Abstract
Background
Frailty is associated with mortality among older adults. We aimed to determine the appropriate time and frailty index (FI) threshold for frailty intervention in Chinese community-dwelling older adults.
Methods
In this prospective cohort study, we used data from the 2011 wave of the Chinese Longitudinal Healthy Longevity Study. Follow-up was performed for seven years from baseline. Using the FI to evaluate frailty and define frailty status, we explored the best time point and FI score for frailty intervention, by comparing the relationships of FI and frailty status with mortality.
Results
From 2011 to 2018, 8642 participants were included and followed-up. A total of 4458 participants died during the study period. After adjusting for variables such as age, sex, marital status, education level, and living conditions, the hazard ratio (HR) of mortality risk based on the FI at baseline was 37.484 (95% confidence interval [CI]: 30.217–46.498; P < 0.001); female sex, living in the city, being married, and living with spouse were found to be protective factors, whereas ageing was a risk factor for frailty. The mortality risk was higher in pre-frail than in frail participants (HR: 3.588, 95% CI: 3.212–4.009, P < 0.001). Piecewise linear regression analysis revealed an FI score threshold of 0.5. When the FI score was > 0.5, the HR of mortality based on the FI was 15.758 (95% CI: 3.656–67.924; P < 0.001); when the FI score was ≤ 0.5, the HR of mortality based on the FI was 48.944 (95% CI: 36.162–66.244; P < 0.001).
Conclusion
Using FI as a continuous variable to predict death is more accurate than frailty status. The advancement of early interventions for mortality risk reduction is more beneficial in pre-frail than in frail patients, and an FI score of 0.5 was found to be the threshold for mortality prediction using the FI.
Similar content being viewed by others
Background
Rapid ageing of the worldwide population has become a major trend in the global demographic structure owing to reduced fertility and increased mortality rates [1, 2]. Frailty is becoming an increasingly obvious and common feature of an ageing older adults; a decline in various physiological functions related to age increases vulnerability to stressors. In addition to disease or disability, frailty is associated with a systemic impairment of physical and cognitive functions, including symptoms, diseases, and life-long deficits [3, 4]. People with frailty are more likely to experience a variety of negative health conditions, such as falls, fractures, hospitalization, need for nursing home placement, disability, poor quality of life, and dementia [5,6,7,8,9].
The frailty index (FI) is one of the most commonly used tools to measure frailty. FI is evaluated based on the concept that frailty is a state caused by a life-long accumulation of health deficits; the higher the number of health deficits, the greater the tendency for frailty. These health deficits include symptoms, disease, disability, abnormal laboratory findings, and social characteristics [10,11,12]. FI is predictive for adverse outcomes and is directly related to survival outcomes [13,14,15]. Moreover, compared with chronological age, FI has a stronger correlation with mortality, especially within short intervals less than four years [16].
FI has been shown to vary with time; thus, it is evaluated using cross-sectional studies that cannot accurately predict mortality risk [17, 18]. Therefore, it is necessary to perform mortality risk reassessment using dynamic FI changes [19, 20]. Moreover, frailty is not only associated with age but is also affected by risk factors including impairment of activities of daily living, chronic diseases, depression, poor lifestyle habits, and geriatric syndromes [21, 22]. Effective prevention and treatment can reduce occurrence of frailty in older adults [23]. Hence, mortality risk prediction and early intervention to treat debilitating conditions can prolong survival time, thereby alleviating the pressure on medical care [24].
We aimed to collect and evaluate longitudinal data at different time points, and to accurately determine the best time point for frailty intervention using a long follow-up duration. Our findings will potentially enhance decision-making regarding frailty intervention and the effective utilization of medical resources.
Methods
Participants
The Chinese Longitudinal Healthy Longevity Survey (CLHLS) is a nationwide longitudinal survey conducted in a randomly selected half of the counties and cities in 22 of the 31 provinces in China. All the participants provided written informed consent [25]. We used the data from the 2011 wave of the CLHLS, which was followed-up in 2014 and 2018. The medical ethics committee of Tongji University approved this study. Participants were excluded if more than 30% of FI variables were missing or if they died before the 2014 follow-up. Moreover, we excluded individuals who had 80% missing data on cognitive function and less than 30 variables for FI calculation.
Frailty index
Health deficits were evaluated using the FI. We selected 42 items on self-related health, physical function, psychological and cognitive function, comorbidity, and social deficits [25, 26]. Cognitive function was measured using the Mini-Mental State Examination (MMSE) scale [27]. Binary variables were encoded as 0 or 1. For ordered and continuous variables, encoding was based on the distribution. A score of 2 was assigned if the respondent had suffered from more than one serious disease in the past two years. The FI score was calculated as the ratio of health deficits present to the total number of deficits considered, with values ranging between 0 and 1. Higher scores indicated a higher degree of frailty; FI scores < 0.25 and ≥ 0.25 were considered to indicate non-frailty and frailty statuses, respectively [28, 29]. To find the best intervention site, the non-frailty status is sub-divided into robust and pre-frailty stages according to FI score ≤ 0.1 and 0.1 < FI score < 0.25, respectively.
