Abstract
Background
The number of elderly patients diagnosed with breast cancer is increasing worldwide. However, treatment decisions for these patients are highly variable. Although researchers have identified the effects of surgery, radiotherapy, endocrine therapy, and chemotherapy in elderly patients with breast cancer, clinicians still struggle to make appropriate decisions for these patients.
Methods
We identified 75,525 female breast cancer patients aged ≥ 70 years in the Surveillance, Epidemiology, and End Results (SEER) database treated between January 1, 2010, and December 31, 2016. The patients were further divided into training and testing cohorts. The cumulative occurrence of breast cancer-specific deaths (BCSDs) and other cause-specific deaths (OCSD) was calculated using the cumulative incidence function. In the univariate analysis, risk factors were screened using the Fine-Gray model. In the multivariate analysis for competing risks, the sub-distribution hazard ratio with a 95% confidence interval for each independent predictor associated with BCSD was calculated for the construction of nomograms. Based on the above analyses, a competing risk nomogram was constructed to predict the probability of BCSD in the 1st, 3rd, and 5th years after treatment. During validation, the concordance index (C-index) was selected to quantify the predictive ability of the competing risk model.
Results
A total of 33,118 patients were included in this study, with 24,838 in the training group and 8,280 in the testing group. Age, race, marital status, cancer grade, tumor stage, node stage, estrogen receptor status, progesterone receptor status, human epidermal growth factor receptor--2 status, and treatment including surgery, radiation, and chemotherapy were used to establish a nomogram. The C-index of 0.852 (0.842-0.862) in the training cohort and 0.876 (0.868-0.892) in the testing cohort indicated satisfactory discriminative ability of the nomogram. Calibration plots showed favorable consistency between the nomogram predictions and actual observations in both the training and validation cohorts.
Conclusions
Our study identified independent predictors of BCSD in elderly patients with breast cancer. A prognostic nomogram was developed and validated to aid clinical decision-making.
Similar content being viewed by others
Background
Breast cancer is the most common cancer and cause of cancer-related death in women, and its incidence is positively correlated with age [1, 2]. Approximately 50% of new breast cancer cases are recorded in women ≥ 60 years (https://gco.iarc.fr/). However, treatment decisions for elderly patients with breast cancer are highly variable [3, 4]. On the one hand, aging is accompanied by fragility and comorbidities [5, 6]. On the other hand, prospective studies supporting specific treatments for elderly patients with breast cancer are lacking owing to ethical requirements [7]. Therefore, no uniform treatment guidelines have been established for the elderly [8, 9].
Some studies have been conducted in elderly patients with breast cancer whose primary option is surgery [10]. The choice of surgical method is affected by age; that is, the acceptance rate of breast-conserving surgery decreases with age, and some studies have focused on the unwillingness of patients to receive the necessary radiotherapy after breast-conserving surgery [11, 12]. Elderly patients exhibit a negative attitude toward their choice of treatment strategy, with a low reception of radiotherapy and chemotherapy. Among patients with indications for radiotherapy, only two thirds patients aged 71–80 years received this treatment [13]. Many older patients receive inadequate chemotherapy treatment [14]. A study showed that the proportion of patients aged ≥ 65 years with breast cancer who received a sufficient number of chemotherapy courses was significantly lower than that in younger patients (P < 0.001 [15]. Despite an understanding of breast cancer treatment in elderly individuals, clinicians still struggle to make appropriate treatment decisions for these individuals. To solve this problem, we analyzed the competing risks of breast cancer patients over 70 years of age using the Surveillance, Epidemiology, and End Results (SEER) database, identified independent predictors of breast cancer-specific death (BCSD), and constructed a nomogram of a predictive risk model to aid in clinical decision-making.
