Abstract
Background
The emerging resistance to the extended-spectrum cephalosporins (ESCs) in Neisseria gonorrhoeae together with increasing incidence of gonorrhoea cases in many countries have been global public health concerns. However, in recent years the levels of ESC resistance have decreased in several regions worldwide. We describe the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) data from 2013, and compare them to corresponding data from 2009–2012.
Methods
During 2013, N. gonorrhoeae isolates from 21 participating countries were examined. Antimicrobial susceptibility testing (Etest or agar dilution) was performed for cefixime, ceftriaxone, ciprofloxacin, azithromycin, spectinomycin and gentamicin. Statistical analyses were performed to identify significant changes in resistance between years and to investigate associations between patients with resistant gonococcal isolates and collected epidemiological variables.
Results
In total, 93 (4.7 %) of 1994 isolates displayed resistance to cefixime, representing an increase compared to the 3.9 % detected in 2012 (p = 0.23). Cefixime resistance was detected in 13 (61.9 %) of the 21 countries. Cefixime resistance among men who have sex with men was only 1.2 %, compared to 5.6 % and 6.1 % in females and male heterosexuals, respectively. The univariate analysis confirmed that isolates resistant to cefixime were more likely to be from females (OR 4.87, p < 0.01) or male heterosexuals (OR 5.32, p < 0.01). Seven (0.4 %) isolates displayed ceftriaxone resistance (in addition to cefixime resistance) compared to three and 10 isolates in 2012 and 2011, respectively. All 93 isolates with cefixime resistance were additionally resistant to ciprofloxacin and 16 (17.2 %) were also resistant to azithromycin. Among all tested isolates (n = 1994), the ciprofloxacin resistance level (52.9 %) was higher than in 2012 (50.1 %; p = 0.08), and azithromycin resistance (5.4 %) increased since 2012 (4.5 %; p = 0.16).
Conclusions
In 2013, the ESC resistance was again slightly increasing in Europe. This emphasises the importance of implementing the actions outlined in the European and additional response plans, particularly activities strengthening the surveillance of antimicrobial resistance. Ceftriaxone combined with azithromycin remains a satisfactory option for the first-line treatment of gonorrhoea. However novel antimicrobials (new derivatives of previously developed antimicrobials or newly developed antimicrobials) for effective monotherapy or at least inclusion in new dual antimicrobial therapy regimens (combined with previously developed antimicrobials or novel antimicrobials) will likely be required.
Similar content being viewed by others
Background
The in vitro resistance emerging in Neisseria gonorrhoeae internationally to the last remaining options for empiric first-line treatment of gonorrhoea, the extended-spectrum cephalosporins (ESCs) cefixime and ceftriaxone, which has translated into clinical treatment failures, has been well documented in recent years [1–14]. Some well-characterised multidrug-resistant N. gonorrhoeae (MDR-NG) strains have accounted for most of this in vitro and clinical resistance to the ESCs worldwide, such as the N. gonorrhoeae multi-antigen sequence type (NG-MAST) ST1407 or genetically closely related STs [3–8, 10, 12]. This developing situation along with the rising incidence of reported gonorrhoea cases in many particularly high-income countries [15–17], the associated morbidity of untreated gonorrhoea such as ectopic pregnancy and infertility, and the fact that gonorrhoea can substantially increase HIV transmission [18–20], make gonorrhoea a global public health problem.
N. gonorrhoeae has a well-documented history of acquiring and developing antimicrobial resistance (AMR) to all drugs used therapeutically for gonorrhoea [2] and it could therefore be predicted that N. gonorrhoeae would develop resistance to the ESCs. In this emergent situation, there have been significantly increased efforts aiming to retain gonorrhoea as a treatable infection. National and international action plans, such as the World Health Organization (WHO) global action plan [21, 22], the Centers for Disease Control and Prevention (CDC) response plan [23] and the European Centre for Disease Prevention and Control (ECDC) response plan to the threat of MDR-NG [24] have emphasised the need to scale up AMR surveillance globally, the need for updating national and international gonorrhoea management guidelines to include introduction of dual antimicrobial therapy [25], and information dissemination about the situation to healthcare professionals, patients and the public. AMR in N. gonorrhoeae has also been frequently highlighted as a major concern in the broader AMR reports and strategies [26–28].
