Abstract
Background
Little is known about the association of longitudinal dynamics between cognitive function and frailty in Chinese older adults. The temporal sequences between cognitive function and frailty remains unclear. Our study investigates this directionality association using longitudinal data.
Methods
Latent growth and multivariate latent growth models were employed to examine dynamics of cognition and frailty and their association among 2824 older adults in China. Cross-lagged panel analyses were used to assess the temporal sequences between frailty and cognition. The relation between cognitive domains and frailty was also examined using aforementioned methods.
Results
Cognitive function was negatively associated with frailty status. Higher initial level of cognition indicated lower baseline level (β=-0.175, P < 0.001) and change rate (β=-0.041, P = 0.002) of frailty. We observed a reciprocal association between frailty and cognitive function rather than a unidirectional causal relationship. The initial cognitive performance for all components were negatively associated with baseline (β ranged between − 0.098 to -0.023) and change rate (β ranged between − 0.007 to -0.024) of frail status. No consistent associations between change rate of cognitive components and either initial level or change rate of frailty were detected.
Conclusions
Our study detected a reciprocal association between cognition and frailty rather than a unidirectional causal relationship. Our results also revealed different connections between cognitive performance and frailty across diverse cognitive domains.
Similar content being viewed by others
Background
The development of effective medications and socio-economic conditions improve the health indicators of older populations [1]. However, with the acceleration of ageing and extended lifespans, the suffering of older people from geriatric diseases may be prolonged, increasing the pressure on health-care systems worldwide [1, 2]. China has the fifth largest number of older people worldwide [3]. The increasing burden of ageing populations creates health-care challenges in China [1, 3].
Frailty is a geriatric syndrome, which is characterized by decreased physiological functions, reduced physiological reserve, and increased susceptibility to endogenous or exogenous shock [4, 5]. Previous studies considered frailty a potentially reversible and dynamic entity, and the level of frailty can change bidirectionally over time [2]. Frailty significantly impairs the functional independency of older adults [3] and is associated with unmet care needs, falls and fractures, disability, hospitalizations, lowered quality of life, and mortality [6, 7]. The older population in China over 65 years old showed an 8% frailty prevalence [8].
Cognitive function is the basis of an individual’s capacity to implement appropriate strategies for optimal living [9]. Cognitive impairment is another common geriatric syndrome and often coexists with frailty. This coexistence leads to a vicious cycle in which physical and cognitive decline is further accelerated [10]. Recently, an international consensus group has recognized “cognitive frailty” as the clinical symptom of simultaneous presence of both physical frailty and cognitive impairment in the absence of dementia [11].
Evidence has been accumulating to link frailty with worse global cognitive function [9, 12]. Moreover, associations between frailty and increased risk of future cognitive impairment, incident dementia, and mild cognitive impairment (MCI) have also been reported [7, 13]. Studies showed that the exacerbation of frailty was associated with the rate of cognitive decline among older persons, with a corresponding link between the rates of frailty and cognition changes [14,15,16]. Besides, subjects with cognitive impairment were independently associated with increased risk of frailty [17]. Recent studies show that frailty and cognitive impairment share common etiologies [1], including oxidative stress, genetic alternations, immune dysfunction, and neuroinflammation [10]. Studies also detected the connection between frailty and certain specific cognitive domains. However, it is unknown which domain is linked to frailty [18, 19].
Convincing evidence from longitudinal analyses of the temporal or causal sequences between frailty and cognition is still lacking [20]. Additionally, most studies on frailty and cognition have been conducted in developed countries; however, little is known about the relationship between frailty and cognition in Chinese community-dwelling older adults [21]. Therefore, the present study investigated the relationship between frailty and global cognitive function and the association between their dynamic changes over time in older Chinese community-dwelling adults. The temporal sequences between frailty and cognition were assessed using a cross-lagged panel design. We also examined the association between domain-specific cognitive performance and frailty.
Methods
Data and study participants
The participants were enrolled from the China Health and Retirement Longitudinal Study (CHARLS), which was conducted among Chinese community-dwelling residents aged ≥ 45 years [22, 23]. The baseline survey was conducted in wave 2011 and covered 450 villages and urban communities in China. The participants were resurveyed through face-to-face interviews in waves 2013, 2015, and 2018 [24, 25]. Due to the lack of sufficient information on frailty in wave 2018 [26], this study enrolled participants from waves 2011 to 2015.