Methods
Data sources and patient selection
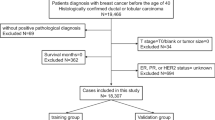
This study was based on SEER database data released in November 2020. Our target patients were extracted from SEER*Stat Version 8.3.9.2 (SEER ID: 26588-Nov2019), which included population-based data from 18 cancer registries covering approximately 28% of the United States (U.S.) cancer population between 1975 and 2018 and provided complete data regarding patient demographics, tumor characteristics, diagnosis, first course of treatment, and follow-up of vital status. Given that the data released by the SEER database are publicly available, this study did not require informed patient consent or ethical approval. We extracted data on patients with breast cancer, including chemotherapy records, who were treated between January 1, 2010, and December 31, 2016. A total of 252,472 breast cancer cases were identified in the database during this period (Supplementary 1). Among them, patients who met any of the following criteria were excluded:1) male sex; 2) aged <70 years at diagnosis; 3) breast cancer was not the first primary cancer diagnosed; 4) paired site; 5) without histologic confirmation; 6) missing stage or stage 0; 7) missing molecular type; 8) missing grade; 9) distant metastasis; or 10) death or loss to follow-up within six months of diagnosis. Ultimately, 33,118 eligible patients were included in the analysis. These patients were randomized at a 2:1 ratio into the training and testing groups.
Data acquisition
We collected patient data, including age at diagnosis, race (white, Black, other, or unknown), marital status (married, divorced, separated, single, widowed, unmarried, domestic partner, or unknown), insurance status (insured, insured/no specifics, any medical, uninsured, or insurance unknown), grade (G1, G2, or G3), stage (I, II, III, or IV), tumor/node/metastasis (TNM) stage (T0–T4, N0–N3, or M0–M1), estrogen receptor (ER) status (negative, positive, or borderline), progesterone receptor (PR) status (negative, positive, or borderline), human epidermal growth factor receptor02 (HER2) status (negative, positive, or borderline), breast surgery procedure (partial mastectomy with or without axillary dissection, simple and subcutaneous mastectomy, modified radical mastectomy, radical and extended radical mastectomy with or without breast reconstruction, and other mastectomy or unknown), and chemotherapy and radiotherapy records. We defined the TNM stage according to the 7th edition guidelines of the American Joint Committee on Cancer (2010–2015). Detailed information about the variables can be found on the official SEER website (https://seer.cancer.gov/data-software/documentation/seerstat/nov2020/), and we strictly followed these definitions while conducting the analysis.
Outcomes
We defined BCSD as the time from diagnosis to death due to breast cancer. Death from other causes was defined as other cause-specific death (OCSD). We used the description from “SEER cause-specific death classification” to define the patient's cause of death.
Statistical analysis
We used a chi-square test to compare categorical variables. The cumulative occurrence of BCSD and OCSD was calculated using the cumulative incidence function (CIF). The difference between BCSD and OCSD CIFs in different subgroups, including those defined by age, race, insurance, marital status, grade, T stage, N stage, ER status, PR status, HER2 status, surgery method, and treatment with radiation or chemotherapy, were first compared using Gary’s test. Subsequently, the 1-, 3-, and 5-year CIFs for BCSD and OCSD in patients with breast cancer in the training cohort were calculated. In univariate analyses, risk factors were screened using the Fine-Gray model, and values with P < 0.05 were included in the subsequent multifactor analysis [16]. In the multivariate analysis for competing risks, the sub-distribution hazard ratio (sdHR) with a 95% confidence interval (CI) of each independent predictor associated with BCSD was calculated for the construction of nomograms. Based on the above analyses, a competing risk nomogram was constructed to predict the probability of BCSD in the 1st, 3rd, and 5th years after treatment [17]. The nomograph was verified using the 1000 resampling guidance method to assess its performance internally and externally. The concordance index (C-index) was chosen to quantify the predictive ability of the competing risk model [18]. The C-index ranges from 0.5 to 1, with values greater than 0.7 indicating better discrimination performance. And calibration curves were used to compare the predicted probability and observed frequencies, and the location of curve is closer to a 45° diagonal line, meaning a better-calibration. All analyses were performed using R software (version 4.1.3), and all tests were two-sided, and statistical significance was set at P < 0.05.