In the European Union (EU)/European Economic Area (EEA), surveillance of AMR in N. gonorrhoeae is performed through the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) [24, 29, 30]. The programme documented a statistically significant increase in cefixime resistance from 2009 (5.1 %) to 2010 (8.7 %), however after 2010 the cefixime resistance levels started to decrease to a low of 3.9 % in 2012 [29, 30]. Similar decreases in the resistance to ESCs have also been documented in other regions [15, 16, 31–36].
The aims of the present study were to describe the Euro-GASP data from 2013, and compare them to the Euro-GASP data from 2009–2012.
Methods
Neisseria gonorrhoeae isolates and the Euro-GASP
During 2013, N. gonorrhoeae isolates from 21 participating countries were examined in the Euro-GASP (Table 1). Number of countries participating in Euro-GASP increased over time: in 2009, 16 countries participated in the Euro-GASP; Cyprus, Hungary, Ireland and Norway joined in 2010; Iceland joined in 2013; Romania participated only in 2010 and 2011. Isolates were collected during two time periods; from April to May, and from October to November. Nine (43 %) of the countries participating in 2013 followed a centralised testing model, i.e., all antimicrobial susceptibility testing was performed centrally (in laboratories at Public Health England or Örebro University Hospital, Sweden) by the Etest for cefixime and ceftriaxone and agar dilution breakpoint method for ciprofloxacin, azithromycin and spectinomycin, and the full agar dilution method for gentamicin. The remaining 12 (57 %) countries followed a decentralised testing model, that is, the antimicrobial susceptibility testing using the Etest or agar dilution method was performed in their own national reference or local laboratory (Table 1). To ensure quality and comparability of data, all countries performing decentralised testing fulfilled strict quality criteria, all countries participated in an annual external quality assessment programme, and all countries used identical international reference strains for quality controls. Further details regarding the Euro-GASP have been published elsewhere [29]. The resistance breakpoints stated by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) were used (cefixime/ceftriaxone minimum inhibitory concentration (MIC) > 0.125 mg/L, azithromycin MIC > 0.5 mg/L and ciprofloxacin MIC > 0.06 mg/L) [37]. The following epidemiological variables were collected and subsequently categorised as follows: age (< 25 years or ≥ 25 years), sexual orientation and gender (men who have sex with men (MSM), male heterosexuals and all women), previous gonorrhoea (yes or no), and concurrent chlamydial infection or no chlamydial infection.
Statistical analysis
The statistical significance of any changes in the proportion of isolates with resistance to tested antimicrobials between years was determined by the Z-test or Fisher’s exact test if cell numbers were less than 5. A univariate analysis and multivariable logistic regression analyses of odds ratios (OR) with 95 % confidence intervals (CI) were calculated to investigate associations between patients infected with an isolate displaying resistance to cefixime, ciprofloxacin or azithromycin, and the collected epidemiological variables. A Pearson χ2-test was used to test if these odds ratios were significantly different from one, with a P-value of < 0.05 indicating significance. Statistical analysis was performed in STATA v12.1 (StataCorp LP, TX, USA).
Ethics
The present study was a surveillance study, using data from the Euro-GASP which is implemented through the ECDC Framework Contract No. ECDC/2013/015. All examined gonococcal isolates were cultured and preserved as part of the routine diagnostics (standard care) and no patient identification information was available in the present study. Ethical approval was therefore not required.