Statistical analysis
Cox proportional hazards regression and piecewise linear regression [30] were used to evaluate the relationship between FI and mortality, and the Kaplan–Meier survival function curve was used to estimate the seven-year survival in relation to the FI and frailty status. The areas under the receiver-operating characteristic (ROC) curves (AUCs) of FI and frailty status were calculated to compare the effects of these parameters on death outcomes during the follow-up period. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), IBM SPSS Statistics version 20 (SPSS Inc., Chicago, IL, USA), and R statistical software version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
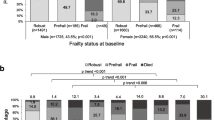
A total of 8642 older people participated in the baseline survey in 2011. Table 1 shows the participant characteristics and frailty status at baseline. Participants had a median age of 85.6 ± 11.3 years, with a range of 50–114 years. At baseline, 2020 (23.4%), 2802 (32.4%), and 3820 (44.2%) participants were robust (FI score ≤ 0.1), pre-frail (0.1 < FI score < 0.25), and frail (FI score ≥ 0.25), respectively.
In addition, 4458 participants died during the study period, as observed in 2018. The AUC of FI at baseline was 0.768 (95% CI: 0.758–0.778, P < 0.001), whereas the AUC of frailty status was 0.537 (95% CI: 0.524–0.549, P < 0.001), thereby showing a weaker prediction with mortality (Fig. 1).
The hazard ratio (HR) of mortality according to the FI at baseline was 37.484 (95% confidence interval [CI]: 30.217–46.498), P < 0.001). Female sex (HR: 0.624, 95% CI: 0.584–0.666, P < 0.001), living in the city (HR: 0.864, 95% CI: 0.792–0.943, P = 0.001), being married and living with spouse (HR: 0.797, 95% CI: 0.736–0.864, P < 0.001) were found to be protective factors, whereas ageing (HR: 1.057, 95% CI: 1.053–1.061, P < 0.001) was a risk factor for mortality (Table 2).
We further classified frailty as non-frailty (FI < 0.25) and frailty (FI ≥ 0.25), and analysed the HR for mortality in different states of frailty. The HR of mortality according to the FI was 2.209 (95% CI: 2.064–2.364, P < 0.001) when the frailty status was dichotomized. The female sex, education level, being married, and living with spouse were found to be protective factors, whereas ageing was a risk factor of frailty. The HR for mortality was higher in pre-frail (HR: 3.588, 95% CI: 3.212–4.009, P < 0.001) than in frail (HR: 1.820, 95% CI: 1.640–2.021, P < 0.001) participants, when the frailty status was evaluated as robust, pre-frailty, and frailty. The female sex, being married, and living with spouse were found to be protective factors, whereas ageing was a risk factor of frailty (Table 3).
Due to the inconsistency of the different frailty status classifications, we reconsidered FI as a continuous variable. We found that the curves of FI at baseline and seven-year survival rate could be divided into two segments around an FI score of 0.5 (Fig. 2), where the partial regression coefficients were 3.891 and 2.757, respectively. To further explore the effect of a unit increase in FI on the mortality risk, piecewise regression analysis was performed by segment within the FI score ranges between 0–0.5 and 0.5–1. When FI score was > 0.5, the HR of mortality based on FI was 15.758 (95% CI: 3.656–67.924, P < 0.001); however, when the FI score was ≤ 0.5, the HR was 48.944 (95% CI: 36.162–66.244, P < 0.001). The female sex, living in the city, being married, and living with spouse were found to be protective factors, whereas ageing was a risk factor of frailty (Table 4).
Discussion
Previous studies have investigated the relationship between FI and mortality and predicted the mortality risk based on the static and dynamic FI [20, 26]. However, the relationship between the frailty status and mortality risk has not been studied [31]. To examine the relationship of the FI and frailty status with survival time, we used Kaplan–Meier survival curves to determine whether FI was more strongly associated with mortality than frailty status by calculating the AUCs, and to find that the frailty status was a weaker predictor than using FI with mortality. Previous studies have reported a correlation between FI and short-term mortality; furthermore, our findings demonstrated that FI can be used to predict the seven-year survival rate [21].
Impairment in activities of daily living, chronic diseases, depression, poor lifestyle habits, and geriatric syndromes are risk factors for frailty [32]. Similarly, our study revealed that female sex, living in a city, being married, and living with a spouse are predictive factors of frailty. This is probably attributed to the fact that marital status and living conditions of older adults are related to their mental health and access to medical resources [33]. Previous research had shown a relationship between frailty and type of death; hence, we used survival analysis to evaluate the association between FI and mortality. Our findings provide evidence that clinicians should perform frailty interventions to reduce preventable suffering before death; moreover, these interventions should be performed based on the known risk factors associated with FI [22].
