Abstract
Background
Frailty is a marker of poor prognosis in older adults with hematologic malignancies and contributes to the severe vulnerability of the aging population to adverse health outcomes. This study aimed to determine the association between frailty and outcomes in hospitalized patients with chronic myeloid leukemia (CML).
Methods
The International Classification of Diseases (ICD-10) identified data on hospitalized patients 20 years or older admitted with CML between 2016 and 2018 in the US National Inpatient Sample (NIS) database. The cohort was further divided into groups of patients with or without frailty. Logistic regression analysis was performed to determine associations between study variables and clinical outcomes. A stratified analysis of the association between frailty and in-hospital mortality by age group was also performed.
Results
A total of 13,849 hospitalized patients with CML were included, 49.6% of whom had frailty. The mean age of the patients was 65.1 years, and 7,619 (56.2%) of them were male. Frailty was associated with nearly 4 times the risk of in-hospital mortality, 3 times the risk of unfavorable discharge, 3 times the risk of prolonged LOS,, and significantly more in total hospital costs. In addition, frailty was associated with a significantly increased risk of in-hospital mortality in all age subgroups (< 40 years, 40–59 years, and > 60 years) compared with no frailty.
Conclusions
Frailty strongly predicts poor clinical outcomes in US patients with CML.
Similar content being viewed by others
Background
The hematologic malignancies are mostly and increasingly diagnosed in older adults [1-4]. However, although age is strongly associated with malignant hematologic diagnoses, it might not precisely reflect the condition of individual patients. Therefore, several tools and assessments from the geriatrics discipline are being incorporated into routine oncology care. Frailty has been recognized as an essential marker of poor outcomes in older adults with hematological malignancy by clinicians [2]. It is most often defined as an aging-related geriatric syndrome of physiological decline, characterized by significant vulnerability to adverse health outcomes. Frail patients often present an increased age-related impairment in function and physiological capacity, followed by medical complexity and reduced tolerance to medical and surgical interventions. Despite aging, there are other paths that may lead to physical frailty, one of which is chronic disease. Evolving chronic diseases including cancers have been suggested to contribute to the development of physical frailty [5, 6]. The exposures to cancer treatments can also lead to frailty as well [7].
Routine measurement of frailty in hematology practice is feasible, and several measures such as the Geriatric 8 (G8), comprehensive geriatric assessment (CGA) and Clinical Frailty Scale (CFS) are available [8-10]. There is heterogeneity in measuring frailty in hematologic malignancies, with most studies using a Geriatric Assessment (GA) to identify frailty [11]. More recently, studies have reported the predictive value of GA domains in patients with certain types of hematologic malignancies, such as acute myelogenous leukemia (AML) [12], myelodysplastic syndrome (MDS) [12, 13], diffuse large B cell lymphoma (DLBCL) [14, 15], as well as patients who were undergoing hematopoietic cell transplantation (HCT) [16]. However, evidence regarding the association between frailty and clinical outcomes in patients with chronic myeloid leukemia (CML) is limited. In this study, we aimed to evaluate the prevalence, characteristics, and impact of frailty in hospitalized patients with chronic myeloid leukemia (CML) in general and in different age groups, using a nationally representative large cohort of the US.
Methods
Data source
This population-based, retrospective study extracted all data from the US Nationwide Inpatient Sample (NIS) database, the largest continuous inpatient care database including about 8 million hospital stays each year [17]. The database is administered by the Healthcare Cost and Utilization Project (HCUP) of the US National Institutes of Health (NIH). Patient data such as patient demographics, procedures, diagnoses, admission and discharge status, duration of hospital stay, and hospital characteristics were obtained. The 2016 HCUP NIS includes all discharge data from 4,573 hospitals. This 2016 NIS sampling frame is comprised of 46 states and the District of Columbia, covering more than 97% of the US population and includes almost 96% of dischargers in the US community hospitals. More details on the design and data framework of the HCUP NIS could be found on: https://health.gov/healthypeople/objectives-and-data/data-sources-and-methods/data-sources/healthcare-cost-and-utilization-project-national-nationwide-inpatient-sample-hcup-nis.