Results
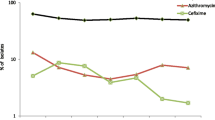
The results of all the antimicrobial susceptibility testing are summarised in Table 1. Of the 1994 examined N. gonorrhoeae isolates collected in 21 EU/EEA countries in 2013, 4.7 % (93 isolates) displayed resistance to cefixime (Table 1, Fig. 1). This represented a slight, but not statistically significant, increase compared to the 3.9 % of resistant isolates detected in 2012 (Z-test, p = 0.233). Cefixime resistance was detected in 13 (61.9 %) of the 21 countries, and in these countries cefixime resistance levels ranged from 0.8 % (United Kingdom) to 15.1 % (Spain) (Table 1). Nineteen (1.0 %) isolates had a MIC of cefixime of ≥ 0.5 mg/L compared to three isolates in 2012, and 13 isolates in 2011 (Fisher’s Exact test p = 0.001 and p = 0.379, respectively). These 19 isolates were from Spain (n = 7), Denmark (n = 3), Germany (n = 3), Austria (n = 2), Greece (n = 1), Hungary (n = 1), Slovakia (n = 1), and United Kingdom (n = 1). Seven (0.4 %) isolates displayed ceftriaxone resistance (six from Spain and one from Germany; all were additionally resistant to cefixime) compared to three isolates and 10 isolates in 2012 and 2011, respectively. All 93 isolates with cefixime resistance were additionally resistant to ciprofloxacin and 16 (17.2 %) were also resistant to azithromycin. Finally, of the seven ceftriaxone-resistant isolates, one (0.05 %) was also resistant to azithromycin and five (0.3 %) had a MIC (0.5 mg/L) exactly at the breakpoint for resistance.
Furthermore, the MIC distribution for ceftriaxone in 2013 compared to the ones in 2011 and 2012 showed a decreased proportion of highly susceptible gonococcal isolates (MIC ≤ 0.002 mg/L) as well as increased proportions of isolates with higher MICs such as 0.064 mg/L and 0.125 mg/L (Fig. 2), which is exactly at the resistance breakpoint.
Overall, the ciprofloxacin resistance level of 52.9 % detected in 2013 was slightly higher than the 50.1 % of resistance detected in 2012 (Z-test, p = 0.082), continuing the gradually increasing trend observed since 2011 (Fig. 1). For the first time since 2008, azithromycin resistance increased, although the increase was not statistically significant (4.5 % in 2012, 5.4 % in 2013; Z-test, p = 0.159). However, only one isolate displayed high-level resistance to azithromycin (MIC ≥ 256 mg/L; collected in Ireland); high-level azithromycin-resistant isolates were also detected in 2006 (n = 1), 2007 (n = 4), 2011 (n = 2) and 2012 (n = 3). Finally, the modal MIC of gentamicin was 8 mg/L (MIC range: from 1 to 16 mg/L) and no resistance to spectinomycin (MIC range: from 0.5 to 64 mg/L) was demonstrated.
In Table 2, the number of patients with isolate susceptibility data (number of resistant isolates) linked with epidemiological data is summarised. In the univariate analysis, the only significant associations with cefixime resistance were being male heterosexual (OR male heterosexual vs. MSM 5.32, CI 2.12–13.3, p = 0.0001) or female (OR female vs. MSM 4.87, CI 1.89–12.6, p = 0.0003). For azithromycin, the univariate analysis revealed associations between azithromycin resistance and male heterosexuals (OR male heterosexual vs. MSM 2.39, CI 1.21–4.69, p = 0.0094). Associations were also observed between ciprofloxacin resistance and higher age (OR ≥ 25 years vs. < 25 years 1.36, CI 1.12-1.66, p = 0.0022), male heterosexuals (OR male heterosexual vs. MSM 1.7, CI 1.3–2.24, p = 0.0001), females (OR females vs. MSM 1.34, CI 1.01–1.79, p = 0.0437) and no concurrent chlamydia infection (OR no concurrent chlamydia vs. concurrent chlamydia 1.44, CI 1.03-2.02, p = 0.0313). In the ciprofloxacin multivariable analysis, the only significant associations remaining were for ciprofloxacin resistance and being male heterosexual (aOR: 1.57, CI 1.13–2.18, p = 0.007), and ciprofloxacin resistance and no concurrent C. trachomatis infection (aOR: 1.57, CI 1.09-2.24, p = 0.016).
