Abstract
Background
Failure to detect cognitive impairment (CI) in hospitalised older inpatients has serious medical and legal implications, including for the implementation of care planning. This mixed methods study aimed to determine amongst hospital in-patients aged ≥ 65 years: (1) Rates of documentation of screening for CI, including the factors associated with completion of screening; (2) Rates of undocumented CI amongst patients who had not received screening during their admission; (3) Healthcare provider practices and barriers related to CI screening.
Methods
A mixed methods study incorporating a clinical audit and interviews with healthcare providers was conducted at one Australian public hospital. Patients were eligible for inclusion if they were aged 65 years and older and were admitted to a participating ward for a minimum of 48 h. Patient characteristics, whether CI screening had been documented, were extracted using a template. Patients who had not been screened for CI completed the Montreal Cognitive Assessment (MoCA) to determine cognitive status. Interviews were conducted with healthcare providers to understand practices and barriers to screening for CI.
Results
Of the 165 patients included, 34.5% (n = 57) had screening for CI documented for their current admission. Patients aged > 85 years and those with two or more admissions had greater odds of having CI screening documented. Among patients without CI screening documented, 72% (n = 78) were identified as cognitively impaired. While healthcare providers agreed CI screening was beneficial, they identified lack of time and poor knowledge as barriers to undertaking screening.
Conclusions
CI is frequently unrecognised in the hospital setting which is a missed opportunity for the provision of appropriate care. Future research should identify feasible and effective strategies to increase implementation of CI screening in hospitals.
Similar content being viewed by others
Background
Whilst older adults aged 65 years and over comprise just 16% of the Australian population [1] they are overrepresented as hospital inpatients. In 2020, adults aged over 65 years accounted for 40% of all hospital admissions and 41% of all overnight hospitalisations [2]. Current projections estimate the number of older Australians will more than double by 2066 [3]. This will have significant impacts on the care provided in hospitals, and the healthcare system more broadly.
Cognitive impairment (CI) refers to changes in cognition with deficits in one or more domains of memory, language, executive function, attention, perceptual-motor, and social cognition. CI can range from age related cognitive decline, to mild cognitive impairment and dementia. In complex acute-care settings proper diagnosis of dementia is challenging due to the presence of other causes of cognitive dysfunction (e.g., delirium, head injury), and full diagnostics for assessment may not be feasible or appropriate. Hence the term CI is adopted to broadly describe cognitive dysfunction in the acute setting without the requirement to ascribe a diagnosis [4]. In Australia, it is estimated that 29-38% of older patients admitted to hospital have CI [5, 6] and 25% aged over 85 years have dementia [7].
Patients admitted to hospital with CI have higher rates of hospital morbidity and mortality; higher incidence of hospital acquired complications including hospital acquired infections, and pressure injuries; longer hospital stays; greater functional decline; and higher rates of discharge into residential aged care than patients without CI [6,7,8,9,10,11,12]. Failure to identify CI in hospital can lead to insufficient or inappropriate care including assumed cognitive capability to understand complex medication regimens and not implementing delirium preventative strategies. Early and timely detection of CI in older inpatients therefore has the potential to both reduce adverse events during hospitalisation (i.e. delirium, falls) and provide opportunities to engage patients and their support persons in advanced care planning to promote autonomy and control over future health care decisions [13,14,15].
Because of the known benefits of detecting CI amongst older inpatients, routine screening for CI is recommended. The Australian Commission on Safety and Quality in Healthcare’s National Safety and Quality Health Service Standards for Delirium [16] and Guide for providing care for patients with CI at risk of delirium [17] specify that screening for CI should be embedded into routine clinical care for patients aged ≥ 65 years (or ≥ 45 years for Aboriginal and Torres Strait Islander people). This is reflected in local hospital guidelines. For example, the Hunter New England Local Health District policies ‘Screening, Assessment and Management of Delirium in Adults’ [18] and ‘Care of Cognitively impaired Older people’ [19] specify that all patients aged ≥ 65 (≥ 45 for Aboriginal and Torres Strait Islander peoples) require a cognitive screen within 24 hours of admission using either the 4AT, Abbreviate Mental Test Score (AMTS), the Six Item Screener or the Rowland Universal Dementia Scale (RUDAS). While policies recommend that CI screening should be part of routine care [16, 17], previous studies show this rarely occurs in routine practice [6, 20].
