Abstract
Background
The effects of dietary intervention in managing sarcopenic obesity are controversial, and behavior change techniques are lacking in previous studies which are important for the success of dietary intervention. This study aimed to evaluate the feasibility and preliminary effects of a dietary behaviour change (DBC) intervention on managing sarcopenic obesity among community-dwelling older people in the community.
Methods
A two-armed, RCT was conducted. Sixty community-dwelling older adults (≥ 60 years old) with sarcopenic obesity were randomised into either the experimental group (n = 30), receiving a 15-week dietary intervention combined with behaviour change techniques guided by the Health Action Process Approach model, or the control group (n = 30), receiving regular health talks. Individual semi-structured interviews were conducted with 21 experimental group participants to determine the barriers and facilitators of dietary behaviour changes after the intervention.
Results
The feasibility of the DBC intervention was confirmed by an acceptable recruitment rate (57.14%) and a good retention rate (83.33%). Compared with the control group, the experimental group significantly reduced their body weight (p = 0.027, d = 1.22) and improved their dietary quality (p < 0.001, d = 1.31). A positive improvement in handgrip strength (from 15.37 ± 1.08 kg to 18.21 ± 1.68 kg), waist circumference (from 99.28 ± 1.32 cm to 98.42 ± 1.39 cm), and gait speed (from 0.91 ± 0.02 m/s to 0.99 ± 0.03 m/s) was observed only in the experimental group. However, the skeletal muscle mass index in the experimental group decreased. The interview indicated that behaviour change techniques enhanced the partcipants’ compliance with their dietary regimen, while cultural contextual factors (e.g., family dining style) led to some barriers.
Conclusion
The DBC intervention could reduce body weight, and has positive trends in managing handgrip strength, gait speed, and waist circumference. Interestingly, the subtle difference between the two groups in the change of muscle mass index warrants futures investigation. This study demonstrated the potential for employing dietary behaviour change interventions in community healthcare.
Trial registration
Registered retrospectively on ClinicalTrailas.gov (31/12/2020, NCT04690985).
Similar content being viewed by others
Background
Low muscle mass/function and excess adiposity, known as sarcopenic obesity, often coexist in older adults [1,2,3]. Sarcopenic obesity is triggered by a variety of unhealthy lifestyles (e.g., a sedentary life and an unhealthy diet) and physiological factors (e.g., a decline in growth hormones, insulin resistance, an increase in oxidative stress), that occur during the ageing process [4,5,6]. Sarcopenic obesity can significantly increase the risk of developing cardio-metabolic diseases, fatigue, physical disability, institutionalization, and mortality [7,8,9] when compared with either obesity or sarcopenia alone. The prevalence of sarcopenic obesity in China can be up to 20.4% in women and 27.0% in men [10].
A systematic review [11] of 12 randomised controlled studies (n = 863) on people with sarcopenic obesity showed that the diagnostic criteria of sarcopenic obesity in previous studies were various with great heterogeneity, which leads to lacking representativeness of participants and might affect the true effects of the interventions. Additionally, effective intervention forms on managing sarcopenic obesity are still not clear, whereas the role of nutritional interventions cannot be ignored. Adequate protein intake is essential for building muscles [4, 12, 13], and caloric restriction is effective at reducing fat mass [11].
Among the different nutritional interventions, dietary modification is a good way of managing sarcopenic obesity and may produce longer-term benefits than oral supplements alone [5], which is also recommended by the Dietary Guidelines for Chinese [14] and Americans [15]. To date, only two studies have been conducted by applying pure nutritional intervention and produce inconsistent results [16, 17], the effective doses of protein intake and caloric restriction for sarcopenic obese older people are still controversial, we have to synthesise evidence from relevant studies.
Two RCTs showed that a dose of 1.2 g/kg of body weight/day of protein intake could achieve significant increases in muscle mass for sarcopenic older adults [16, 17]. Additional guidelines and recommendations [18,19,20] indicated that 0.8–1.5 g/kg body weight/day of protein is recommended for older people who want to maintain optimal muscle function as they age. Therefore, a dose of 1.2–1.5 g/kg body weight/day of protein intake could be useful for sarcopenic obese older people during caloric restriction. With regard to caloric restrictions for older people, it is noteworthy that caloric restrictions varied significantly between studies. Stringent caloric restrictions are harmful and could exacerbate muscle loss in older people with sarcopenic obesity [5]. A two-year RCT with 218 participants showed that a moderate 11.9% reduction in calorie intake could promote a sustained average weight reduction, simultaneously safeguarding muscle mass [21]. Therefore, a diet of a 12% reduction in calorie intake and protein intake of 1.2–1.5 g/kg body weight/day may lead to fat loss while preserving muscle mass.