Results
Clinicopathological and baseline characteristics of patients
A total of 33,118 patients were included in this study, with 24,838 in the training group and 8,280 in the testing group. Clinicopathological and baseline characteristics are presented in Table 1.
Among all patients, 21,954 (66.3%) were aged between 70 and 80 years, 9,710 (29.3%) were aged between 80 and 90 years, and 1,454 (4.4%) were older than 90 years. Among all patients, 12.5% were ER-negative, 23.9% were PR-negative, and 90% were HER2-negative. In the entire population, a vast majority of patients underwent surgery, 82.8% of whom underwent partial mastectomy and 12.3% underwent mastectomy. Approximately half of the patients were treated with radiation; however, only a small proportion (15.9%) received chemotherapy.
Univariate and multivariate analyses
In the training cohort, the number of patients (12.4%; 3,083/24,838) who died from non-breast cancer-related causes was higher than that of patients (5.7%; 1,412/24,838) who died from breast cancer. The 1-, 3-, and 5-year CIFs of BCSD and OCSD in patients with breast cancer in the training cohort are shown in Table 2 and Supplementary Table 2, respectively. The 1-, 3-, and 5-year CIFs of BCSD among the patients were 0.94%, 4.51%, and 7.23%, respectively, and those of OCSD were 1.46%, 8.11%, and 16.06%, respectively, which were almost twice the CIFs of BCSD. In the univariate analyses, most variables (P < 0.05) strongly correlated with the CIF of the BCSD, except for insurance (P = 0.110). Differences in the CIF of the BCSD are shown in Fig. 1. There was no relationship between CIF and ER status (P = 0.680), PR status (P = 0.069), HER2 status (P = 0.771), or N stage (P = 0.919). Multivariate analysis revealed the independent risk factors (age, race, marital status, grade, T stage, N stage, ER status, PR status, HER2 status, and treatment with surgery, radiation, or chemotherapy) associated with BCSD for the subsequent construction of a nomogram. The results of the multivariate analysis of competing risks in the training group are presented in Table 3. Among the identified factors, age, grade, T stage, and N stage positively correlated with the CIF of BCSD. Black and unmarried women have a higher risk of developing BCSD than those of other races and martial statuses. According to the sdHRs with 95% CI, the possibility of BCSD increased with grade, as observed in the T and N stages. Compared to patients who did not receive radiation or chemotherapy, those who underwent radiation (sdHR 0.760 [0.661–0.873]) or chemotherapy (sdHR 0.580 [0.514–0.653]) had a reduced probability of BCSD.
Construction and validation of the competing risk nomogram
After model validation, all independent risk factors, including age, race, marital status, grade, T stage, N stage, ER status, PR status, HER2 status, and treatment with surgery, radiation, or chemotherapy were incorporated to construct a nomogram to predict the 1-, 3-, and 5-year CIFs of BCSD, as shown in Fig. 2. The probabilities of BCSD at 1-, 3-, and 5-years were predicted using the total score in the nomogram. As shown in Fig. 2, the N stage had the strongest effect on BCSD, followed by the T stage, age, and breast surgery method.
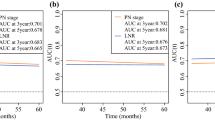
In the internal and external validation, the nomogram showed great predictive ability, with a C-index of 0.852 (0.842-0.862) in the training cohort and 0.876 (0.868-0.892) in the testing cohort, which indicated satisfactory discriminative ability of the nomogram. Calibration plots showed favorable consistency between the nomogram predictions and actual observations in both the training and validation cohorts. The calibration results are shown in Fig. 3.