Ethics statement
All data were obtained from the Online HCUP Central Distributor (https://www.distributor.hcup-us.ahrq.gov/), which administers the database (certificate # HCUP-4T39K81HZ). This study conforms to the NIS data-use agreement with HCUP. Because this study analyzed secondary data from the NIS database, patients and the public were not involved directly. The protocol of this study was submitted and exempted to the Institutional Review Board (IRB) of our Hospital. Due to all data in the NIS database are de-identified, informed consent was also waived.
Study population
Data of hospitalized patients aged 20 years or older admitted with CML between 2016 and 2018 were identified in the NIS database through the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes: C92.10, C92.11, C92.12, C92.20, C92.21, C92.22. Individuals without complete data on main study variables and outcomes were excluded. The cohort was further categorized into patients with or without frailty. The method/criterion for defining frailty is detailed below.
Study variables
Study endpoints
Study endpoints were: 1) in-hospital mortality; 2) unfavorable discharge, defined as discharged to a nursing home or long-term facility; 3) prolonged length of stay (LOS) defined as > 75th LOS; and 4) total hospital cost.
Definition of frailty
To define frailty, we adapted the hospital frailty risk score (HFRS), a previously developed algorithm by Gilbert et al. to identify frailty traits in an electronic database [18]. The HFRS has the advantage of being derived from ICD-10 codes, so it can be used wherever ICD-10 coding systems are in place. This algorithm was validated and increasingly utilized recently in various clinical settings across different countries [19-21]. In the present study, patients who had an HFRS > = 5 were considered frail, whereas patients with an HRFS < 5 were regarded as non-frail. The codes used to assess HFRS are summarized in Supplementary Table S1.
Covariates
Data of patients’ demographic characteristics included age, gender, race, household income quartiles, insurance status (primary payer). Household income quartiles were obtained from the NIS, estimated from the household income of residents in the patient's ZIP Code (https://hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp). Since these estimates are updated annually, the value ranges categories vary by year. The ranges of household income quartiles are summarized in Supplementary Table S4. In addition, individual’s clinical characteristics, including CML status (in remission, not having achieved remission, in relapse), comorbidities, Charlson Comorbidity Index (CCI), and treatments (hematopoietic stem cell transplantation (HSCT) or chemotherapy), were identified using ICD-10 codes. Finally, hospital-related characteristics (bed size, location/teaching status, and hospital region) were also obtained as part of the comprehensive data available for all participants. The codes used to identify the comorbidities, treatments, and CCI are summarized in Supplementary Table S2 and S3.
Statistical analysis
The NIS database includes a 20% sample of US annual inpatient admissions and as suggested by the guidelines of the database, weighted samples (DISCWT), stratum (NIS_STRATUM), and cluster (HOSPID) were used to derive the national estimates. The SURVEY procedure in SAS performs analysis for sample survey data. Descriptive statistics are presented as number (n) and weighted percentage (%) or mean and standard error (SE). Categorical data was analyzed by PROC SURVEYFREQ statement and continuous data was analyzed by PROC SURVEYREG statement. Logistic regression analyses were performed and determined the associations between study variables and in-hospital mortality, unfavorable discharge, and prolonged LOS. To minimize the differences in baseline characteristics, in the regression analyses, we further excluded 2,057 (15.2%) patients who were in remission and 312 (2.3%) patients in relapse to focus on those not having achieved remission. For the associations between study variables and total hospital cost, natural log-transformed ordinary least squares (OLS) regression were performed to address the potential skewness in distribution of cost. Multivariate regression was adjusted for the significant variables in the univariate regression model. All p values are two-sided, and p < 0.05 is considered statistically significant. All statistical analyses were performed through SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). In addition, stratified analyses on the association between frailty and in-hospital mortality by different age groups were also performed.