A national evaluation in four hospitals across Australia [6] estimated that while 40% of older adults who receive screening using a validated tool are found to have CI, screening is conducted less than 60% of the time in hospital [6]. A range of factors that impede screening for CI have been identified. At the healthcare provider level, barriers include limited time within existing workloads, onerous tools, lack of confidence in screening, limited human resources, on over reliance on clinical knowledge/skills, a reluctance to discuss a sensitive topic, and lack of access of specialists to refer to if CI is identified [4, 21,22,23]. Patient level factors include not being able to participate in cognitive screening (e.g., aphasia) and a fear of the outcomes of screening [4, 21,22,23]. Understanding the barriers to screening for CI is important to guide and inform interventions to improve rates of screening. To date, no studies have comprehensively examined the prevalence of screening for CI amongst older hospitalised inpatients, while also quantifying rates of undocumented CI and practices and barriers to screening amongst health professionals in the same setting.
This mixed methods study with a prospective clinical record audit for quantitative data and healthcare interviews for qualitative data aimed to:
1. Determine amongst hospital in-patients aged ≥ 65 years:
-
a)
rates of documentation of screening for CI, including the factors associated with completion of screening for CI.
-
b)
rates of undocumented CI amongst patients who had not received screening as part of their current admission.
2. Understand healthcare provider perceptions of current practices and barriers to screening for CI in the hospital setting.
Methods
Design
A mixed methods study that included a prospective audit of clinical records of geriatric inpatients and interviews with healthcare providers was conducted. The interviews were performed to provide complementary perspectives on current practices and barriers to screening for CI in real world clinical practice. The COREQ checklist was used in reporting qualitative study findings [24].
Setting
Medical inpatient wards at a Public acute group B hospital (AIHW classification) in the Hunter New England Local Health District were eligible for inclusion in the clinical audit. The Hunter New England Local Health District provides services to 12% of the NSW population with over 2.7 million patients supported each year and more than 225,000 stays per year [25]. The average length of stay is 3 days with 42% of admissions aged ≥ 65 years. These statistics are consistent with the national average length of stay and age of admissions [2]. The population of patients serviced by the Hunter New England Local Health District is diverse including a mix of metropolitan, regional, rural and remote communities and mirrors the Australian healthcare system [26].
Clinical record audit
Eligibility
Patients were eligible for inclusion in the clinical record audit if they were aged ≥ 65 years; were inpatients admitted to a participating ward; had been admitted for a minimum of 48 h to enable adequate time for completion of assessment; were determined to be physically well enough to participate by the Nurse Unit Manager; and were determined to not be in a severe delirium that limited capacity to consent to the cognitive assessment or impacted their ability to complete the Montreal Cognitive Assessment as assessed by the geriatrics trainee.
Recruitment
Inpatients admitted at the participating hospital who met the eligibility criteria as assessed by the geriatrics trainee were selected at random for inclusion in the audit before undertaking data extraction.
Data collection
De-identified data was collected from the clinical records of patients using an audit template from February to September 2020. A systematic sampling approach was adopted where the geriatric trainee approached patients in odd numbered beds. This method of sampling was adopted to eliminate bias from over or under sampling close observation bay beds or single infectious rooms in the medical ward that may have been more likely to have patients with cognitive impairment.
Measures
The following information was extracted from each record.
Demographic characteristics. Age, gender, relationship status, number of admissions in the past 12 months were recorded.
Clinical information. The following information was recorded: [1] whether the patient had the following medical conditions (current or active; yes/no): dementia or mild cognitive impairment; cancer; heart conditions; respiratory conditions; chronic kidney conditions; endocrine conditions; nutritional or metabolic disorders; rheumatological conditions; gastrointestinal conditions; neurological conditions; urinary or reproductive conditions; mental health conditions; [2] whether the patient was currently receiving palliative care (yes/no/unknown); and [3] the patient’s estimated Eastern Cooperative Oncology Group (ECOG) performance status. While most commonly used in practice and in research for oncology and patients, ECOG has been used in national multi-centre health record audits including with patients with dementia as a proxy for functional status given it is a relatively simple and quick assessment tool [27, 28, 29]. Following review of the clinical information recorded, the auditor noted the patient’s likely functional status using five defined categories: 0 if fully active, able to carry out all daily living activities; 1 if restricted in physically strenuous activity, but ambulatory and able to carry out work of a light or sedentary nature; 2 if ambulatory and capable of all self-care but unable to carry out any work activities; 3 if capable of only limited self-care, confined to bed or chair more than 50% of waking hours; 4 if completely disabled, unable to carry out any self-care, totally confined to bed or chair. If they were unable to estimate likely degree of disability, they selected ‘insufficient information available’.