Discussion
Breast cancer is the most common malignant tumor and the main cause of death in elderly women [19]. Despite comprising a large proportion of breast cancer cases, the elderly are underrepresented in clinical trials [20], which is related to frailty in the elderly. Understanding the risk factors for BCSD in elderly patients with breast cancer could help comprehensively evaluate the status of patients and is of great importance for treatment decision-making.
The choice of treatment for elderly cancer patients is often complicated by the presence of multiple chronic comorbidities. When discussing the impact of breast cancer on the survival of older patients, deaths from other causes may occur before the event of interest, leading to the exclusion of relevant events. Considering this, a competing risk model was selected to address competing risk events.
In this study, we extracted information on 33,118 elderly patients with breast cancer from the SEER database and constructed a competitive risk model to screen 12 independent risk factors related to BCSD, making the results highly reliable. The probability of BCSD is correlated with age and tumor characteristics, such as molecular classification, tumor grade, and tumor stage. Compared to previous articles that included ten risk factors [21], more risk factors were included in study that impact treatment choices, including radiation and chemotherapy, and BCSD in elderly patients. Elderly patients are likely to choose to forego chemotherapy and radiation because of the higher likelihood of adverse effects. We considered these two treatment approaches; thus, this nomogram can be an effective tool for predicting the CIF of patients with BCSD and appropriate treatment strategies.
In our study, we found that older age was an independent risk factor for higher BCSD probabilities. The inclusion of age as an independent predictive factor for the prognosis of patients with breast cancer has been a subject of ongoing controversy [22]. For patients with breast cancer, especially those younger than 35 years old, younger age is associated with poor prognosis [23, 24]. For elderly breast cancer patients, it is generally observed that the prognosis tends to worsen with increasing age, which is consistent with our results [21, 25]. Regarding tumor factors, tumor stage and grade were important predictive risk factors, having a positive correlation with the CIF of BCSD, consistent with previously reported results [26, 27].
Among these results, the effect of treatment on BCSD in elderly patients was our main focus. Among all treatment methods, surgery showed the greatest impact on BCSD in elderly patients, and this finding is similar to previous studies [28,29,30]. One study showed that in early stage breast cancer, surgical treatment led to similar 5-year survival rates in both elderly and young patients [28]. Some reports have also shown that age, comorbidities, cognition, functional status, and tumor size are correlated with the preference for operative treatment [31]. With increasing age, few patients are recommended breast-conserving surgery, possibly because of clinicians’ concern that elderly patients cannot tolerate radiotherapy [11, 32, 33]. However, our results suggest that patients who underwent mastectomy had a higher incidence of BCSD than those who underwent breast-conserving surgery.
In our analysis, chemotherapy significantly reduced the incidence of BCSD in elderly patients with breast cancer. Previous studies have reported that the toxicity and side effects of chemotherapy are severe, and the life expectancy of the elderly is short; therefore, the elderly are considered to benefit minimally from chemotherapy [34]. In our study, only 15.4% of patients received chemotherapy, which is an extremely small proportion. Chemotherapy significantly reduces disease-free survival and prolongs overall survival in patients aged < 70 years old [35]. In recent studies, chemotherapy was found to prolong disease-free survival and reduce the relative risk of recurrence among patients with breast cancer aged ≥ 65 years [36]. In addition, chemotherapy has no significant effect on the cognitive function or quality of life in elderly patients receiving this treatment [37, 38]. Therefore, chemotherapy is safe and suitable for elderly patients with breast cancer and has a negligible effect on their quality of life.
Our results show that radiotherapy is more effective than chemotherapy [39, 40]. In early-stage, ER-positive patients aged > 70 years, adjuvant radiotherapy combined with endocrine therapy after breast-conserving surgery or mastectomy can significantly reduce the incidence of local recurrence but has no effect on overall survival [41, 42]. In contrast, ER-negative patients with early-stage breast cancer have better overall survival when treated with radiotherapy [43]. However, due to limited information in the database, regional radiotherapy and postoperative whole-breast radiotherapy cannot be distinguished; therefore, the impact of different radiotherapy modalities on outcomes could not be further analyzed when analyzing the effect of radiotherapy on BCSD. In general, radiotherapy may be recommended for disease control in elderly patients with a life expectancy of 5–10 years, radiotherapy might be recommended to control the disease [44].