In addition, poor adherence and high dropout rates were often reported in previous dietary interventional trials of older adults, leading to inconsistent results. Successful dietary modifications require participants to adhere to a diet regimen [22, 23]. Therefore, behaviour change techniques grounded on a tested effective theoretical model can be incorporated within a diet modification intervention to improve the adherence of participants.
The primary aims of this pilot trial is to evaluate the feasibility and acceptability of the dietary behaviour change intervention (12% caloric reduction/day + 1.2–1.5 g/kg body weight/day of protein intake) for 15 weeks among community-dwelling older adults with sarcopenic obesity. The secondary aim is to evaluate the preliminary effects of the intervention on body composition, muscle strength, and physical performance.
Methods
This study was reported according to the Consolidated Standards of Reporting Trials (CONSORT) for randomised pilot and feasibility trials [24] (CONSORT Checklist please see Supplemental Material 1).
Trial design
This study was conducted as a prospective, two-armed, assessor-blinded, parallel-group, pilot randomised controlled trial (RCT) with an allocation ratio of 1:1. A qualitative interview of the experimental group was conducted after the pilot RCT. This trial was conducted between Jun 2020 and Feb 2021, and it has been retrospectively registered with ClinicalTrial.gov (31/12/2020, NCT04690985).
Participants
Participants were recruited from June 2020 to November 2020 by convenience sampling. The study was promoted by displaying the posters in three largest community health centres which provided a free annual physical health examination to all citizens from the age of 60 in Nanjing, China, and community staffs also helped introduce the research project to older people who came to receive the free physical health check. Initially, participants were identified to be overweight or obese [25] by the community staffs. Then, the screening of sarcopenia was conducted by the research assistant by referring to the consensus of Asian Sarcopenia Working Group (ASWG) [26]. The final enrolment screening was conducted by the first author (YHY) according to the inclusion and exclusion criteria. The community physician ascertained whether the participant was in suitable physical health for the study. Eligible participants were interviewed to obtain their informed consent and socio-demographic data, as well as to establish a baseline assessment prior to randomisation. Participants were screened according to the inclusion and exclusion criteria shown in the Table 1.
Sample size
The primary objective of a pilot study is to explore the feasibility of the study. Therefore, a formal calculation of sample size is not required [27]. Hertzog suggested that a minimum of 30 participants per group would be required for a meaningful pilot study [28]. Therefore, a total of 60 participants were recruited in this pilot study.
Intervention
The experimental group received a 15-week dietary behaviour change (DBC) programme. They were recommended to follow a moderate hypocaloric diet with adequate daily protein intake. A moderate hypocaloric diet (i.e., a 12% reduction in calories from the estimated daily energy expenditure) [21] was suggested to promote a sustained reduction in average weight while simultaneously safeguarding muscle mass. The daily energy expenditure was calculated based on the basic metabolic rate (BMR) and physical activity level. The BMR was assessed via the bioelectrical impedance analysis (BIA), and physical activity level was assessed via the International Physical Activity Questionnaire Short-form (IPAQ-SF). In addition, to compensate for the blunted anabolic responses to muscle protein synthesis, a dose of 1.2–1.5 g/kg body weight/day of protein intake was recommended [18,19,20].
The experimental group were taught behaviour change techniques (BCTs) developed according to the health action process approach (HAPA) model [29]. The HAPA model is a psychological, behavioural change model that is used to describe and predict improvements in health-related behaviours. The model builds a bridge between motivation and action by planning and helps the participants to successfully transform their motivation into action [29]. The model contains two phases: motivation and volition [29]. The motivation phase refers to the goal initiation phase. ‘Self-efficacy’, ‘outcome expectancies’, and ‘increased risk awareness’ are the three attributes that motivate individuals to form an intention/goal to change their unhealthy lifestyle for a healthy lifestyle. The volition phase refers to the process of implementing intentions into actual behaviour through careful planning and action execution. The HAPA model has been found to be effective in previous studies, such as in promoting physical activity [30] or healthy eating habits [31].