Screening for CI. The auditor recorded whether the patient had received screening for CI (yes/no) during the admission. If screening had been conducted, they also recorded which tool was used, and if the score indicated CI. CI was indicated by a score of > 4 on the 4AT, > 8 on the 6 Item Screener, and ≤ 6 on the AMTS.
Assessment of CI. Patients that did not have screening for CI documented in their clinical record were administered the Montreal Cognitive Assessment (MoCA) by the geriatrics trainee. The MoCA is a validated tool to detect CI that contains 30 questions assessing eight cognitive domains: orientation, short term memory, delayed memory, executive function, visuospatial ability, abstraction, language, and attention. It can be administered in approximately 10 min and is scored out of 30. It has adequate psychometric properties (sensitivity = 83.9%, specificity = 74.6, internal consistency α = 0.78, and test re-test reliability 0.88) [30]. A score of ≤ 25 was considered as indicative of CI. Given it is a concise, validated tool that has a higher sensitivity of picking up mild cognitive impairment, MoCA was chosen over other brief screening tools mentioned in the policy (4AT,6 Item Screener, AMTS or RUDAS) [31,32,33,34].
Analysis
Statistical analyses were programmed using SAS v9.4 [35]. A priori, statistical significance was set at p-value < 0.05. Descriptive statistics are reported for all relevant variables, including frequencies and percentages for categorical variables, and means, standard deviations, medians, and ranges for continuous variables. A multivariable logistic regression analyses was conducted to examine the factors associated with having screening for CI documented in the clinical record.
Qualitative interviews
Eligibility
Healthcare providers were eligible for participation in interviews if they were medical officers or members of the Aged Care Services in Emergency Team at the participating hospital.
Recruitment
An email was sent to all Medical Officers and Aged Care Service Emergency Team nurses at the participating hospital. The email included a detailed study information statement and asked those interested in participating to contact a member of the research team to schedule a convenient time for an interview.
Data collection
Interviews focused on current practices for screening for cognitive impairment and perceived barriers to screening. Each interview was guided by a semi-structured interview guide developed with input from geriatricians and health behaviour researchers. The final interview guide (Appendix A) covered the following domains: awareness of hospital policies regarding CI screening, CI screening tools used, frequency of screening, benefits of CI screening and barriers to routine CI screening.
All interviews were conducted face-to-face, at the participating hospital and audio recorded with participant consent by the geriatrics advanced trainee (RR).
Analysis
Audio recordings of interviews were transcribed by a professional transcription service. Data were analysed using Nvivo software. An inductive qualitative content analysis approach was chosen. This method was considered particularly suited to gain in-depth insights into participants’ perspectives which are grounded in the actual data, rather than researchers’ preconceived categories and theoretical perspectives [36]. Each whole interview was considered as a unit of analysis. An experienced researcher (JB: PhD) read the transcripts line by line and examined, compared, and categorized their content to apply a paraphrase or label (a “code”) which described what was interpreted in each section as important. Based on the initial codes, more abstract categories were developed [37]. The codes and categories were used to form a coding matrix which was reviewed by all members of the research team. Based on the coding matrix, we generated threads of meaning across categories (i.e. themes) and thus analysed both latent and manifest content derived from the data [38]. The robustness of the conclusions was tested on the basis of each case by comparing codes within each interview, as well as independently of cases by comparing codes between interviews [39].
Ethics approval
Ethics approvals were obtained from the Hunter New England Human Research Ethics Committee (2021/ETH00319 and 2021/STE00569).
Results
Clinical record audit sample
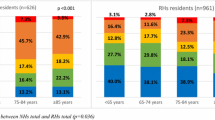
A total of 175 participants were assessed for eligibility, 167 of which were eligible to participate. Two participants were excluded due to incomplete data. Complete data was obtained for 165 participants. Participant characteristics by CI screening status and are presented in Table 1. The mean age of participants was 81.89 years (SD = 7.71).
