalle VA, Gandhi S, Lole KS, Chadha MS, Gupte GM, Lokhande MU. An outbreak of hepatitis B with high mortality in India: association with precore, basal core promoter mutants and improperly sterilized syringes. J Viral Hepat. 2011;18(4):e20–28.
Papatheodoridis GV, Hadziyannis SJ. Diagnosis and management of pre-core mutant chronic hepatitis B. J Viral Hepat. 2001;8(5):311–21.
Zhong YW, Di FL, Liu C, Zhang XC, Bi JF, Li YL, et al. Hepatitis B virus basal core promoter/precore mutants and association with liver cirrhosis in children with chronic hepatitis B virus infection. Clin Microbiol Infect. 2016;22(4):379. e1–8.
Kamijo N, Matsumoto A, Umemura T, Shibata S, Ichikawa Y, Kimura T, et al. Mutations of pre-core and basal core promoter before and after hepatitis B e antigen seroconversion. World J Gastroenterol. 2015;21(2):541–8.
Tseng TC, Liu CJ, Yang HC, Chen CL, Yang WT, Tsai CS, et al. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut. 2015;64(2):292–302.
Park YM, Jang JW, Yoo SH, Kim SH, Oh IM, Park SJ, et al. Combinations of eight key mutations in the X/preC region and genomic activity of hepatitis B virus are associated with hepatocellular carcinoma. J Viral Hepat. 2014;21(3):171–7.
Acknowledgements
This study was funded by the Swedish International Development Agency through the Cooperation with the University of Rwanda, Directorate of Research, Consultancy and Postgraduate Studies. We are grateful to Gustaf Pettersson, Marie Karlsson and Anette Roth for skillful technical assistance.
Funding
This study is supported by grant no 51160027–8 from the Swedish International Development Cooperation Agency (SIDA).
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request. The sequences obtained are deposited in GenBank with accession numbers KX982108-KX982165.
Authors’ contributions
TT was involved in study design, carried out the experiments and performed analysis of the data and writing the manuscript; GS assisted in collection of the blood donor samples and in study design; TDW; ML, TB and JBG were involved in study design, analyzing the data and writing, and HN planned the project and study design, analyzed the data and assisted in the writing. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All study subjects were older than 18 years and provided informed consent to participate. The Rwanda National Ethics Committee approved the study (No 243/RNEC/2014). A material transfer agreement for samples’ shipment to and analysis in Sweden was signed between the University of Gothenburg, CML, Sweden, and the University of Rwanda, Directorate of Research, Consultancy and Postgraduate Studies, Rwanda.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
List of primers used to sequence the S-gene. Table S2 Distribution of blood donors according to the HBeAg reactivity and associated factors. Table S3 Factors associated with the 18 amino acid preS2 deletions compared to strains without. (DOCX 24 kb)
Additional file 2: Figure S1.
Phylogenetic tree of the small S-gene encoding for HBsAg of 527 strains. The branch with 52 of the 58 A1 strains from this study and additional 7 strains from Rwanda and 13 strains from other African countries is enlarged. The strains sequenced in this study are shown in red. Strains obtained from GenBank are given with accession number and country of origin at the nodes. Strains with an 18 amino acid deletion in preS2 are marked with a red arrowhead at the nodes. (PPTX 93 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Twagirumugabe, T., Swaibu, G., Walker, T.D. et al. Hepatitis B virus strains from Rwandan blood donors are genetically similar and form one clade within subgenotype A1. BMC Infect Dis 17, 32 (2017). https://doi.org/10.1186/s12879-016-2149-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-016-2149-z