To help the participants implement the dietary intervention, each participant was given a guidebook (Supplemental Material 2), which was developed through an evidence-based literature review and expert consultations. The delivery of the intervention contained three phases, with six face-to-face sessions alternating with weekly telephone calls, details of the interventions is shown in the Table 2: Health Action Process Approach-based DBC intervention.
The intervention was delivered in the community healthcare centres by a registered nurse, who is also a qualified weight management coach from the Chinese Nutrition Society. The interventionist used an intervention checklist to ensure the fidelity of the delivery. The interventionist also checked the participants’ compliance with the intake of calories and protein according to their food diary. Each participant was given a food diary notebook, they needed to write down the food type and amount they consumed every day. Training of food recording was provided during the face-to-face meetings, and continuous guidance was provided throughout the intervention. The food diary was checked during each face-to-face meeting, and the interventionist gave the participants further suggestions based on the food diary. If the participant did not keep a food diary, a three-day food recall method, a commonly used method in nutritional studies [32], was used to assess their food intake.
The control group received regular health talks to control for the effects of social interaction The control group were asked to continue with their usual dietary habits. A research assistant (RA), who was not involved in other procedures in this study, contacted the participants to offer health talks according to a standard manual. The content of the health talks was unrelated to sarcopenic obesity or diet. The number and duration of contacts for the control group were similar to those for the experimental group.
Outcomes
Outcome measurements were conducted by a trained research assistant, who was blinded to the group allocations, at baseline (T0) and immediately after the intervention (T1).
Feasibility of the intervention. The feasibility outcomes were measured as rates of: recruitment (i.e., length of recruitment, recruitment rate, to determine ease of recruitment), attendance (attendance in the face-to-face sessions), retention (complete follow-up), and adherence (adherence to keeping a food diary and to dietary instructions). The adherence to keeping a food diary was rated as ‘good’, ‘moderate’, or ‘bad’, according to the following average reports on frequency: ‘6–7 days/week’, ‘3–5 days/week’, and ‘0–2 days/week’, respectively. The adherence to the dietary instructions (protein and calorie intake) was assessed based on the food diary. Compliance with adequate protein intake was measured by the percentage of people whose adequate protein intake score in the Dietary Quality Index-International (DQI-I) was 5. In addition, the average amount of daily protein intake for the participants were calculated based on the food diary. Compliance with calorie control was assessed by calculating the average number of calories consumed monthly based on the food diary and three-day food recall exercise. In addition, adverse events were recorded via the CONSORT Extension for Harms checklist [33].
Acceptability of the intervention. Acceptability of the intervention was assessed via individual semi-structured interviews, which were arranged after the intervention for the participants from the experimental group based on their level of adherence (low, moderate, high), with the aim to better understand the participants’ perceptions about the intervention process and to characterise the facilitators and barriers to changing their dietary behaviour. There was a total of 21 participants that received interview by according to the data saturation principle, as no more new information occurred during the interviews. The development of interview script was based on the MRC framework which contains the key points of developing and implementing an intervention. The interviews were conducted by the interventionist in a private room in the community centre.
Preliminary effects of the intervention. Outcomes included the parameters to reflect the condition of sarcopenic obesity: body composition that measured via bioelectrical impedance analysis (BIA) with multiple frequencies (InBody 270, Korea), which included body mass index, percentage of body fat, body fat mass, skeletal muscle mass index, skeletal muscle mass adjusted by weight, waist circumference, and body weight; handgrip strength that measured via a handheld Jamar Hydraulic Hand Dynamometer; and physical performance measured via the Short Physical Performance Battery (SPPB). Additionally, nutrition self-efficacy, dietary quality, nutritional status, and health status were also measured. The detailed descriptions of outcome measurements are listed in Table 3. Additionally, participants’ physical activity status was measured at T0 and T1, using the IPAQ-SF [34], to take into account the effects of potential confounding factors.