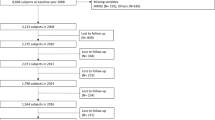
A total of 17,616 community-dwelling adults aged ≥ 45 years participated in CHARLS baseline survey, followed by 18,484 in wave 2013 and 20,991 in wave 2015. In the present study, individuals were eligible if they fulfilled the following criteria: (a) age ≥ 60 years at baseline, (b) without cognitive impairment or frailty at baseline, (c) participated in all three waves from 2011 to 2015, and (d) missed at most one cognitive function measurement or frailty measurement among the three waves. This study included a total of 2,824 older adults; among these, 2,037 had complete data with no missing cognitive or frailty measurements at all three time points. Figure 1 shows the selection process of the analytical sample.
Global cognitive function measurement
The Telephone Interview of Cognitive Status (TICS-10) was adopted to examine global cognitive function [27,28,29]. The TICS-10 included the components of time orientation (naming the month, day, year, week, and season), working memory (sequential subtraction of 7 from 100 five times), visual and spatial abilities (redrawing two overlapping pentagons), immediate recall test of memory, and delayed recall test of memory. The participants were asked to recall as many words as they could immediately after hearing a list of ten Chinese nouns. The number of correct words was defined as the immediate recall scores. Several minutes later, the participants were asked to recall the words again; this was considered delayed recall. The scores for global cognitive function were the sum of the correct answers or words and ranged from 0 to 31, with a higher score indicating better global cognition. To eliminate the influence of cognitive impairment at baseline, the participants were grouped every five years of age, and individuals with cognitive scores less than mean-standard deviation (SD) in each age group were excluded [27, 30].
Frailty measurement
Frailty was measured using Fried’s Physical Frailty Phenotype (PFP), as in previous studies in the CHARLS cohort [31]. Five criteria were used to define frailty: slowness, weakness, exhaustion, inactivity, and weight loss. The participants were asked to walk over a 2.5 m course twice, with slowness defined as an average speed below or equal to the 20th percentile after adjusting for sex and height [31]. A handheld dynamometer was used to assess the handgrip strength twice for each hand, with weakness defined as a maximum of four readings below or equal to the 20th percentile after adjusting for sex and body mass index (BMI). Participants responding ‘a moderate amount of time; 3 to 4 days’ or ‘most of the time; 5 to 7days’ when asked ‘How often during the last week did you feel this way’ to two statements in the Center for Epidemiological Studies-Depression (CESD) scale: ‘I could not get going’ and ‘I felt everything I did was an effort’ met the exhaustion criterion. Participants who walked continuously for < 10 min during a usual week met the criterion for inactivity. Participants who self-reported over 5kg weight loss in the past year or with a current BMI ≤ 18.5kg/m2 met the criterion for weight loss. The number of criteria met was used to assess frailty status. Individuals who met no criteria were considered ‘non-frail’ or ‘robust’; those who met 1–2 criteria were deemed ‘prefrail’; and those who met ≥ 3 criteria were defined as ‘frail’. Individuals who missed two or more of the five frailty criteria were excluded.
Covariates
The potential covariates included baseline measurements of age, sex, residence region, education level, marital status, status of current smoking and drinking, number of comorbidities, and depression in wave 2011. As mentioned in previous studies, the comorbidities included hypertension, diabetes mellitus, dyslipidemia, heart disease, stroke, cancer, lung disease, arthritis, kidney disease, digestive disease, and asthma [28, 32]. Depression was measured using 10 items of the CESD scale. The scores ranged from 0 to 30, with greater values representing higher levels of depressive symptoms [33].
Statistics analyses
Mean with SD and frequencies with percentages were calculated as descriptive statistics for continuous and categorical variables, respectively. Comparisons among the three waves were performed using repeated-measures analysis of variance and the Friedman’s test. Linear regression was employed to assess the impact of frailty on cognition at each cross-sectional time point, after adjusting for the covariates. Additionally, ordinal regression was utilized to investigate the influence of cognition on frailty.
The latent growth model was used to describe the course of cognition and frailty across the three waves after adjusting for the effects of baseline covariates. Longitudinal changes were examined using intercepts and slopes as latent variables. The intercept represented the average level at baseline, while the slope represented the average rate of change per unit time over the follow-up period. Additionally, the variances of the intercept and slope were estimated to indicate individual differences. The parameters were estimated using maximum likelihood estimation. We used a multivariate latent growth model to evaluate the relationship of longitudinal dynamics between cognition and frailty [34]. The unstandardized pathway coefficients between the parameters were also estimated.