Screening for CI
Of the 165 patients included, 34.5% (n = 57) had screening for CI documented in their medical record for their current admission. The most frequently used tools to conducted CI screening were the 4AT (n = 31, 54.4%), followed by the 6 Item Screener (n = 21, 36.8%) and the AMTS (n = 5, 8.7%). Factors associated with having screening completed are provided in Table 2. Those aged 85 years or older were 3.52 times more likely to have CI screening completed compared to those aged 84 or less (95% CI: 1.70, 7.30, p = 0.0007). Those who had two or more admissions in the preceding year were 0.24 times more likely to have CI screening completed compared to those with one or no previous admissions (95% CI: 0.11,0.50, p = 0.0001). Gender, functional status ECOG or number of comorbid conditions were not significantly associated with screening for CI.
Rates of undocumented CI
Documentation of screening for CI by MoCA test result is provided in Table 3. Of patients who had screening documented, 75% (n = 43) were also identified as cognitively impaired following testing using the MoCA. Amongst patients who had no CI screening documented, 72% (n = 78) met criteria for CI using the MoCA.
Qualitative sample
Interviews were conducted with a total of 20 healthcare providers. Participants included Junior Medical Officers (n = 10), Senior Resident Medical Officers (n = 8) and registered nurses of the Aged Care Service Emergency team (n = 2). Interview duration varied between 7 and 20 min.
Perceived benefits to CI screening
Participants universally agreed that there were benefits to undertaking CI screening. These included ensuring patients received appropriate care, identifying delirium and/or dementia, establishing a baseline so that any deterioration during admission could be identified, providing information about capacity to make treatment decisions while in hospital, and providing critical information for care planning including the types of services patients may need following discharge.
“You have a baseline you can see when there’s a deterioration from their baseline and also, you can identify issues earlier. So if there are any issues that need to be addressed, you can get processes and things in place to help them” (P16, JMO, < 1 year experience).
“In the short term, the benefit to screening is that you have patients that are higher risk of becoming, like delirious or agitated or aggressive, but then you also have the added benefit of, if you pick up a patient that you didn’t realise has a MoCA of 18, they probably should have an outpatient geriatrician follow-up, or have further assessment while they’re an inpatient… I think screening is always good” (P8, JMO, < 1 year experience).
“Screening would have higher sensitivity than just pure concern would. So, you might pick up patients that might not have the capacity that you think they do that’s actually happened to us last week. Shocking cognitive impairment in someone that we assumed was perfectly independent” (P5, SMRO, 3 years’ experience).
Current practices for CI screening
Commonly used tools for screening identified by participants were the 4AT, Mini Mental State Exam (MMSE), MoCA, and CAM, and several participants identified the suitability of the RUDAS for non-native English speakers. Healthcare providers reported that the conduct of screening was variable. While some participants reported screening every patient aged over 65 years, others reported that whether screening was completed depended largely on the symptoms of the presenting patient.
“I think it really just depends on what they come in with, right? I wouldn’t do it - I don’t think- for every patient that I’ve seen, but if I start getting concerned that someone might be delirious, I’ll start the CAMs” (P16, JMO, 1 year experience).
“I think it would be dependent on the patient, definitely. Like, I’m not going to do a cognitive screen on someone who seems very with-it when I’m talking to them, and can fully recount a history and that kind of thing, but, if someone comes in, and they’re confused, especially if they’re confused and they’re over 50, over 60, then I would be performing it” (P19, JMO, 1 year experience).
Barriers to CI screening
Lack of time and poor healthcare provider knowledge and awareness of CI screening were the two most commonly mentioned barriers to routine CI screening. In the emergency department, healthcare providers reported not having enough time to consistently undertake CI screening for patients aged 65 and older. Managing acute problems and bed block in stretched and busy emergency departments meant CI screening was not seen as a priority and was “one of those things that just slips under the radar” (P3, JMO, 1 year experience).
“ I don’t think it is a lack of resources, I just think it is maybe a lack of sort of, it just - the hospital being an acute environment and you can get quite busy, it gets missed.” (P4, JMO, < 1 years experience).
There was a clear lack of awareness among some participants about policies and expectations for screening. Participants reported they “didn’t know about the policy, because I hadn’t done a medical term.” (P15, JMO, 1 year experience) and even experienced staff reported they weren’t “aware there was a policy for screening people over 65” (P18, SMRO, 3 years experience).