Randomisation and blinding
The block randomisation method (block size = 4) was utilized, to ensure that an equally balanced number of participants were allocated to each study group (i.e., the experimental or control groups). The randomisation table was obtained from the Research Randomiser software (https://www.randomiser.org/). A random sequence code was generated by a research assistant who was not involved in the implementation of the intervention or in assessing the outcome. Allocation concealment was ensured by using sealed envelopes, and upheld until the group assignment was completed. Because of our proposed intervention structure, it was impossible to implement the steps to blind the participants. Therefore, only the outcome assessor was blinded to the group allocation throughout the whole process.
Statistical methods
Descriptive statistics (absolute number and percentage of participants) were used to present the length of recruitment, recruitment rate, retention rate, adherence rate, and completion rate of all the measurements. The recruitment rate of 50% and the adherence rate of 60% indicated an acceptable level, and the proportion of missing data for each variable was suggested to be less than 5% [35]. The SPSS version 26.0 was used to analyse the acquired data.
For the acceptability outcomes, the semi-structured interviews were digitally audio-recorded and transcribed verbatim. NVivo 12 software was used to manage the data and help identify common codes from the transcripts. Content analysis was employed inductively to synthesise the categories and themes. Two researchers (the first author and RA) worked independently on the coding and on identifying codes by following the guideline of content analysis [36]. Both coders had received research training and had experience in coding. The bracketing strategy [37] was followed during the data analysis. The coding scheme and identified themes were discussed among the research team to achieve a consensus on the final themes.
For the analysis of the preliminary effects data, the intention-to-treat (ITT) principle was followed in the data analyses. Descriptive statistics were used to present the demographic data and the feasibility outcomes. Normality assumptions were checked for variables. The homogeneity of the two groups was examined by comparing the demographic and baseline outcomes using an independent t-test or the Mann-Whiteney U test for continuous data at baseline, and the Chi-square test or Fisher’s exact test for dichotomous data. The missing variables were caused by dropouts, which were checked by using missing completely at random (MCAR) method. The generalized estimating equation (GEE) was employed to estimate the time and group effects on the clinical outcomes measured pre- and post-intervention. Two heterogeneous variables, i.e., level of education and body height, were adjusted during the statistical analysis. Considering the covariate effects, all of the GEE analyses were adjusted for three covariates (the variables related to height, level of education, and physical activity level) by considering the significant heterogeneity between the groups. A p-value of < 0.05 was considered statistically significant.
Results
Characteristics of the participants
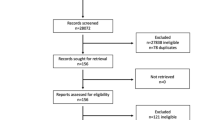
One hundred and five people were found to be eligible after 2,000 people were screened. Sixty of them (mean age = 68.13 ± 6.12 years old) agreed to participate (please see the CONSORT flow chart in Fig. 1). The demographic characteristics of the participants are presented in Table 4. There were no significant differences in demographics among the participants except for level of education (χ2 = 8.20, p = 0.041) and body height (t=-2.10, p = 0.035). These two heterogeneous variables were adjusted during the statistical analysis.
Feasibility of the intervention
The recruitment process lasted for around six months. The recruitment rate was 57.1% (60/105), and the overall retention rate was 83.3% (50/60) (see Fig. 1). The response rate of all the questionnaires among the participants who fully involved the trial was 100%. The percentage of people who attended the face-to-face sessions at least five out of six times was 73.3%, and the percentage of those whose rate of adherence to keeping a food diary was moderate or above was 26.7%. The rate of compliance with adequate protein intake was 66.7%, and the participants had a protein intake of 1.3 ± 0.2 g/kg/d. Calorie intake for male participants decreased from 1715 ± 284 kcal/day to 1571 ± 267 kcal/day, and for female participants from 1696 ± 231 kcal/day to 1451 ± 195 kcal/day. No adverse events were reported by the participants throughout the study.