Using the SEER database, we constructed a nomogram to predict the CIF of BCSD in elderly patients in the 1st, 3rd, and 5th years after diagnosis. Compared with previous nomograms, our nomogram only focused on elderly patients and included additional clinical risk factors, particularly treatment modalities. Data on clinical factors can be collected from the medical histories at any time. The prediction accuracy of our nomogram was confirmed using the C-index and calibration curves, and the results proved that our nomogram is convenient and reliable. The use of a high-quality and large-sample database to conduct competitive risk analysis makes our study highly reliable.
In the future, clinicians may use this tool to accurately assess the prognosis of elderly patients with breast cancer and provide them with targeted and individualized treatments. Through this nomogram, patients can intuitively understand the benefits of different treatment methods and their prognoses. For example, based on our nomogram, the 1-, 3-, and 5-year BCSD of a 87-year-old patient, who is unmarried, white and with grade III triple-negative breast cancer staged T2 and N0, with partial mastectomy, was 3.84%, 20.5% and 34.7%, respectively.
However, this study has some limitations. Although an extremely small fraction, some cases with missing information were excluded from our analysis, possibly causing selection bias. In addition, our analysis was based on reported data, which may contain information bias. Finally, systemic treatments are being developed, and an increasing number of targeted drugs are being administered in clinics, both of which have a great impact on patient recovery. Although studies have shown that the use of endocrine therapy in elderly patients with breast cancer has become common practice [45], our present work lacks data on endocrine therapy in these patients. Regardless, the lack of data on endocrine therapy did not affect the judgment of the overall results. Finally, the effect of comorbidities on prognosis was not considered in this study. To externally validate our nomogram, a large amount of data from prospective cohort studies is needed.
Conclusions
Our study identified independent predictors of BCSD in elderly patients with breast cancer and developed and validated a prognostic nomogram to aid clinical decision-making.
Availability of data and materials
Since data released by the SEER database (https://gco.iarc.fr/) was publicly available, ethics approval and informed patient consent was not required for this study.
Abbreviations
- BCSD:
-
Breast cancer-specific death
- C-index:
-
Concordance index
- CI:
-
Confidence interval
- CIF:
-
Cumulative incidence function
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor-2
- HR:
-
Hazard ratio
- PR:
-
Progesterone receptor
- SEER:
-
Surveillance, Epidemiology, and End Results
References
Nasrazadani A, Marti JLG, Kip KE, Marroquin OC, Lemon L, Shapiro SD, Brufsky AM. Breast cancer mortality as a function of age. Aging (Albany NY). 2022;14(3):1186–99.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Jenkins EO, Deal AM, Anders CK, Prat A, Perou CM, Carey LA, Muss HB. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist. 2014;19(10):1076–83.
Petkov VI, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer. 2016;2:16017.
Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–11.
Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92.
Parks RM, Holmes HM, Cheung KL. Current challenges faced by cancer clinical trials in addressing the problem of under-representation of older adults: a narrative review. Oncol Ther. 2021;9(1):55–67.
Varghese F, Wong J. Breast cancer in the elderly. Surg Clin North Am. 2018;98(4):819–33.
Baban CK, Devane L, Geraghty J. Change of paradigm in treating elderly with breast cancer: are we undertreating elderly patients? Ir J Med Sci. 2019;188(2):379–88.
Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkler IH, Cardoso MJ, Cheung KL, de Glas NA, Trimboli RM, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327–40.