Despite this, several participants acknowledged that CI screening could be completed relatively quickly using short tools, and that “it’s pretty easy, so there shouldn’t really be a reason not to do it” (P2, JMO, < 1 years experience). Screening was more often thought to occur on wards where there was sufficient time and often a dedicated healthcare provider who took responsibility for completing screening, although some participants also mentioned that screening was routinely conducted in the emergency department.“Most of the patients up here on this ward tend to receive them, because I think the staff-to-patient ratio up here is more appropriate” (P8, JMO, 1 year experience). “We start the process here in ED, and then the idea is if they’re triggering any red flags that we then pop them onto a pathway that forms part of our handover to the ward and then they continue to monitor their cognition.” (P12, Nurse, 2 years experience).
While almost all healthcare providers were aware of the various CI screening tools, almost one quarter were unaware of hospital policy for CI screening to occur for overnight admitted patients aged 65years and over. Participants reported that lack of knowledge of policies and differences in practice across the hospital contributed to low rates of CI screening
“I wasn’t aware there was a policy for screening people over 65” (P18, SMRO, 3 years experience).
“If it’s new staff to the department, they haven’t had that education. So, they’ve come from another team who don’t routinely screen for cognition and delirium. They may not know that we do that as par for the course here. (P12, Nurse, 2 years experience).
Participants sometimes reported confusion about which CI screening tool was appropriate to use in different situations, and how to appropriately administer them. There was also some confusion about whose responsibility it was to complete screening, with some participants seeing it as a responsibility or nurses or allied health providers.
“From doing MoCAs in the past, what I struggled with was knowing when a patient will get the answer, like how much time you give them, and how patient you are with them being - with them answering and how much is okay. Also, how many hints you can give before it not counting as a point?” (P15, JMO, 1 year experience).
“I don’t think we’ve specifically been told to screen… I’ve seen allied health get involved by maybe the nurses taking initiative and getting OTs involved… I haven’t done a lot of screening myself” (P15, JMO, 1 year experience).
“My understanding, I think, was that if the patient is for discharge, then they’ll be seen by our set nurse, and they do it. But I guess in terms of patients being admitted, I have never really thought about who’s doing it. I guess that should fall to me.” (P2, JMO, < 1 years experience).
“For me, it was just mainly having an unclear idea of when to do it and if I’m to do it.” (P2, JMO, < 1 years experience).
Discussion
The routine and accurate detection of CI is key to improving quality of healthcare provided to older adults in hospitals. This mixed methods study aimed to understand completion of CI screening and care planning in the clinical record of hospital inpatients aged 65 years and older, as well as understand healthcare provider perspectives on practices and barriers to CI screening.
Overall, only 34.5% of inpatients had screening for CI documented in their clinical records, despite a policy directive requiring screening for all overnight admitted patients aged ≥ 65 years [16,17,18,19]. Failure to detect CI has serious medical and legal implications. Healthcare providers may wrongly assume capacity to understand complex risk–benefits of treatments, and CI is associated with increased hospital-acquired complications, increased length of stay and unplanned readmissions [8, 9, 12, 40,41,42]. However, identification of CI through screening can prompt the provision of “dementia-friendly” hospital care [6, 14, 43] including non-pharmacological interventions to prevent delirium, avoiding medications with cognitive side-effects, improved communication with patients, inclusion of caregivers and/or SDM in decision making, early medical and psychosocial interventions to patients with CI and their families [44, 45] and supported adequate discharge management [6, 15, 43]. Previous research has found that low rates of screening may result from the lack of a single endorsed validated tool for cognitive screening [46, 47], poor staff awareness of hospital policies [4] and understanding of the importance of screening in improving health outcomes, insufficient time to undertake screening, and cognitive assessment being considered a lower priority compared to physical care. Similar barriers were identified by healthcare professionals in our study, who identified lack of awareness of CI screening policies, lack of knowledge about which screening tool to use as key barriers to routine implementation of CI screening. These findings highlight the need for ongoing training for clinical staff in the use of available short screening tools.