Discussion
The 2013 Euro-GASP data show that the encouraging recent trends of decreasing cefixime, ceftriaxone, ciprofloxacin and azithromycin resistance in N. gonorrhoeae across the EU/EEA region have not been maintained, although the increases observed in 2013 were not statistically significant. Further data are needed to establish if the increasing trend will continue. The 2013 data emphasize the need for continued expansion and improvement in quality-assured surveillance of gonococcal AMR and treatment failures, as described in the European and other response plans [21–24]. The data also stress that widespread implementation of current European management guidelines [25] or similar therapeutic regimens [38, 39] recommending ceftriaxone in combination with azithromycin for first-line treatment of all cases of uncomplicated gonorrhoea, remains crucial.
As well as decreases in the level of resistance to ESCs in the EU/EEA from 2010 to 2012, decreases have also been observed in other regions with well-developed surveillance programmes globally [15, 16, 31, 33–36]. Furthermore, despite some rare ESC treatment failures verified in recent years [3–14], the anticipated number of reported gonorrhoea treatment failures has not yet materialised, which could also be partly due to a lack of identification and under-reporting of treatment failures. The decrease in ESC resistance in Europe (2010–2012) and in several additional regions is an extremely interesting and perplexing situation as decreasing resistance trends have never been documented for any previously used antimicrobial in N. gonorrhoeae. The situation is even more confusing as similar decreasing trends have been witnessed in countries that have not used cefixime as any main therapeutic agent or have not changed their recommended treatment regimens [31, 33, 35, 36]. The reasons explaining all these decreases in ESC resistance are most likely multi-factorial and complex. A number of recent changes in the diagnostics and management of gonorrhoea may have contributed to the decreases in ESC resistance. In many countries, for example, increased use of more sensitive molecular diagnostics and increased testing of extra-genital sites, including the pharynx, among MSM might have contributed to reducing the reservoir of strains such as the NG-MAST ST1407 as effective diagnostics allows the administration of appropriate antimicrobial therapy; implementation of updated management guidelines recommending dual antimicrobial therapy could similarly have more effectively targeted resistant strains; education to healthcare professionals is also likely to have contributed to a more effective implementation of testing and treatment guidelines. The decrease in ESC resistance may also be due to an epidemiologic replacement of the MDR-NG ST1407 clone by other STs, which might potentially have been affected also by some type of partial immunity to the widely spread ST1407 from prior infection (17 % - 21 % of patients reported having had a previous gonorrhoea infection during years 2009 – 2013 [29]). Clearly, sufficient understanding regarding the gonococcal population dynamics, including epidemiologic curves for single strains, is lacking. In the Euro-GASP, a molecular typing study examining the 2013 isolates and associated AMR and epidemiologic data is currently underway. This study should further elucidate the perplexing situation with the initially decreasing ESC resistance trends that started to once again increase in 2013.
It is also crucial to emphasize that the resistance level to both cefixime and ciprofloxacin is higher in the heterosexual community. In 2013, cefixime resistance was significantly associated with heterosexual orientations: among MSM only 1.2 % of isolates were resistant to cefixime, compared to 5.6 % and 6.1 % in females and male heterosexuals, respectively. In the Euro-GASP, this trend was also earlier identified analysing material from 2009 to 2011 [40]. Due to the severe complications and sequelae resulting from ascending infection, the risk of untreated or inappropriately treated gonorrhoea is of particular concern in women where the infection is also much harder to diagnose and is often asymptomatic in nature. Hopefully, the ongoing molecular typing study examining the 2013 European gonococcal isolates and associated AMR and epidemiologic data will be able to further elucidate also the associations between AMR and sexual orientation, as well as additional epidemiological variables.