The temporal sequences between cognition and frailty were examined using a cross-lagged model after adjusting for the effect of baseline covariates without restricting the cross-lagged effects across waves to be equal. The standardized autoregressive and cross-lagged pathway coefficients were also estimated. The differences between the two cross-lagged pathway coefficients in each time span were tested using Fisher’s Z-test [35].
Sensitivity analyses were performed using the subset of complete data. The main analyses of the multivariate growth and cross-lagged models were repeated to validate the robustness of the results. Additionally, the dynamics and associations between cognitive domains, with the components of TICS-10 as the indicators, and frailty were explored using the aforementioned methods.
The comparative fit index (CFI), standardized root mean square residual (SRMR), and root mean square residual (RMR) were used to evaluate the goodness of fit of each model. Indices for CFI ≥ 0.90 and SRMR and RMR ≤ 0.08 indicated an acceptable fit. The full information maximum likelihood (FIML) method was used to handle missing data.
All analyses were conducted in R software version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org). All tests were two-tailed and statistical significance was set at P < 0.05.
Results
Demographic characteristics and cross-sectional associations between cognitive function and frailty
Baseline demographic characteristics of the participants are shown in Table 1. The mean age of participants was 67.069 ± 5.867 years and 55.17% were male. The majority were rural residents, with a relatively low education level, never smoker, and non-drinkers. The scores of global cognitive function and frailty status in the three waves are shown in Table 2. The results showed that cognitive function decreased over time (P < 0.001). The cross-sectional associations are shown in Additional file 1. In every wave of the survey, significant negative associations were identified between cognition and frailty, and the unstandardized regression coefficients (β) ranged from − 0.045 to -1.400 (P < 0.001).
Dynamics of cognitive function and frailty
For cognition, the latent growth model showed an acceptable fit for the data (CFI = 1.000, SRMR = 0.002, RMR = 0.013). The initial average TICS-10 score was 11.512 (P < 0.001) and decreased with a rate of 0.641 (P < 0.001) at each time point during the follow-up period. Additionally, the intercept and slope were not significantly correlated (β=-0.200, P = 0.296). For frailty, the model fit to the data well (CFI = 0.988, SRMR = 0.005, and RMR = 0.002). The mean intercept and slope were 0.698 (P < 0.001) and − 0.054 (P = 0.041), respectively. We observed no significant association between the intercept and slope (β = 0.004, P = 0.211).
Associations of dynamics between cognitive function and frailty
The indices indicated a good fit between the model and the data (CFI = 0.997, SRMR = 0.006, and RMR = 0.028). As shown in Fig. 2, no significant relationship was observed between the intercept and slope for frailty or cognition (P = 0.405 and P = 0.139, respectively). The intercept of cognition was negatively associated with the intercept of frailty (P < 0.001), and the slope of frailty (P = 0.002). Additionally, we observed a negative association between the slope of cognition and that of frailty (P = 0.007), suggesting that a higher rate of change in cognition was associated with a lower rate of frailty. The intercept of frailty was not associated with the slope of cognition (P = 0.355), indicating that initial frailty status did not influence changes in cognition.
Associations of dynamics between cognitive function and frailty. The models were adjusted for baseline covariates, including age, sex, residence region, marital status, education level, current smoking and drinking status, number of comorbidities, and depression symptoms. For brevity, the covariates are omitted in this figure. The dashed lines indicate the non-significant path coefficients. C, cognitive function; F, frailty; **P < 0.01; ***P < 0.001
Temporal sequence between cognitive function and frailty
Figure 3 shows the cross-lagged model estimates of the reciprocal association between cognition and frailty. The results suggested an acceptable fit for the data (CFI = 0.991, SRMR = 0.010, and RMR = 0.041). Significant associations were detected in the autoregressive pathways for both cognition and frailty. That is, initial cognition predicted future cognition, and baseline frailty predicted future frailty status. We observed the significant negative cross-lagged effect between cognition and frailty. This indicated that lower levels of cognition subsequently predicted higher frailty scores and vice versa. The standardized path coefficient from cognition in 2011 to frailty in 2013 was greater than that from frailty in 2011 to cognition in 2013 (-0.099 vs. -0.069, respectively); however, the difference between these two coefficients was not significant (P = 0.256). Similarly, the coefficient from cognition in Wave 2013 to frailty in Wave 2015 was greater than that from frailty to cognition (-0.082 vs. -0.062); however, the difference was not statistically significant (P = 0.450). This indicated a reciprocal association between cognition and frailty, rather than a unidirectional causal relationship.