Results
During 2016 and 2018, in the NIS database, a total of 13,849 hospitalized CML patients were identified. After exclusion for missing data of sex (n = 5), age < 20 (n = 176) and no information on study endpoints (n = 111), the remaining 13,557 patients were included as the primary cohort. Of them, 49.6% (n = 6,719) were frail.
Baseline characteristics
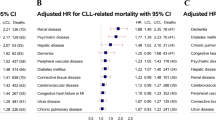
Baseline characteristics of the study population are summarized in Table 1. Patients’ mean age and HFRS were 65.1 years and 5.6, respectively, and 7,619 (56.2%) were males. As compared with non-frail patients, frail patients were older (69.1 vs. 61.2 years, p-value < 0.001), had more females (45.0% vs. 42.6%, p = 0.006), had a white race (74.7% vs. 71.2%, p < 0.001), with a more significant proportion of insurance covered by medicare/medicaid (80.7% vs. 68.4%, p < 0.001), with higher CCI scores (2–3: 32.0% vs. 23.5%; 4 + : 37.2% vs. 16.8%, p < 0.001). Significantly higher frequencies of coronary artery disease, congestive heart failure, diabetes, hypertension, cerebrovascular disease, chronic pulmonary disease, drug abuse, severe liver disease, moderate or severe renal disease, and rheumatic disease were observed among frail patients. There were also significant differences in hospital location/teaching status and hospital region between frail and non-frail patients (p < 0.001) (Table 1).
In-hospital outcomes
The mean total hospital cost was 74,773 US dollars. In-hospital mortality, the rate of unfavorable discharge, and prolonged LOS of the study population were 4.4, 18.8, and 29.6%, respectively. In frail patients, greater frequencies of in-hospital death, unfavorable discharge, prolonged LOS, and higher total hospital cost were observed (all p-value < 0.001). (Table 2).
Associations between in-hospital mortality, unfavorable discharge, prolonged LOS, total hospital cost and frailty
The relationship between frailty and in-hospital mortality, unfavorable discharge, prolonged LOS, and total hospital cost are summarized in Table 3. In multivariate analyses after adjustment, frailty was significantly and independently associated with increased risks for in-hospital mortality (adjusted odds ratio [aOR], 3.81; 95% CI: 2.99–4.86), unfavorable discharge (aOR, 2.90; 95% CI: 2.58–3.27), prolonged LOS (aOR, 2.97; 95% CI: 2.70–3.27), and higher total hospital cost (adjusted beta, 0.33; 95% CI: 0.29–0.37) than non-frailty. (Table 3).
Association between frailty and in-hospital mortality stratified by age
Table 4 shows the relationship between frailty and in-hospital mortality stratified by age. After adjusting by insurance status, CML status, congestive heart failure, diabetes, hypertension, cerebrovascular disease, obesity, drug abuse, severe liver disease, moderate or severe renal disease, CCI, hospital bed size, and hospital region, frailty remained significantly associated with more significant risks for in-hospital mortality in all age -groups, while the most significant risk was observed among patients aged 40–59 years old (aOR, 5.90; 95% CI: 3.08–11.31) (Table 4).
Discussion
Half of hospitalized CML patients in the US were frail defined by HFRS. Frailty is significantly and independently associated with nearly 4-time risk of in-hospital mortality, 3-time of unfavorable discharge, 3-time of prolonged LOS, and significantly higher cost during admission. Furthermore, the impact of frailty on in-hospital death is seen in both elderly and non-elderly patients. These findings indicate that frailty is a strong independent predictor for adverse in-patient outcomes in hospitalized CML patients.
A review study by Handforth et al. reported 42 and 43% prevalence of frailty and pre-frailty in older adults with solid tumor and hematological malignancy, respectively [22]. Atakul et al. reported a frailty prevalence of 42.2% in older patients undergoing treatment for hematological malignancies [23]. Patel et al. reported a 41.3 and 29.9% prevalence of prefrailty and frailty [24]. The present study found a higher prevalence of frailty (52.8%) in CML patients aged over 60 than in the previous studies. It may be explained by this study utilized an acute care cohort, thereby the prevalence of frailty is likely to be higher.