The main inherent limitations in the Euro-GASP have been detailed previously in the European response plan [24], and mostly include issues regarding the number and representativeness of gonococcal isolates and associated patients. Efforts are underway to address these limitations: in 2014, several additional countries have joined the Euro-GASP, an increased number of isolates with associated epidemiologic metadata are available from many countries, and a comprehensive review of the longitudinal AMR and epidemiological surveillance data to identify areas where representativeness needs to be improved is in progress. Nevertheless, in 2013, 21 (68 %) of the 31 EU/EEA countries were already participating in the Euro-GASP, which should provide a relatively effective evidence base for the gonococcal AMR situation in the EU/EEA region.
Conclusions
Even though slight decreases in the ESC resistance were documented in recent years in Europe and several other regions globally, in 2013 the ESC resistance was once again increasing in Europe. Overall, this might be a time of ‘calm before the storm’ and efforts to keep gonorrhoea as a treatable infection need to be sustained. N. gonorrhoeae has already shown its capacity to develop high-level resistance to ceftriaxone [2, 6, 9] and, based on the well-documented history regarding all antimicrobials previously used for gonorrhoea treatment, it is likely that it is only a matter of time before ceftriaxone-resistant strains with sufficient biological fitness emerge and start to spread internationally. National and international surveillance of N. gonorrhoeae AMR (including collection of appropriate associated epidemiologic metadata), treatment failures and ideally also antimicrobial use/misuse, as well as implementation of recommended dual antimicrobial treatment regimens (ceftriaxone plus azithromycin) needs to be maintained and in several areas further strengthened to provide more reliable evidence for policy makers and to inform antibiotic prescription protocols. The work outlined in the European [24] and additional response plans [21–23] needs to be further implemented, and a close collaboration between the Euro-GASP and additional GASPs internationally [41] in liaison with the WHO Global GASP is crucial. The number of cases of gonorrhoea reported to the ECDC has been increasing annually since 2008; 52,995 cases were reported in 2013, which is an 11 % increase from the previous year (47,641 cases in 2012) [42]. This increasing gonorrhoea incidence, along with the slight rise of ESC resistance described in this study, confirms the importance of antimicrobial susceptibility surveillance for this disease. Presently, ceftriaxone combined with azithromycin remains a satisfactory option for the first-line treatment of gonorrhoea. However, novel antimicrobials (new derivatives of previously developed antimicrobials or newly developed antimicrobials) for effective monotherapy or at least inclusion in new dual antimicrobial therapy regimens (combined with previously developed antimicrobials or other novel antimicrobials) will ultimately be required if the upturn seen in 2013 is sustained into future years.
References
Chen Y, Stevens K, Tideman R, Zaia A, Tomita T, Fairley CK, et al. Failure of 500 mg of ceftriaxone to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother. 2013;68:1445–7.
Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613.
Golparian D, Ohlsson A, Janson H, Lidbrink P, Richtner T, Ekelund O, et al. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill. 2014;19.
Lewis DA, Sriruttan C, Muller EE, Golparian D, Gumede L, Fick D, et al. Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J Antimicrob Chemother. 2013;68:1267–70.
Unemo M, Golparian D, Potocnik M, Jeverica S. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill. 2012;17.
Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56:1273–80.
Unemo M, Golparian D, Stary A, Eigentler A. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 2011;16.
Unemo M, Golparian D, Syversen G, Vestrheim DF, Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill. 2010;15.
Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance toceftriaxone. Antimicrob Agents Chemother. 2011;55:3538–45.
Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill. 2011;16.
Forsyth S, Penney P, Rooney G. Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations. Int J STD AIDS. 2011;22:296–7.
Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA. 2013;309:163–70.
Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill. 2011;16.
Read PJ, Limnios EA, McNulty A, Whiley D, Lahra MM. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500 mg ceftriaxone in Sydney, Australia. Sex Health. 2013;10:460–2.
Kirkcaldy RD, Kidd S, Weinstock HS, Papp JR, Bolan GA. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the USA: the Gonococcal Isolate Surveillance Project (GISP), January 2006-June 2012. Sex Transm Infect. 2013;89 Suppl 4:5–10.
Martin I, Sawatzky P, Liu G, Mulvey MR. Antimicrobial resistance to Neisseria gonorrhoeae in Canada: 2009–2013. CCDR. 2015;41:S4. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/15vol41/dr-rm41-02/surv-4-eng.php (Accessed: June 14, 2015).
European Centre for Disease Prevention and Control. ECDC SURVEILLANCE REPORT: Sexually Transmitted Infections in Europe 2012 . 2014. http://ecdc.europa.eu/en/publications/Publications/sexually-transmitted-infections-europe-surveillance-report-2012.pdf (Accessed: June 14, 2015).
Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. 2012;206:6–14.
Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group Lancet. 1997;349:1868–73.
Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as riskfactors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7(1):95–102.
Ndowa F, Lusti-Narasimhan M, Unemo M. The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex Transm Infect. 2012;88:317–8.
World Health Organisation. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. 2012. http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf (Accessed: June 14, 2015).
Centers for Disease Control and Prevention. Cephalosprin-resistant Neisseria gonorrhoeae Public Health Response Plan. 2012. http://www.cdc.gov/std/treatment/ceph-r-responseplanjuly30-2012.pdf (Accessed: June 14, 2015).
European Centre for Disease Prevention and Control. Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe. Stockholm: ECDC; 2012. 2012. http://ecdc.europa.eu/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf (Accessed: June 14, 2015).
Bignell C, Unemo M. On behalf of the European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85–92.
UK AMR High Level Steering Group. UK 5 Year Antimicrobial Resistance (AMR) Strategy 2013–2018. 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322358/Outcome_measures.pdf (Accessed: June 14, 2015).
World Health Organisation. Antimicrobial resistance: global report on surveillance. 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf (Accessed: June 14, 2015).
Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. 2013. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (Accessed: June 14, 2015).
European Centre for Disease Prevention and Control. Gonococcal antimicrobial susceptibility surveillance in Europe 2012. 2014. http://www.ecdc.europa.eu/en/publications/Publications/gonococcal-antimicrobial-susceptibility-surveillance-Europe-2012.pdf (Accessed: June 14, 2015).
Cole M, Spiteri G, Chisholm S, Hoffmann S, Ison C, Unemo M, et al. Emerging cephalosporin and multidrug-resistant gonorrhoea in Europe. Euro Surveill. 2014;19.
Bala M, Kakran M, Singh V, Sood S, Ramesh V. Monitoring antimicrobial resistance in Neisseria gonorrhoeae in selected countries of the WHO South-East Asia Region between 2009 and 2012: a retrospective analysis. Sex Transm Infect. 2013;89(4):28–35.
Public Health England. GRASP 2012 Report: The Gonococcal Resistance to Antimicrobials Surveillance Programme. 2013. http://194.74.226.162/webc/HPAwebFile/HPAweb_C/1317140152190 (Accessed: June 14, 2015).
Kubanova A, Kubanov A, Frigo N, Solomka V, Semina V, Vorobyev D, et al. Russian gonococcal antimicrobial susceptibility programme (RU-GASP)--resistance in Neisseria gonorrhoeae during 2009–2012 and NG-MAST genotypes in 2011 and 2012. BMC Infect Dis. 2014;14:342.
Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, et al. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect Dis. 2013;13:762–8.