Sensitivity analyses
The results suggested an acceptable fit for the data in the sensitivity analyses (multivariate growth model: CFI = 0.997, SRMR = 0.006, and RMR = 0.024; cross-lagged model: CFI = 0.989, SRMR = 0.010, and RMR = 0.039). As shown in Additional files 2 and 3, the results are similar to those of the main analyses, suggesting the robustness of the present study.
Associations between cognitive components and frailty
We conducted multivariate growth and cross-lagged model analyses for the dynamics of frailty and the five cognitive components of the TICS-10. The fit indices, shown in Additional file 4, indicated that the models were acceptable for the data.
Table 3 lists the parameters of the multivariate growth model. The slope for each cognitive component decreased over time. The intercept of each cognitive component was negatively associated with both the intercept and slope of frailty, indicating that higher initial levels for different cognitive domains were associated with lower baseline and change rate of frailty. Moreover, no consistent relationship between the slope of cognitive performance and either the frailty intercept or frailty slope was identified across the components. Figure 4 shows the results of the cross-lagged model. Different cross-lagged pathways were observed in five cognitive components. No significant difference was detected between the cross-lagged coefficients. The reciprocal associations appeared to differ between diverse cognitive domains and frailty status.
Associations between cognitive components and frailty. The models were controlled for baseline covariates. The solid lines indicate that the path coefficient is statistically significant (P < 0.05). (A) COG 1 for the cognitive component of time orientation; (B) COG 2 for the cognitive component of working memory; (C) COG 3 for the cognitive component of visual and spatial abilities; (D) COG 4 for the cognitive component of the immediate recall test of memory; (E) COG 5 for the cognitive component of the delayed recall test of memory
Discussion
This study explored the association between cognitive function and frailty in Chinese older adults. We confirmed the reciprocal association between frailty and cognitive function and identified diverse connections between cognition and frailty across different cognitive domains.
In this study, the change rates of both cognition and frailty did not depend on their initial levels; therefore, the change rate may be either fast or slow, even if the initial level is high. Currently, there was a contentious debate regarding the relationship between baseline cognitive function and the subsequent changes. In Ma’s research, older adults with subjective memory decline with higher global cognition showed a less rapid cognitive decline [36]. However, in another study, baseline cognition was positive associated with cognitive decline [37]. Besides, previous studies have demonstrated that the level of frailty can change bidirectionally over time [2]. This, to some extent, explains the absence of an identified correlation between baseline frailty and its change rate in our research. It seems that regardless of the baseline cognition or frailty status of older adults, their subsequent change rates exhibit notable heterogeneity. This suggests that regular monitoring of global cognitive function and frailty status in community-dwelling older adults is crucial for preventing cognitive impairment and adverse health outcomes.
Cognitive impairment and frailty share common biological pathways. For example, oxidative stress contributes to frailty and impaired cognitive performance. Reactive oxygen species (ROS) induce changes at the cellular level and lead to systemic responses that influence frailty [38]. Meanwhile, the brain is particularly susceptible to the harmful effects of oxidative damage owing to its deficiency in free radical protective antioxidant compounds and the non-renewability of nervous tissue [14, 15, 39]. In the present study, older adults with better cognitive function had a lower change rate of frailty as well as initial frailty status. We inferred that higher cognitive function may indicate a lower level of adverse factors, more neural reserve, stronger tolerance, or more stability for degeneration of physiological systems caused by ageing. This strong association further confirms that cognitive impairment and frailty share a common pathological basis.