The prevalence of undocumented CI as assessed by the MoCA was alarmingly high at 72%. While we are, to the best of our knowledge, the first to describe rates of undocumented CI by comparing CI screening tests at admission with a validated CI screening test,, this rate of undocumented CI is higher than previously described in literature both nationally and internationally. A prospective observational cohort study examining CI amongst Australians aged over 70 years admitted to four hospitals in Queensland reported 29.4% had CI and 20.1% dementia [5]. Of those who were diagnosed with dementia, more than half did not have documentation of this in their clinical record [5]. International studies with varying methodology show a broad range of rates of undocumented CI when compared to medical records between 46 and 62% [48, 49] and undocumented dementia between 46 and 64% [11, 20, 50, 51] in admitted older hospital inpatients. These differences likely reflect variability in demographics of patient populations, the clinical setting, and the screening and diagnostic assessment methods used to determine CI. While some studies used MMSE, in our study the MoCA was used given its greater sensitivity at detecting MCI [30, 52,53,54], early vascular CI [55] and CI due to Parkinson’s disease [56]. Studies also administered screening at different timepoints following admission, which is important given screening is best done once acute illness has resolved. Our study population was older than other studies and included patients from institutionalized care, where rates of CI are higher [7, 57, 58]. Nevertheless, our data suggests there are significant numbers of older people who have CI that are not recognised, and therefore may not be receiving appropriate patient-centred care.
Strengths and limitations
A strength of this study is the use of the MoCA to screen for CI rather than the MMSE, as the MoCA has been shown to be more sensitive to milder forms of CI, vascular CI and CI related to Parkinson’s disease. The face-to-face administration of the MoCA by a trained geriatric trainee to a large sample of patients, and systematic sampling of patients, are also strengths of this study.
Several limitations should be noted. Firstly, the study was conducted in a single centre and therefore findings may not be generalisable to other healthcare settings. Secondly, screening for CI was limited to a single MoCA assessment but vital information gained from collateral history of subjective memory complaints from caregivers was not incorporated. The MoCA is designed as a stand-alone screening tool, but further testing is generally required to make a formal diagnosis of CI. Thirdly, this is a prospective clinical record audit hence some information about cognitive screening performed at the bedside but not documented in the clinical record may have been missed. Finally, interviews were limited to medical officers and ASET aged care nursing staff, but excluded other relevant health professionals such as occupational therapists and medical ward nursing staff. The small number of nurses included is a limitation given nurses often have a key role to play in screening for CI during admission.
Conclusion
Medical admissions offer a timely opportunity to identify CI to ensure high-quality care is provided and strategies are implemented to minimise adverse events associated with CI. Despite policy recommendations for routine screening of hospitalised inpatients aged over 65 years, many patients are not screened. With the rapidly growing ageing population, further research is warranted to identify feasible and effective strategies to increase implementation of CI screening, to provide quality patient-centred care.
Data Availability
The datasets used and/or analysed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
- AMTS:
-
Abbreviate Mental Test Score
- CI:
-
Cognitive impairment
- ECOG:
-
Eastern Cooperative Oncology Group
- MMSE:
-
Mini Mental State Exam
- MoCA:
-
Montreal Cognitive Assessment
- RUDAS:
-
Rowland Universal Dementia Scale
References
Australian Instutue of Health and Welfare. Older australians. Australian Government; 2021.
Australian Instutue of Health and Welfare. Australian hospital statistics. Admitted patient care 2019–20. 2020.
Statistics ABo. Population projections, Australia. ABS cat. no. 3222.0. Canberra: ABS; 2018.
MacDermott S, McKechnie R, LoGiudice D, Morgan D, Blackberry I. Barriers and facilitators to Screening for Cognitive Impairment in Australian Rural Health Services: a pilot study. Geriatr (Basel). 2022;7(2).
Travers C, Byrne G, Pachana N, Klein K, Gray L. Prospective observational study of Dementia and delirium in the acute hospital setting. Intern Med J. 2013;43(3):262–9.
MacDermott S, Yates M, Theobald M, Morvell M, Mohebbi M, West E, Jebramek J, Watts J. National Rollout and evaluation of the Dementia Care in hospitals Program (DCHP). Ballarat Health Services. Ballarat, Victoria Australia,2017.
Draper B, Karmel R, Gibson D, Peut A, Anderson P. The Hospital Dementia Services Project: age differences in hospital stays for older people with and without Dementia. Int Psychogeriatr. 2011;23(10):1649–58.
Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of Dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–55.
Fogg C, Griffiths P, Meredith P, Bridges J. Hospital outcomes of older people with cognitive impairment: an integrative review. Int J Geriatr Psychiatry. 2018.