Jeverica S, Golparian D, Maticic M, Potocnik M, Mlakar BX, Unemo M. Phenotypic and molecular characterization of Neisseria gonorrhoeae isolates from Slovenia, 2006–12: rise and fall of the multidrug-resistant NG-MAST genogroup 1407 clone? J Antimicrob Chemother. 2014;69:1517–25.
Chen SC, Yin YP, Dai XQ, Unemo M, Chen XS. Antimicrobial resistance, genetic resistance determinants for ceftriaxone and molecular epidemiology of Neisseria gonorrhoeae isolates in Nanjing, China. J Antimicrob Chemother. 2014;69:2959–65.
European Committee on Antimicrobial Susceptibility Testing - EUCAST. Breakpoint tables for interpretation of MICs and zone diameters. 2012. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf (Accessed: June 14, 2015).
Bignell C, Fitzgerald M. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS. 2011;22:541–7.
Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
Cole MJ, Spiteri G, Town K, Unemo M, Hoffmann S, Chisholm SA, et al. Risk factors for antimicrobial-resistant Neisseria gonorrhoeae in Europe. Sex Transm Dis. 2014;41:723–9.
Ndowa FJ, Ison CA, Cole MJ, Lusti-Narasimhan M. Gonococcal antimicrobial resistance: challenges for public health control. Sex Transm Infect. 2013;89 Suppl 4:3–4.
European Centre for Disease Prevention and Control. ECDC SURVEILLANCE REPORT: Sexually Transmitted Infections in Europe 2013. 2015. (In press).
Acknowledgements
We are grateful to the whole European STI surveillance network for its contribution in the development and implementation of the Euro-GASP and the submission of gonococcal isolates and epidemiological data. The authors would like to acknowledge and thank the following ECDC staff who reviewed the manuscript: Julien Beaute, Phillip Zucs, Denis Coulombier, Andrew Amato and Mike Catchpole. And a special thank you to Cathy Ison who has contributed a great deal to the creation, implementation and co-ordination of the Euro-GASP over the years.
The study was funded by the European Centre for Disease Prevention and Control (Framework Contract No. ECDC/2013/015).
Euro-GASP network
Austria: Angelika Stary, Maria Haller; Belgium: Ruth Verbrugge, Tania Crucitti; Cyprus: Soteroulla Soteriou, Panayiota Maikanti-Charalambous; Denmark: Susan Cowan, Steen Hoffmann; France: Guy La Ruche, Agathe Goubard; Germany: Peter Kohl, Susanne Buder, Viviane Bremer; Greece: Eva Tzelepi, Vasileia Konte; Hungary: Eszter Balla, Mária Dudás; Iceland: Guðrún Sigmundsdóttir, Guðrún Svanborg Hauksdóttir; Ireland: Derval Igoe, Brendan Crowley; Italy: Barbara Suligoi, Paola Stefanelli; Latvia: Gatis Pakarna, Violeta Mavcutko; Malta: Christopher Barbara, Jackie Maistre Melillo; Netherlands: Alje Van Dam, Birgit Van Benthem, Ineke Linde; Norway: Hilde Kløvstad, Gaute Syversen; Portugal: Jacinta Azevedo, Maria José Borrego; Slovak Republic: Peter Pavlik, Peter Truska; Slovenia: Irena Klavs, Samo Jeverica; Spain: Julio Vazquez, Mercedes Diez; Sweden: Inga Velicko; United Kingdom: Stephanie Chisholm, Gwenda Hughes, Kirstine Eastick.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MC, GS and MU designed, initiated and coordinated the study. SJ, RP, VG and Network members coordinated and performed the laboratory analyses. Patient data was supplied by the Network members. MC, GS and MU analysed and interpreted all the data, and wrote a first draft of the paper. All authors read, commented and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Cole, M.J., Spiteri, G., Jacobsson, S. et al. Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect Dis 15, 321 (2015). https://doi.org/10.1186/s12879-015-1013-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-015-1013-x