Studies showed frailty can provide a better measure of vulnerability of worse outcomes than age in hematologic malignancies. Facon et al. investigated the outcomes of patients from the large, phase 3 FIRST trial in newly diagnosed multiple myeloma (NDMM) based on frailty using scores for age, Charlson Comorbidity Index (CCI), and Eastern Cooperative Oncology Group performance status (ECOG PS) [25]. The authors of that study concluded simplified frailty scale predicts worse progression-free and overall survival among transplant-ineligible patients with newly diagnosed multiple myeloma [25]. Abel et al. reviewed the outcomes of different blood cancers in patients by the methods of Vulnerable Elders Survey (VES-13), G8, Geriatric Assessment in Hematology (GAH), CFS, Timed Up and Go (TUG), and International Myeloma Working Group (IMWG) Frailty Score, indicating that frailty assessment rather than age could help hematologist in practice [10]. Scheepers et al. suggested older patients with hematologic malignancy with geriatric impairments had a higher risk of treatment-related toxicity, treatment non-completion, and healthcare services utility, indicating that frailty assessments should be considered before starting treatment in older patients with hematologic malignancies.
Individuals with a cancer are dealing with the interacting effects of the biologic and physiologic changes of aging, multimorbidity, effects of the cancer per se, and the effects of the cancer treatments, among which chemotherapy has the most pervasive effect [26]. It was also documented that frailty in blood cancers may originate from various conditions including cancer issues (e.g., weight loss, cachexia), comorbidities, immunosuppression, or treatment-related toxicity [27]. In particular, high-intensity therapeutic exposures, chronic graft-versus-host disease (GvHD), and chronic health conditions after hematopoietic stem cell transplantation (HSCT) serve as substantial stressors, increasing the risk of frailty even among nonelderly [28]. Of note, the proportion of patients who received chemotherapy or HSCT in this study cohort was very low, probably due to not properly coded in the database.
Many molecular targeting drugs have been used in the clinic and might have the potential to replace conventional chemotherapy and HSCT [29]. For example, tyrosine kinase inhibitors (TKIs), such as imatinib or dasatinib, are thought to be the mainstay of CML treatment and drastically improved outcomes of CML [29,30,31]. Treatment of CML with TKIs results in near-normal life expectancy. However, studies have reported that TKI may lead to skeletal muscle loss in cancer treatment, affecting patients' health with more extended treatment [30,31,32]. The NIS database does not provide information on medication used, which hindered further evaluation on the potential causal influence between TKIs and frailty. Since there is currently no data regarding whether and how TKIs interact with frailty in patients with hematologic cancers, it is important to conduct such investigations in the future.
The present study included non-elderly CML patients. It is demonstrated that the prevalence of frailty is 27 and 38.6% in patients aged 20–39 years and 40–59 years, respectively. Importantly, we found that frailty is strongly associated with increased risk for in-hospital mortality not only in elderly patients but also in the non-elderly. In young CML patients, it is postulated that frailty is more likely related to CML treatments. Future studies focused on the origin and impact of frailty in non-elderly CML patients is warranted.
Strengths and limitations
The strength of the present study is the use of a very large sample that represents a nationwide population. One of the major limitations was that we were not able to distinguish disease-related frailty, which may be a consequence of CML and its treatment, from age-related frailty. HFRS has yet to be formally validated in population under 75 years old as well as in cancer patients. Nevertheless, a list of recent studies did have assessed the prognostic role of HFRS and expanded its coverage to younger population aged 20–75 years [33, 34]. Other limitations include the possibility of coding errors during use of the ICD-10 coding systems for defining CML, comorbidities and complications. Possible confounding variables not collected by the NIS could not be included in the analyses. Targeted therapies are crucial for the management of CML. However, they could not be captured through the coding system thus could not be analyzed. The study also lacks follow-up data after discharge, precluding the evaluation of late morbidity and mortality.