Reynish EL, Hapca SM, De Souza N, Cvoro V, Donnan PT, Guthrie B. Epidemiology and outcomes of people with Dementia, delirium, and unspecified cognitive impairment in the general hospital: prospective cohort study of 10,014 admissions. BMC Med. 2017;15(1):140.
Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195(1):61–6.
Fogg C, Meredith P, Bridges J, Gould GP, Griffiths P. The relationship between cognitive impairment, mortality and discharge characteristics in a large cohort of older adults with unscheduled admissions to an acute hospital: a retrospective observational study. Age Ageing. 2017;46(5):794–801.
Dubois B, Padovani A, Scheltens P, Rossi A, Dell’Agnello G. Timely diagnosis for Alzheimer’s Disease: A literature review on benefits and challenges. J Alzheimers Dis. 2016;49(3):617–31.
Borson S, Frank L, Bayley PJ, Boustani M, Dean M, Lin PJ, et al. Improving Dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9(2):151–9.
Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: The benefits of early diagnosis and intervention.; 2018.
Care AACSQH. Delirium Clinical Care Standard. Sydney: ACSQHC; 2021.
Sydney AACoSaQiHC. Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards—User Guide for Health Service Organisations Providing Care for Patients with cognitive Impairment or at Risk of Delirium. In: Sydney ACoSaQiHC, editor. Sydney, Australia2019.
District HNELH, Screening. Assessment and Management of Delirium in Adults Hunter New England Local Health District Pol 21_04:PCP 1 – Policy Compliance Procedure (PCP) 2021.
District HNELH. Care of Cognitively Impaired Older People in Hunter New England Local Health District Hospitals. CG 19_42. 2019.
Laurila JV, Pitkala KH, Strandberg TE, Tilvis RS. Detection and documentation of Dementia and delirium in acute geriatric wards. Gen Hosp Psychiatry. 2004;26(1):31–5.
Abzhandadze T, Buvarp D, Lundgren-Nilsson Ã, Sunnerhagen KS. Barriers to cognitive screening in acute Stroke units. Sci Rep. 2021;11(1):19621.
Swartz RH, Bayley M, Lanctôt KL, Murray BJ, Cayley ML, Lien K, et al. Post-stroke depression, obstructive sleep apnea, and cognitive impairment: Rationale for, and barriers to, routine screening. Int J Stroke. 2016;11(5):509–18.
Kirk Wiese L, Williams CL, Tappen RM. Analysis of barriers to cognitive screening in rural populations in the United States. ANS Adv Nurs Sci. 2014;37(4):327–39.
Bryant J, Sellars M, Sinclair C, Detering K, Buck K, Waller A et al. Inadequate completion of advance care directives by individuals with Dementia: national audit of health and aged care facilities. BMJ Support Palliat Care. 2021.
Buck KDK, Sellars M, Ruseckaite R, Kelly H, Nolte L. Prevalence of Advance Care Planning Documentation in Australian Health and residential aged care services, short report. Advance Care Planning Australia, Austin Health, Melbourne.; 2017.
Buck K, Detering KM, Pollard A, Sellars M, Ruseckaite R, Kelly H, et al. Concordance between self-reported completion of Advance Care Planning Documentation and availability of documentation in Australian Health and residential aged care services. J Pain Symptom Manage. 2019;58(2):264–74.
Tong A, Sainsbury PJC. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
NSW Department of Health. The NSW Health Annual Report 2019-20. 2020.
Hunter New England Local Health District. Hunter New England Local Health District Strategic Plan: Towards 2018. Newcastle2015.
De Roeck EE, De Deyn PP, Dierckx E, Engelborghs S. Brief cognitive screening instruments for early detection of Alzheimer’s Disease: a systematic review. Alzheimers Res Ther. 2019;11(1):21.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Emery A, Wells J, Klaus SP, Mather M, Pessoa A, Pendlebury ST. Underestimation of cognitive impairment in older inpatients by the abbreviated Mental Test score versus the Montreal Cognitive Assessment: cross-sectional observational study. Dement Geriatr Cogn Dis Extra. 2020;10(3):205–15.
Brymer C, Sider C, Evans A, Lee BY, Taneja J, Morgenstern RN. Montreal Cognitive Assessment Vs. Rowland Universal Dementia Assessment Scale for cognitive screening. Innov Ageing. 2017;1(1):468.