The temporal sequence of cognitive function and frailty remains controversial owing to limitations in study design and analysis methods [40,41,42,43]. Our results indicated a reciprocal relationship between cognitive function and frailty rather than a unidirectional causal association. In Zhao’s study, physical function was assessed through three tests capturing the Chinese older people’s physical performance, including “stand up from sitting in a chair without using hands”, “stand up to pick up a book from floor”, and “turn around 360° without help” [44]. The results demonstrated a positive reciprocal relationship between physical and cognitive functions, and showed no evidence suggesting that the predictive effect of physical performance on subsequent cognition was significantly larger than that of cognition on subsequent physical performance or vice versa [44]. Our findings are consistent with Zhao’s study [44]. Our findings demonstrate that the interventions for preventing against frailty, such as leading a physically active lifestyle, contribute to decelerate cognitive decline; Conversely, interventions aimed at preserving cognitive function or decelerating cognitive decline will also contribute to enhance the physical function of older adults and sustaining their independence in daily activities. Results from randomized controlled trials also demonstrates that physical activities can enhance cognitive function in older adults, and cognitive training improve their physical function [45,46,47]. Attributed to the common biological pathways and etiologies, cognitive decline and frailty may coexist or occur simultaneously, which create the construct of cognitive frailty [48]. As a complete understanding of the underpinning biological basis of cognitive decline and frailty remains fragmented [49], further longitudinal studies are needed to elucidate the relationship between these disorders.
Cognitive domains are reportedly associated with frailty; however, whether the link differs by cognitive domains remains unclear. Chen et al. reported that all domains measured using Montreal Cognitive Assessment were associated with frailty status, except for orientation, when comparing frail and non-frail individuals [50]. In another recent study in older adults in US communities, frail individuals showed significant declines in cognitive function compared to non-frail adults across all domains except for immediate word recall [51]. In our study, the association between the change rate of cognitive performance with level or change rate of frailty was diverse across cognitive domains. The pathways in the cross-lagged models for every cognitive component also supported the idea that the diverse connections between cognitive performance and frailty across different cognitive domains. Further studies on the underlying biological mechanisms are required.
This study had several limitations. First, the CHARLS lacked neuroimaging data and neuropsychological tests to assess performance in cognitive domains. We used the components of the TICS-10, which correlate well with the Mini-Mental State Examination, as substitute indicators of cognitive domains, which have been validated in other studies [52, 53]. Second, the measurement of global cognitive function and frailty was difficult in older adults. This study excluded individuals who lacked one cognitive function measurement or information on frailty. Selection bias could, to some extent, contribute to the overestimation or underestimation of the association between cognition and frailty. Furthermore, due to the lack of relevant data in CHARLS database, the influence of residual confounding was unable to be eliminated in this study, such as the apolipoprotein E genotype. Finally, the follow-up time of 4 years was relatively short across the life course. Longitudinal studies with longer observation periods are warranted.
Conclusions
The results of our study illustrated the relationship between the dynamics of cognitive function and frailty among community-dwelling older adults in China. We observed a reciprocal association between cognition and frailty rather than a unidirectional causal relationship. Our results also revealed different connections between cognitive performance and frailty across diverse cognitive domains. Accordingly, regular monitoring of global cognitive function and frailty is crucial for the ageing Chinese population.
Data availability
The data that support the findings of this study are available in the China Health and Retirement Longitudinal Study (CHARLS) repository, http://charls.pku.edu.cn.
Abbreviations
- MCI:
-
Mild cognitive impairment
- CHARLS:
-
China Health and Retirement Longitudinal Study
- TICS:
-
Telephone Interview of Cognitive Status
- SD:
-
Standard deviation
- PFP:
-
Physical Frailty Phenotype
- BMI:
-
Body mass index
- CESD:
-
Center for Epidemiological Studies-Depression
- CFI:
-
Comparative fit index
- SRMR:
-
Standardized root mean square residual
- RMR:
-
Root mean square residual
- FIML:
-
Full information maximum likelihood
- ROS:
-
Reactive oxygen species
References
Zhao X, Chen Q, Zheng L, et al. Longitudinal relationship between Frailty and cognitive impairment in chinese older adults: a prospective study. J Appl Gerontol. 2022;41(12):2490–8.
Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–86.
Chen X, Giles J, Yao Y, et al. The path to healthy ageing in China: a Peking University-Lancet Commission. Lancet. 2022;400(10367):1967–2006.
Ma L, Chhetri JK, Chan P. Frailty in China: from research to practice. J Nutr Health Aging. 2021;25(4):479–83.
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Junius-Walker U, Onder G, Soleymani D, et al. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10.
Ge M, Zhang Y, Zhao W, et al. Prevalence and its Associated factors of physical Frailty and Cognitive Impairment: findings from the West China Health and Aging Trend Study (WCHAT). J Nutr Health Aging. 2020;24(5):525–33.
He B, Ma Y, Wang C, et al. Prevalence and risk factors for Frailty among Community-Dwelling Older People in China: a systematic review and Meta-analysis. J Nutr Health Aging. 2019;23(5):442–50.