Peters E, Anzeneder T, Jackisch C, Dimpfl T, Kunz G, Katalinic A, Waldmann A. The treatment of primary breast cancer in older women with adjuvant therapy: a retrospective analysis of data from over 3000 patients from the PATH Biobank, with two-year follow-up. Dtsch Arztebl Int. 2015;112(35–36):577–84.
LeMasters TJ, Madhavan SS, Sambamoorthi U, Vyas AM. Disparities in the initial local treatment of older women with early-stage breast cancer: a population-based study. J Womens Health (Larchmt). 2017;26(7):735–44.
Algan O, Zhao YD, Herman T. Radiotherapy in patients 70 years and older with triple-negative breast cancer. Clin Breast Cancer. 2016;16(4):e99–106.
Schwentner L, Van Ewijk R, Kühn T, Flock F, Felberbaum R, Blettner M, Kreienberg R, Janni W, Wöckel A, Singer S. Exploring patient- and physician-related factors preventing breast cancer patients from guideline-adherent adjuvant chemotherapy-results from the prospective multi-center study BRENDA II. Support Care Cancer. 2016;24(6):2759–66.
Wallwiener CW, Hartkopf AD, Grabe E, Wallwiener M, Taran FA, Fehm T, Brucker SY, Krämer B. Adjuvant chemotherapy in elderly patients with primary breast cancer: are women ≥65 undertreated? J Cancer Res Clin Oncol. 2016;142(8):1847–53.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–61.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Marinopoulos S, Dimitrakakis C, Kalampalikis A, Zagouri F, Andrikopoulou A, Rodolakis A. Adjuvant treatment of elderly breast cancer patients: offer the best chances of cure. Breast Care (Basel). 2022;17(1):71–80.
Lu X, Li X, Ling H, Gong Y, Guo L, He M, Sun H, Hu X. Nomogram for predicting breast cancer-specific mortality of elderly women with breast cancer. Med Sci Monit. 2020;26:e925210.
Anders CK, Fan C, Parker JS, Carey LA, Blackwell KL, Klauber-DeMore N, Perou CM. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29(1):e18-20.
Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–46.
Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ. 2000;320(7233):474–8.
Chen HL, Zhou MQ, Tian W, Meng KX, He HF. Effect of age on breast cancer patient prognoses: a population-based study using the SEER 18 database. PLoS One. 2016;11(10):e0165409.
Wang Z, Cheng Y, Chen S, Shao H, Chen X, Wang Z, Wang Y, Zhou H, Chen T, Lin N, et al. Novel prognostic nomograms for female patients with breast cancer and bone metastasis at presentation. Ann Transl Med. 2020;8(5):197.
Chu J, Yang D, Wang L, Xia J. Nomograms predicting survival for all four subtypes of breast cancer: a SEER-based population study. Ann Transl Med. 2020;8(8):544.
Lee CM, Zheng H, Tan VK, Tan TJ, Kanesvaran R, Wong FY, Sim YR, Yong WS, Madhukumar P, Ong KW, et al. Surgery for early breast cancer in the extremely elderly leads to improved outcomes - an Asian population study. Breast. 2017;36:44–8.
Cortadellas T, Córdoba O, Gascón A, Haladjian C, Bernabeu A, Alcalde A, Esgueva A, Rodriguez-Revuelto R, Espinosa-Bravo M, Díaz-Botero S, et al. Surgery improves survival in elderly with breast cancer. A study of 465 patients in a single institution. Eur J Surg Oncol. 2015;41(5):635–40.
Ward SE, Richards PD, Morgan JL, Holmes GR, Broggio JW, Collins K, Reed MWR, Wyld L. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer-specific survival. Br J Surg. 2018;105(11):1454–63.
Morgan JL, Walters SJ, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW, Wyld L. What influences healthcare professionals’ treatment preferences for older women with operable breast cancer? An application of the discrete choice experiment. Eur J Surg Oncol. 2017;43(7):1282–7.