Acceptability of the intervention
Twenty-one participants (mean age = 68.19 ± 6.30 years old) from the experimental group attended the semi-structured interview. The adherence of the interviewees to keeping a food diary was diverse, from moderate to good (8/21) and bad (13/21). Four themes with corresponding sub-themes were synthesised from the data: (1) overall perceptions of the DBC intervention; (2) barriers to participating in the DBC intervention; (3) facilitators in implementing the DBC intervention; (4) suggestions for a future programme (see Table 5). The participants reported the DBC intervention was helpful for their health and they were motivated to change their dietary behaviour. The facilitators for their dietary behaviour change included the support from their family, the concerns about own health, the concerns about own body image, and the support from the researchers. However, some barriers for dietary behaviour change were also reported which included the barriers to keeping a food diary, the difficulties to estimate the food amount, yield to offspring’s taste, and overeat due to being unwilling to waste leftovers.
Preliminary effects of the intervention
Participants in the experimental group experienced a significant reduction in body weight (Wald χ2 = 4.90, p = 0.027, d = 1.22) and improvement in dietary quality (Wald χ2 = 12.66, p < 0.001, d = 1.31) after the intervention compared with those in the control group. A non-significant decrease in waist circumference (from 99.28 ± 1.32 cm to 98.42 ± 1.39 cm) and increase in handgrip strength (from 15.37 ± 1.08 kg to 18.21 ± 1.68 kg) and gait speed (from 0.91 ± 0.02 m/s to 0.99 ± 0.03 m/s) was observed within the experimental group from baseline to post-intervention. Reduction was observed in skeletal muscle mass index (from 7.31 ± 0.16 kg/m2 to 7.23 ± 0.19 kg/m2) in the DBC group, although this change shows no statistical significance. Table 6 showed the preliminary effects of the DBC intervention on outcomes over 15 weeks from baseline.
Discussion
This study demonstrated that the DBC intervention is feasible and acceptable among the target population, as reflected by the relatively high attendance and retention rates, and by the positive feedback from the interviews. This study showed that the DBC could effectively reduce body weight and improve dietary quality among older adults with sarcopenic obesity. However, the effects on building muscles were nonsignificant.
Feasibility and acceptability of the intervention
We screened around 2,000 people and only 105 people were eligible. The relatively low eligibility rate (5.25%) indicates that extensive screening may be needed in a future study. It is difficult to compare the eligibility rate in this study with those of previous interventional studies because the eligibility rate varied greatly among the different studies. For example, the eligibility rate in some studies could reach 62.36% [38], while in others it was only 3.21% or 7.62% [3, 39]. The variability could be due to a lack of standardisation in the diagnostic criteria for sarcopenic obesity, as various diagnostic criteria for screening the participants were used in previous studies. In addition, one previous investigation [40] showed that the prevalence of sarcopenic obesity among 101 males (aged 80 or above) in Beijing, China was 40.0% when using relative appendicular skeletal muscles for screening, and 95% when using a skeletal muscle mass index. In contrast, Du [41] reported that the prevalence of sarcopenic obesity was 7.0% in males and 2.4% in females after screening 213 males and 418 females in Shanghai, China, figures that were obtained using the old Asian criteria for sarcopenic obesity. A potential reason for the relatively low eligibility rate in this study compared to those of previous prevalence studies was that the older people were reluctant to visit the community health centres due to a fear of being infected by COVID-19. The collecting of data was suspended for six months due to the pandemic. We might not have obtained a comprehensive sample. In addition, the average age of the participants was 68.13 ± 6.12 years old. However, sarcopenia is more prevalent among the older population (i.e., 70 years or above) because muscle loss and fat accumulation are positively related to an increase in age [5]. This may have contributed to the higher prevalence found in previous cross-sectional studies (average age = 88.8 ± 3.7 years old) [40].
The findings of the semi-structured interview revealed both positive and negative aspects of the intervention process. On the one hand, the participants recognised the positive role of the BCTs in encouraging them to modify their dietary habits from unhealthy to healthy. The continuous support from the interventionist during the intervention process was reported as being a crucial factor in bringing about changes in behaviour. Previous studies on lifestyle modifications tended to focus on the provision of knowledge, materials, and professional education, which may be insufficient for making behavioural changes [42]. Instead, providing alternative strategies to deal with obstacles in actual practice, such as making the participants more aware of risky behaviour or facilitating their ability to self-monitor, might be more effective at changing behaviour [29].