Conclusions
Frailty is a strong predictor for increased in-hospital mortality, unfavorable discharge, prolonged LOS, and more hospital cost in CML patients in the US. Frailty not only poses greater risk for in-hospital death in older patients but also in non-elderly. Future studies that include data of targeted therapies are warranted.
Availability of data and materials
All data analysed during this study are included in this published article.
Abbreviations
- CML:
-
Chronic myeloid leukemia
- NIS:
-
Nationwide Inpatient Sample
- ICD:
-
ICD-10International Classification of Diseases
- CGA:
-
Comprehensive geriatric assessment
- CFS:
-
Clinical Frailty Scale
- AML:
-
Acute myelogenous leukemia
- MDS:
-
Myelodysplastic syndrome
- DLBCL:
-
Diffuse large B cell lymphoma
- HCT:
-
Hematopoietic cell transplantation
- HCUP:
-
Healthcare Cost and Utilization Project
- NIH:
-
National Institutes of Health
References
Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–92.
Koll TT, Rosko AE. Frailty in Hematologic Malignancy. Curr Hematol Malig Rep. 2018;13:143–54.
Graf SA, Samples LS, Keating TM, Garcia JM. Clinical research in older adults with hematologic malignancies: Opportunities for alignment in the Veterans Affairs. Semin Oncol. 2020;47:94–101.
Kanapuru B, Singh H, Kwitkowski V, Blumenthal G, Farrell AT, Pazdur R. Older adults in hematologic malignancy trials: Representation, barriers to participation and strategies for addressing underrepresentation. Blood Rev. 2020;43:100670.
Angioni D, Macaron T, Takeda C, Sourdet S, Cesari M, Virecoulon Giudici K, et al. Can we distinguish age-related frailty from frailty related to diseases? Data from the MAPT study. J Nutr Health Aging. 2020;24:1144–51.
El Haddad K, Rolland Y, Gérard S, Mourey L, Sourdet S, Vellas B, et al. No difference in the phenotypic expression of frailty among elderly patients recently diagnosed with cancer vs cancer free patients. J Nutr Health Aging. 2020;24:147–51.
Brown JC, Harhay MO, Harhay MN. The prognostic importance of frailty in cancer survivors. J Am Geriatr Soc. 2015;63:2538–43.
Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72.
Marchesi F, Cenfra N, Altomare L, Dessanti ML, Mecarocci S, Cerchiara E, et al. A retrospective study on 73 elderly patients (>=75years) with aggressive B-cell non Hodgkin lymphoma: clinical significance of treatment intensity and comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:242–8.
Abel GA, Klepin HD. Frailty and the management of hematologic malignancies. Blood. 2018;131:515–24.
Scheepers ERM, Vondeling AM, Thielen N, van der Griend R, Stauder R, Hamaker ME. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 2020;105:1484–93.
Deschler B, Ihorst G, Platzbecker U, Germing U, März E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98:208–16.
Buckstein R, Wells RA, Zhu N, Leitch HA, Nevill TJ, Yee KW, et al. Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: an MDS-CAN prospective study. Br J Haematol. 2016;174:88–101.
Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma. 2015;56:921–6.
Yoshida M, Nakao T, Horiuchi M, Ueda H, Hagihara K, Kanashima H, et al. Analysis of elderly patients with diffuse large B-cell lymphoma: aggressive therapy is a reasonable approach for “unfit” patients classified by comprehensive geriatric assessment. Eur J Haematol. 2016;96:409–16.
Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–9.
HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nisoverview.jsp.
Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–82.
Nghiem S, Afoakwah C, Scuffham P, Byrnes J. Hospital frailty risk score and adverse health outcomes: evidence from longitudinal record linkage cardiac data. Age Ageing. 2021;50:1778–84.
Gunnarsdottir GM, Helgadottir S, Einarsson SG, Hreinsson K, Whittle J, Karason S, et al. Validation of the Hospital Frailty Risk Score in older surgical patients: A population-based retrospective cohort study. Acta Anaesthesiol Scand. 2021;65:1033–42.