Liu T, Wong GH, Luo H, et al. Everyday cognitive functioning and global cognitive performance are differentially associated with physical frailty and chronological age in older chinese men and women. Aging Ment Health. 2018;22(8):936–41.
Xue H, Huang C, Zhu Q, et al. Relationships among cognitive function, Frailty, and Health Outcome in Community-Dwelling older adults. Front Aging Neurosci. 2021;13:790251.
Dartigues JF, Amieva H. Cognitive frailty: rational and definition from an (I.a.N.a./i.a.g.g.) international consensus group. J Nutr Health Aging. 2014;18(1):95.
Ma W, Wu B, Gao X, et al. Association between frailty and cognitive function in older chinese people: a moderated mediation of social relationships and depressive symptoms. J Affect Disord. 2022;316:223–32.
Kiiti Borges M, Oiring de Castro Cezar N, Silva Santos Siqueira A, et al. The relationship between physical frailty and mild cognitive impairment in the Elderly: a systematic review. J Frailty Aging. 2019;8(4):192–7.
Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007;69(5):483–9.
Buchman AS, Yu L, Wilson RS, et al. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69(12):1536–44.
Boyle PA, Buchman AS, Wilson RS, et al. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–55.
Hsieh TJ, Chang HY, Wu IC, et al. Independent association between subjective cognitive decline and frailty in the elderly. PLoS ONE. 2018;13(8):e0201351.
Kaur S, Banerjee N, Miranda M et al. Sleep quality mediates the relationship between frailty and cognitive dysfunction in non-demented middle aged to older adults. Int Psychogeriatr. 2020:1.
Robinson TL, Gogniat MA, Miller LS. Frailty and cognitive function in older adults: a systematic review and Meta-analysis of cross-sectional studies. Neuropsychol Rev. 2022;32(2):274–93.
Bartoli M, Palermo S, Cipriani GE, et al. A possible Association between Executive Dysfunction and Frailty in Patients with Neurocognitive Disorders. Front Psychol. 2020;11:554307.
Peng C, Burr JA, Yuan Y, et al. Physical frailty and cognitive function among older chinese adults: the mediating roles of activities of Daily Living Limitations and Depression. J Frailty Aging. 2023;12(3):156–65.
Zhao Y, Hu Y, Smith JP, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Wang G, Yao Y, Wang Y, et al. Determinants of COVID-19 vaccination status and hesitancy among older adults in China. Nat Med. 2023;29(3):623–31.
Jin Y, Jing M, Ma X. Effects of Digital device ownership on Cognitive decline in a middle-aged and Elderly Population: longitudinal observational study. J Med Internet Res. 2019;21(7):e14210.
Liang F, Fu J, Moore JB, et al. Body Mass Index, Waist circumference, and Cognitive decline among chinese older adults: a Nationwide Retrospective Cohort Study. Front Aging Neurosci. 2022;14:737532.
Sha S, Chan SHW, Chen L et al. The Association between Trajectories of loneliness and physical Frailty in Chinese older adults: does Age Matter? Int J Environ Res Public Health. 2022;19(9).
Yuan M, Xu C, Fang Y. The transitions and predictors of cognitive frailty with multi-state Markov model: a cohort study. BMC Geriatr. 2022;22(1):550.
Li H, Zhang J, Zou X, et al. The Bidirectional Association between cognitive function and gait speed in chinese older adults: longitudinal observational study. JMIR Public Health Surveill. 2023;9:e44274.
Garcia MA, Ortiz K, Arevalo SP, et al. Age of Migration and cognitive function among older Latinos in the United States. J Alzheimers Dis. 2020;76(4):1493–511.
Hu Y, Peng W, Ren R, et al. Sarcopenia and mild cognitive impairment among elderly adults: the first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. 2022;13(6):2944–52.
Li Y, Xue QL, Odden MC, et al. Linking early life risk factors to frailty in old age: evidence from the China Health and Retirement Longitudinal Study. Age Ageing. 2020;49(2):208–17.
Xu W, Li YX, Hu Y, et al. Association of Frailty with recovery from disability among community-dwelling chinese older adults: China health and retirement longitudinal study. BMC Geriatr. 2020;20(1):119.
Zhao X, Jin L, Sun SB. Early-life interparental relationship quality and late-life depressive symptoms: a mediation analysis. J Affect Disord. 2022;313:137–48.