Contextual cultural factors may pose some barriers to changing dietary behaviour, as reflected by the relatively low rate of adherence to keeping a food diary. According to our qualitative interviews, the barriers included internal factors (e.g., previous eating habits) and external factors (e.g., specific aspects of Chinese dining culture). For example, Chinese families are used to eating together and sharing dishes, which may cause difficulties for participants in controlling the amount of food that they eat compared to individual servings. In addition, some participants were busy taking care of their grandchildren, so they hardly had adequate time to keep a detailed food diary. These external barriers need to be addressed in future research by including family members in the study, to increase the awareness of family members and relieve some of the burden on the participants.
Preliminary effects of the intervention
The findings on preliminary effects demonstrated that body weight could decreased significantly, with a simultaneous decrease in skeletal muscle. This preliminary finding is similar to that in previous interventional studies [16, 17], which showed a decrease in the lean body mass of older people associated with weight reduction after they had followed a hypocaloric diet for 3 or 4 months. Many studies have also reported the phenomenon of muscle loss along with weight reduction [5, 43, 44]. Obese adults could lose 2–10% of their muscle mass in a 8–10% diet-induced weight reduction [45,46,47,48]. There could be several reasons for this result. First, given the relatively small sample size in this pilot study, its statistical power might not have been sufficient to detect differences between the experimental and control groups [49]. Second, the duration of the intervention might have been insufficient to estimate the effects of dietary interventions on body composition. The preliminary results of this pilot study may indicate that a longer time is needed to see the effects in terms of muscle building.
On the other hand, we could observe a non-significant increase in handgrip strength and gait speed and the decrease in waist circumference in the intervention group from baseline to post-intervention. These findings are similar to those of previous nutritional studies [16, 17] in that a high-protein low-caloric diet led to a significant improvement in handgrip strength and decrease in waist circumference within the group after the intervention, even though the between-group effects when compared to those on a normal-protein low-caloric diet were not significant. The role of energy restriction and high protein intake have been proven to be extremely important for functional capacity improvement for healthy ageing [11]. Obesity and lack of activity in older people are strongly related to the physical dysfunction while the adjustment of energy and protein intake can help prevent the process [50].
Limitations and strengths
There were some limitations to this study. First, it was challenging to perform double-blinding (blinding of the interventionist and participants) due to the nature of the study. Although we maintained continuous social contact with the control group to avoid the confounding effects of psychosocial contact, it was not possible to compensate entirely for the Hawthorne effect. Second, the method of assessing food intake in this study might have led to bias in estimating the amount of food that was consumed. Because the participants self-reported the amount of their food intake, there might have been variations between the actual amount and the estimated amount, even though the participants had been trained in measurement methods. However, the food diary is the most widely used method in current dietary intervention studies especially in a community-dwelling setting. We also considered using digital methods (e.g., technological equipment or application programs) to help in recording food intake. However, the accuracy of digital methods is yet to be established, and the problems of self-reporting remain unsolved [51]. Third, we did not conduct the process evaluation and explore the therapeutic mechanism, which could be addressed in a future full trial. Forth, the estimation of the sample size was referred to the rule of thumb without considering the attrition rate which did occur during the data collection. Finally, the interpretation of findings should be treated with caution because this is a pilot trial, but these pilot data can be used to power a future intervention.
This study has implications for both clinical practice and research. First, this study provides a good reference for community health providers to use to play a supervisory role in implementing dietary interventions using behaviour change techniques (e.g., workshops or telephone follow-ups), and then to improve the quality of the diets of older adults. Notably, the intervention in this study used an individualised rather than a uniform approach, which is crucial for dietary interventions considering the heterogeneity among participants in terms of lifestyle, mealtimes, confidence, and family context. Second, this study inspired the design for future research, i.e., a longer intervention duration and better-tailored methods for promoting compliance in the keeping of a food diary. In this pilot study, handgrip strength, waist-hip ratio, and gait speed all showed a non-significant positive change. Supposing these parameters could be significantly changed in a longer intervention duration and a bigger sample size.
Conclusion
This pilot study supports the view that a dietary intervention combined with behaviour change techniques is a feasible and acceptable programme for older adults with sarcopenic obesity. The DBC intervention could reduce body weight, and has positive trends in managing handgrip strength, gait speed, and waist circumference. Interestingly, the subtle difference between the two groups in the change of muscle mass index warrants futures investigation. The effects of the DBC intervention on managing sarcopenic obesity could be further explored in a future study with a bigger sample size and longer intervention duration.