Lees R, Corbet S, Johnston C, Moffitt E, Shaw G, Quinn TJ. Test accuracy of short screening tests for diagnosis of delirium or cognitive impairment in an acute Stroke unit setting. Stroke. 2013;44(11):3078–83.
Institute S, STAT-SAS. Version 9.4. North Carolina: SAS Institute. Cary; 2010.
Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–12.
Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–15.
Przyborski A, Wohlrab-Sahr M. Qualitative Sozialforschung: Ein Arbeitsbuch: De Gruyter. 2014.
Bail K, Berry H, Grealish L, Draper B, Karmel R, Gibson D et al. Potentially preventable Complications of urinary tract Infections, pressure areas, Pneumonia, and delirium in hospitalised Dementia patients: retrospective cohort study. BMJ Open. 2013;3(6).
Timmons S, O’Shea E, O’Neill D, Gallagher P, de Siún A, McArdle D, et al. Acute hospital Dementia care: results from a national audit. BMC Geriatr. 2016;16:113.
Campbell SE, Seymour DG, Primrose WR, Project A. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33(2):110–5.
Weldingh NM, Mellingsæter MR, Hegna BW, Benth JS, Einvik G, Juliebø V, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266.
Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s Disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30(2):161–78.
Mittelman MS, Roth DL, Clay OJ, Haley WE. Preserving health of Alzheimer caregivers: impact of a spouse caregiver intervention. Am J Geriatr Psychiatry. 2007;15(9):780–9.
Cullen B, O’Neill B, Evans JJ, Coen RF, Lawlor BA. A review of screening tests for cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(8):790–9.
Jackson TA, Naqvi SH, Sheehan B. Screening for Dementia in general hospital inpatients: a systematic review and meta-analysis of available instruments. Age Ageing. 2013;42(6):689–95.
Harwood DM, Hope T, Jacoby R. Cognitive impairment in medical inpatients. II: do physicians miss cognitive impairment? Age Ageing. 1997;26(1):37–9.
Joray S, Wietlisbach V, Büla CJ. Cognitive impairment in elderly medical inpatients: detection and associated six-month outcomes. Am J Geriatr Psychiatry. 2004;12(6):639–47.
Timmons S, Manning E, Barrett A, Brady NM, Browne V, O’Shea E, et al. Dementia in older people admitted to hospital: a regional multi-hospital observational study of prevalence, associations and case recognition. Age Ageing. 2015;44(6):993–9.
Bickel H, Hendlmeier I, Heßler JB, Junge MN, Leonhardt-Achilles S, Weber J, et al. The prevalence of Dementia and cognitive impairment in hospitals. Dtsch Arztebl Int. 2018;115(44):733–40.
Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50(5):1039–52.
Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ, Initiative ADN. Relationship between the Montreal Cognitive Assessment and Mini-mental State examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107.
Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa MLG, Ximenes RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491–504.
Koski L. Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36(1):6–18.
Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and Dementia in Parkinson Disease. Neurology. 2009;73(21):1738–45.
van der Flier WM, Scheltens P. Epidemiology and risk factors of Dementia. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v2–7.
Matthews FE, Dening T, Study UMRCCFA. Prevalence of Dementia in institutional care. Lancet. 2002;360(9328):225–6.
Acknowledgements
The authors would like to acknowledge the participants and healthcare providers that gave up their time to contribute to this study.
Funding
This work was supported by a National Health and Medical Research Council-Australian Research Council Dementia Research Development Fellowship (APP1105809) awarded to Dr Bryant, and a National Health and Medical Research Council Dementia Research Teams Grant (APP1095078). Infrastructure support was received from the Hunter Medical Research Institute.
Author information
Authors and Affiliations
Contributions
All authors contributed to the original concept and study design. RR conducted data collection and JB oversaw data collection. RR drafted the paper and JB and RSF reviewed, produced critical comments and suggestions for revision. All authors approve the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with relevant guidelines and regulations including the Declaration of Helsinki. The study was approved by the Hunter
New England Human Research Ethics Committee (2021/ETH00319 and 2021/STE00569). Healthcare providers participating in interviews provided informed consent for participation. A waiver of consent was provided by the Hunter New England Human Research Ethics Committee for the conduct of the clinical audit.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rice, R., Bryant, J. & Fisher, R.S. Documentation of cognitive impairment screening amongst older hospitalised Australians: a prospective clinical record audit. BMC Geriatr 23, 672 (2023). https://doi.org/10.1186/s12877-023-04394-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04394-z