Hamelinck VC, Stiggelbout AM, van de Velde CJH, Liefers GJ, Bastiaannet E. Treatment recommendations for older women with breast cancer: A survey among surgical, radiation and medical oncologists. Eur J Surg Oncol. 2017;43(7):1288–96.
Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, Schäfer P, Kurtz J, Sappino AP, Vlastos G. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21(19):3580–7.
Hamelinck VC, Bastiaannet E, Pieterse AH, de Glas NA, Portielje JE, Merkus JW, den Hoed ID, van de Velde CJ, Liefers GJ, Stiggelbout AM. A prospective comparison of younger and older patients’ preferences for adjuvant chemotherapy and hormonal therapy in early breast cancer. Clin Breast Cancer. 2016;16(5):379–88.
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–31.
Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, Wood WC, Henderson IC, Hudis C, Winer E, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–81.
Brouwers B, Hatse S, Dal Lago L, Neven P, Vuylsteke P, Dalmasso B, Debrock G, Van Den Bulck H, Smeets A, Bechter O, et al. The impact of adjuvant chemotherapy in older breast cancer patients on clinical and biological aging parameters. Oncotarget. 2016;7(21):29977–88.
Extermann M, Leeuwenburgh C, Samiian L, Sehovic M, Xu J, Cubitt C, Jacobsen PB, Pahor M, Grobmyer SR, Manini TM. Impact of chemotherapy on medium-term physical function and activity of older breast cancer survivors, and associated biomarkers. J Geriatr Oncol. 2017;8(1):69–75.
Giugliano FM, Falivene S, Esposito E, Di Franco R, Muto M, D’Aiuto M, Muto P. External radiotherapy for breast cancer in the elderly. Aging Clin Exp Res. 2017;29(Suppl 1):149–57.
Rutter CE, Lester-Coll NH, Mancini BR, Corso CD, Park HS, Yeboa DN, Gross CP, Evans SB. The evolving role of adjuvant radiotherapy for elderly women with early-stage breast cancer. Cancer. 2015;121(14):2331–40.
Kunkler IH, Prescott RJ, Williams LJ, King CC. When may adjuvant radiotherapy be avoided in operable breast cancer? Clin Oncol (R Coll Radiol). 2006;18(3):191–9.
Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, Muss HB, Smith BL, Hudis CA, Winer EP, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7.
Daugherty EC, Daugherty MR, Bogart JA, Shapiro A. Adjuvant radiation improves survival in older women following breast-conserving surgery for estrogen receptor-negative breast cancer. Clin Breast Cancer. 2016;16(6):500-506.e502.
Matuschek C, Bölke E, Haussmann J, Mohrmann S, Nestle-Krämling C, Gerber PA, Corradini S, Orth K, Kammers K, Budach W. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiat Oncol. 2017;12(1):60.
Muss HB. Adjuvant treatment of elderly breast cancer patients. Breast. 2007;16(Suppl 2):S159-165.
Acknowledgements
Not applicable.
Funding
This study is supported by Key projects of Sichuan Provincial Department of science and technology (2019YFS0338), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20192011), the Key Program of National Natural Science Foundation of China (32071284), the Key Foundation of Sichuan Province Health Commission (18ZD044).
Author information
Authors and Affiliations
Contributions
RY and JC designed the study; YW and YQ collected data; XZ and RC checked and sorted out the data; WL and YH analyzed the data; TH drafted the article and revised it; XZ, QL and LZ finally revised the final vision of the manuscript. And manuscript is approved by all authors for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since data released by the SEER database was publicly available, ethics approval and informed patient consent was not required for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary 1.
Patients selection.
Additional file 2: Supplementary 2.
1-, 3-, 5-Year CIF of OCSD among patients with breast cancer in the training cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, R., Wu, Y., Qi, Y. et al. A nomogram for predicting breast cancer specific survival in elderly patients with breast cancer: a SEER population-based analysis. BMC Geriatr 23, 594 (2023). https://doi.org/10.1186/s12877-023-04280-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-04280-8