Data Availability
The data and materials are not publicly availabe as the participants did not consenting to share their data. Further detials about the data and ethical conditions are available from the corresponding author on reasonable request.
Abbreviations
- DBC:
-
Dietary behavioral change
- HAPA:
-
Health action process approach
- RCT:
-
Randomised controlled trial
- RA:
-
Research assistant
- GEE:
-
Generalized estimating equation
- ITT:
-
Intention-to-treat
- BIA:
-
Bioelectric impedance analysis
- SD:
-
Standard deviation
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- SPPB:
-
Short physical performance battery
- MNA:
-
Mini-Nutritional Analysis
- IPAQ-SF:
-
International Physical Activity Questionnaire Short-form
- DQI-I:
-
Dietary quality index-international
References
Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–8.
Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obesity. 2004;12(6):887.
Kemmler W, Teschler M, Weissenfels A, Bebenek M, Von Stengel S, Kohl M, et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Resultsof the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos Int. 2016;27(11):3261–70.
Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones. 2018;17(3):321–31.
Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons–a narrative review. Clin Interv Aging. 2015;10:1267.
Kob R, Bollheimer LC, Bertsch T, Fellner C, Djukic M, Sieber CC, et al. Sarcopenic obesity: molecular clues to a better understanding of its pathogenesis? Biogerontology. 2015;16(1):15–29.
Godziuk K, Prado CM, Woodhouse LJ, Forhan M. The impact of sarcopenic obesity on knee and hip osteoarthritis: a scoping review. BMC Musculoskelet Disord. 2018;19(1):1–10.
Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116(7):1171–8.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity-definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693.
Chen MBH, Wang S, Sun W, Li R, Gao ZH, Sun JQ. Prevalence of sarcopenic obesity among the older people in Shanghai. The 17th Symposium of Danone Institute China; Shanghai2014.
Yin Y-H, Liu JYW, Välimäki M. Effectiveness of non-pharmacological interventions on the management of sarcopenic obesity: a systematic review and meta-analysis. Exp Gerontol. 2020;135:110937.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73.
Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang X-J, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41(2):215–9.
Chinese Society of Nutrition. (2016) The Dietary Guidelines for Chinese Residents.
U.S. Department of Agriculture & U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025 2020 [updated December. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf.
Muscariello E, Nasti G, Siervo M, Di Maro M, Lapi D, D’Addio G, et al. Dietary protein intake in sarcopenic obese older women. Clin Interv Aging. 2016;11:133.
Sammarco R, Marra M, Di Guglielmo ML, Naccarato M, Contaldo F, Poggiogalle E, et al. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: a pilot study. Obes Facts. 2017;10(3):160–7.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36.
Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, et al. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391–6.
Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care. 2014;17(1):5.
Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Das SK, Redman LM, et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. The Lancet Diabetes & Endocrinology. 2019;7(9):673–83.
Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. (2013) Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev(2).
Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes. 2017;7(3):123–35.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8.
Chinese Centre for Disease Control. (2006) Guideline for the prevention and control of obesity in Chinese adults. Beijing. p. 44 – 5.
Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–7. e2.
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1–10.
Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–91.
Schwarzer R, Luszczynska A. How to overcome health-compromising behaviors: the health action process approach. Eur Psychol. 2008;13(2):141–51.
Chiu CY, Lynch RT, Chan F, Berven NL. The Health action process Approach as a motivational model for physical activity self-management for people with multiple sclerosis: a path analysis. Rehabil Psychol. 2011;56(3):171.
Schwarzer R, Renner B. Social-cognitive predictors of health behavior: action self-efficacy and coping self-efficacy. Health Psychol. 2000;19(5):487.
Yang YJ, Kim MK, Hwang SH, Ahn Y, Shim JE, Kim DH. Relative validities of 3-day food records and the food frequency questionnaire. Nutr Res Pract. 2010;4(2):142–8. https://doi.org/10.4162/nrp.2010.4.2.142.
Ioannidis JP, Evans SJ, Gøtzsche PC, O’neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8.
Lee PH, Yu Y, McDowell I, Leung GM, Lam T, Stewart SM. Performance of the international physical activity questionnaire (short form) in subgroups of the Hong Kong chinese population. Int J Behav Nutr Phys Activity. 2011;8(1):1–10.
Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):1–6.
Neuendorf KA. The content analysis guidebook. Sage; 2017.
Chan ZC, Fung Y-l, Chien W-t. Bracketing in phenomenology: only undertaken in the data collection and analysis process. Qualitative Rep. 2013;18(30):1–9.
Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. 2017;65(4):827–32.
Kemmler W, Weissenfels A, Teschler M, Willert S, Bebenek M, Shojaa M et al. (2017) Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: the randomized controlled FranSO study. Clinical interventions in aging.12:1503.
Meng P, Hu YX, Fan L, Zhang Y, Zhang MX, Sun J, et al. Sarcopenia and sarcopenic obesity among men aged 80 years and older in B eijing: prevalence and its association with functional performance. Geriatr Gerontol Int. 2014;14:29–35.
Du Y, Wang X, Xie H, Zheng S, Wu X, Zhu X, et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr Disord. 2019;19(1):1–11.
Van Achterberg T, Huisman-de Waal GG, Ketelaar NA, Oostendorp RA, Jacobs JE, Wollersheim HC. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int. 2011;26(2):148–62.
Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511–9.
Fukushima Y, Kurose S, Shinno H, Thu HC, Takao N, Tsutsumi H, et al. Importance of lean muscle maintenance to improve insulin resistance by body weight reduction in female patients with obesity. Diabetes Metab J. 2016;40(2):147.
Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, et al. Contribution of individual organ mass loss to weight loss–associated decline in resting energy expenditure. Am J Clin Nutr. 2009;90(4):993–1001.
Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133(2):92–103.
Santanasto AJ, Glynn NW, Newman MA, Taylor CA, Brooks MM, Goodpaster BH et al. (2011) Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes.2011.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29.
Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013.
Beavers KM, Nesbit BA, Kiel JR, Sheedy JL, Arterburn LM, Collins AE, et al. Effect of an Energy-Restricted, nutritionally complete, higher protein meal plan on body composition and mobility in older adults with obesity: a Randomized Controlled Trial. The Journals of Gerontology: Series A. 2018;74(6):929–35. https://doi.org/10.1093/gerona/gly146.
Subar AF, Crafts J, Zimmerman TP, Wilson M, Mittl B, Islam NG, et al. Assessment of the accuracy of portion size reports using computer-based food photographs aids in the development of an automated self-administered 24-hour recall. J Am Diet Assoc. 2010;110(1):55–64.
Pavasini R, Guralnik J, Brown JC, Di Bari M, Cesari M, Landi F, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):1–9.
Kim S, Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index-International (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133(11):3476–84.
Research E. (1984) Food Processor® software.
Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002;18(4):737–57.
He X-y, Liu X-q. Evaluation of reliability and validity of mini-nutritional assessment and chinese nutrition screen. Nurs J Chin People’s Liberation Army. 2010;27:894–6.
Li L, Wang H, Shen Y. Chinese SF-36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57(4):259–63.
Acknowledgements
We sincerely thank the community health centres in Nanjing, China for the support in providing data collection settings. We thank faculties of Nanjing Medical University for helping connect the research settings. We also thank the experts involved in Delphi methods for their professional comments, research assistants for their wonderful work, and all the participants for participating in this study.
Funding
This study is funded by The Hong Kong Polytechnic University for supporting postgraduate students, the grant number is not available.
Author information
Authors and Affiliations
Contributions
YHY, JYWL and MV were all involved in the design of the study and initiated the study. YHY was accountable throughout for the data collection and data analysis, and drafted the manuscript. JYWL was accountable for the quality control of the study. JYWL and MV both commented and rewrote the manuscript. All authors read and approve the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the Declaration of Helsinki. This study obtained ethical approval from the research committee of The Hong Kong Polytechnic University (HSEARS20191007001) and the community centres. The written informed consent of the participants was obtained prior to the collecting of data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yin, YH., Liu, J.Y.W. & Välimäki, M. Dietary behaviour change intervention for managing sarcopenic obesity among community-dwelling older people: a pilot randomised controlled trial. BMC Geriatr 23, 597 (2023). https://doi.org/10.1186/s12877-023-04327-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04327-w