Gilbert T, Cordier Q, Polazzi S, Bonnefoy M, Keeble E, Street A, et al. External validation of the Hospital Frailty Risk Score in France. Age Ageing. 2022;51:afab126.
Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–101.
Atakul E, Akyar İ. Frailty prevalence and characteristics in older adults with hematologic cancer: a descriptive study. Asia Pac J Oncol Nurs. 2019;6:43–9.
Patel BG, Luo S, Wildes TM, Sanfilippo KM. Frailty in older adults with multiple myeloma: a study of US veterans. JCO Clin Cancer Inform. 2020;4:117–27.
Facon T, Dimopoulos MA, Meuleman N, Belch A, Mohty M, Chen WM, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34:224–33.
Henderson TO, Ness KK, Cohen HJ. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book 2014;34:e423–30.
Goede V. Frailty assessment in the care of older people with haematological malignancies. Lancet Healthy Longevity. 2021;11:e736–45.
Arora M, Sun CL, Ness KK, Teh JB, Wu J, Francisco L, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the bone marrow transplant survivor study. JAMA Oncol. 2016;2:1277–86.
Shimada A. Hematological malignancies and molecular targeting therapy. Eur J Pharmacol. 2019;862:172641.
Medeiros BC, Possick J, Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: Strategies for monitoring, detecting, and managing. Blood Rev. 2018;32:289–99.
Kakinuma K, Tsuruoka H, Morikawa K, Furuya N, Inoue T, Miyazawa T, et al. Differences in skeletal muscle loss caused by cytotoxic chemotherapy and molecular targeted therapy in patients with advanced non-small cell lung cancer. Thorac Cancer. 2018;9(1):99–104.
Uchikawa S, Kawaoka T, Namba M, Kodama K, Ohya K, Morio K, et al. Skeletal Muscle Loss during Tyrosine Kinase Inhibitor Treatment for Advanced Hepatocellular Carcinoma Patients. Liver Cancer. 2020;9:148–55.
Elsamadicy AA, Koo AB, Reeves BC, Pennington Z, Sarkozy M, Hersh A, et al. Hospital Frailty Risk Score and healthcare resource utilization after surgery for primary spinal intradural/cord tumors. Global Spine J. 2022;0:21925682211069937.
Ramai D, Dang-Ho KP, Kewalramani A, Bandaru P, Sacco R, Giacomelli L, et al. Hospital frailty risk score is independently associated with mortality and encephalopathy in hospitalized patients with hepatocellular carcinoma. Biomedicines. 2021;9:1693.
Acknowledgements
The authors would like to thank Taipei Medical University Hospital(108TMU-TMUH-24) for supporting this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design: HTL, YRL. Acquisition of data: KDL, HET. Analysis and interpretation of data: HTL, KDL. Drafting of the manuscript: YRL, HET. Critical revision of the manuscript: KDL, HET. guarantor of integrity of the entire study: HTL. statistical analysis: KDL. literature research: YRL. Administrative, technical or material support: HTL. Final approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All data were obtained from the Online HCUP Central Distributor (https://www.distributor.hcup-us.ahrq.gov/), which administers the database (certificate # HCUP-4T39K81HZ). This study conforms to the NIS data-use agreement with HCUP. Because this study analyzed, secondary data from the NIS database, patients and the public were not involved directly. The protocol of this study was submitted to and exempted from the Institutional Review Board (IRB) of our Hospital. Due to all data in the NIS database are de-identified, informed consent was also waived.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table S1. ICD-10 codes used to assess HFRS. Supplementary Table S2. ICD-10 codes used to identify CML, comorbidities and treatments. Supplementary Table S3. ICD-10 codes used to define CCI. Supplementary Table S4. Quartile ranges of household income (USD).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huan-Tze, L., Yun-Ru, L., Kuan-Der, L. et al. Frailty in chronic myeloid leukemia: evidence from 2016–2018 Nationwide Inpatient Sample of the US. BMC Geriatr 23, 334 (2023). https://doi.org/10.1186/s12877-023-03962-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03962-7