Liao HP, Pan XF, Yin XQ, et al. Decreased COVID-related adaptive behavior and increased negative affect: a multivariate latent growth curve model. J Health Psychol. 2022;27(9):2115–28.
Zhang T, Zhang H, Li Y, et al. Temporal relationship between Childhood Body Mass Index and insulin and its impact on adult hypertension: the Bogalusa Heart Study. Hypertension. 2016;68(3):818–23.
Ma C, Li M, Wu C. Cognitive function trajectories and factors among chinese older adults with subjective memory decline: CHARLS Longitudinal Study results (2011–2018). Int J Environ Res Public Health. 2022;19(24).
Wolinsky FD, Bentler SE, Hockenberry J, et al. A prospective cohort study of long-term cognitive changes in older Medicare beneficiaries. BMC Public Health. 2011;11:710.
Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R18–36.
Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging. 2011;15(9):756–60.
Li C, Ge S, Yin Y, et al. Frailty is associated with worse cognitive functioning in older adults. Front Psychiatry. 2023;14:1108902.
Guo CY, Sun Z, Tan CC, et al. Multi-concept Frailty predicts the late-life occurrence of Cognitive decline or Dementia: an updated systematic review and Meta-analysis of Longitudinal Studies. Front Aging Neurosci. 2022;14:855553.
Fabricio DM, Chagas MHN, Diniz BS. Frailty and cognitive decline. Transl Res. 2020;221:58–64.
Howrey BT, Al Snih S, Middleton JA, et al. Trajectories of Frailty and Cognitive decline among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2020;75(8):1551–7.
Zhao X, Jin L, Sun SB. The Bidirectional Association between physical and cognitive function among chinese older adults: a mediation analysis. Int J Aging Hum Dev. 2021;92(2):240–63.
Welford P, Osth J, Hoy S, et al. Effects of yoga and Aerobic Exercise on Verbal Fluency in physically inactive older adults: Randomized Controlled Trial (FitForAge). Clin Interv Aging. 2023;18:533–45.
Ng TP, Ling LHA, Feng L, et al. Cognitive Effects of Multi-Domain Interventions among Pre-Frail and Frail Community-Living older persons: Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci. 2018;73(6):806–12.
Ng TP, Feng L, Nyunt MS, et al. Nutritional, Physical, Cognitive, and combination interventions and frailty reversal among older adults: a Randomized Controlled Trial. Am J Med. 2015;128(11):1225–36e1.
Godin J, Armstrong JJ, Rockwood K, et al. Dynamics of Frailty and Cognition after Age 50: why it matters that Cognitive decline is mostly seen in Old Age. J Alzheimers Dis. 2017;58(1):231–42.
Ma L, Chan P. Understanding the physiological links between physical Frailty and Cognitive decline. Aging Dis. 2020;11(2):405–18.
Chen S, Honda T, Narazaki K, et al. Global cognitive performance and frailty in non-demented community-dwelling older adults: findings from the Sasaguri Genkimon Study. Geriatr Gerontol Int. 2016;16(6):729–36.
Chu NM, Xue QL, McAdams-DeMarco MA, et al. Frailty-a risk factor of global and domain-specific cognitive decline among a nationally representative sample of community-dwelling older adult U.S. Medicare beneficiaries. Age Ageing. 2021;50(5):1569–77.
de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry. 2003;18(4):318–24.
Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47(3):193–203.
Acknowledgements
The authors thank the staff and participants of the CHARLS Team for providing the data.
Funding
This work was supported by the Scientific Research Project from Education Department of Liaoning Province (grant numbers LJKMZ20221237).
Author information
Authors and Affiliations
Contributions
K.C. and XY.G. contributed to the conception and design of the study. K.C. and WH.M. collected and analyzed the data. K.C. wrote the manuscript. XN.Z. and ZQ.L. performed the supervision for the work. XN.Z. polished the language. ZQ.L., XN.Z., and XZ.L. contributed to manuscript revision and submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All participants joined CHARLS voluntarily and gave informed written consent before they were interviewed. The CHARLS study data are publicly available and open to researchers worldwide. The studies involving human participants were reviewed and approved by the Institutional Review Board at Peking University (IRB00001052-11015). All methods in this study were performed in accordance with the guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, K., Meng, W., Li, Z. et al. Dynamics, association, and temporal sequence of cognitive function and frailty: a longitudinal study among Chinese community-dwelling older adults. BMC Geriatr 23, 658 (2023). https://doi.org/10.1186/s12877-023-04328-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04328-9