We further explored the relationship between the frailty status and mortality risk at baseline (2011) and during follow-up (2014 and 2018), to establish suitable frailty interventions [29]. When examining the frailty-related mortality risk, we adjusted for demographic (sex and age), and sociological (education level, marital status, and living conditions) factors. When the frailty status was divided into non-frailty and frailty, ageing was considered a risk factor while education level was found to be a protective factor for frailty, in addition to the female sex, being married, and living with spouse. This finding was probably because education increases health literacy. Furthermore, we found that the HR for mortality was higher in pre-frail than in frail individuals. When the FI is > 0.25 as frailty stage, it covers the fraction of FI > 0.5, and thus has less impact on death than pre-frailty stage when the FI is between 0.1 and 0.25. This provides evidence for the possibility of early intervention in pre-frail older adults.
Frailty, defined by phenotype or FI, was found to be significantly associated with an increased risk of all-cause mortality in community-dwelling Chinese older adults based on previous studies [34, 35]. Previous studies showed slightly different results of the relative mortality risk for different frailty levels owing to a lack of a unified frailty classification standard and inconsistencies in frailty status classification [36]. In the present study, we stratified the FI by grade rather than frailty categorization, to perform a more precise risk prediction, and confirm whether 0.5 was the FI threshold. The mortality risk increased with age, and the female sex and being married were found to be protective factors of frailty, which was consistent with previous study findings [37]. Living in the city was found to be a protective factor of frailty when the FI score was < 0.5, indicating that lifespan may be prolonged by exposure to advanced medications in the early state of frailty [38]. When the FI was > 0.5, the effect of frailty on mortality was relatively small because patients with the highest number of health deficits had the highest all-cause mortality rates [26]. A score of 0.5 was the risk threshold when the IF score was close to it, and the risk of death increased significantly with frailty under a score of 0.5.
Conclusion
Frailty is associated with and predictive of all-cause mortality. Although the effect of intervention in the pre-frailty period may be better than that in the frailty period, intervention with FI below 0.5 may be more beneficial. It is recommended to conduct frailty screening and intervention management for the older adults in Chinese communities.
Availability of data and materials
The CLHLS analysed during our study are available in the Peking University Open Research Data, [https://opendata.pku.edu.cn/].
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- FI:
-
Frailty index
- HR:
-
Hazard ratio
- MMSE:
-
Mini-mental status examination
- ROC:
-
Receiver-operating characteristic
- B:
-
Regression coefficients
- SE:
-
Standard error
- Df:
-
Degree of freedom
References
Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017;389:1619–29.
Jean-Pierre M, Matilde L, Mike M, Matthew P. WHO’s report for the decade of healthy ageing 2021–30 sets the stage for globally comparable data on healthy ageing. Lancet Healthy Longev. 2021;2:E121–2.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–36.
Bortz WM 2nd. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–8. https://doi.org/10.1093/gerona/57.5.m283.
Kojima G. Frailty as a predictor of future falls Among community-dwelling older people: A systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16:1027–33. https://doi.org/10.1016/j.jamda.2015.06.018.
Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–82. https://doi.org/10.1016/S0140-6736(18)30668-8.
Kojima G. Frailty as a predictor of nursing home placement Among community-dwelling older adults: A systematic review and meta-analysis. J Geriatr Phys Ther. 2018;41:42–8. https://doi.org/10.1519/JPT.0000000000000097.
Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17:881–8.
Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:716–21.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7.
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–75.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc. 2016;17:1163.e1-1163.e17.
Stenholm S, Ferrucci L, Vahtera J, Hoogendijk EO, Huisman M, Pentti J, et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies. J Gerontol A Biol Sci Med Sci. 2019;74:667–74.
Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet. 2015;385:e7-9.
Kulminski A, Yashin A, Arbeev K, Akushevich I, Ukraintseva S, Land K, et al. Cumulative index of health disorders as an indicator of aging-associated processes in the elderly: results from analyses of the National Long Term Care Survey. Mech Ageing Dev. 2007;128:250–8. https://doi.org/10.1016/j.mad.2006.12.004.
Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Res Rev. 2019;50:81–8. https://doi.org/10.1016/j.arr.2019.01.010.
Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2: e198398.
Jenkins DA, Sperrin M, Martin GP, Peek N. Dynamic models to predict health outcomes: current status and methodological challenges. Diagn Progn Res. 2018;2:23. https://doi.org/10.1186/s41512-018-0045-2.
Chen Q, Tang B, Zhai Y, Chen Y, Jin Z, Han H, et al. Dynamic statistical model for predicting the risk of death among older Chinese people, using longitudinal repeated measures of the frailty index: a prospective cohort study. Age Ageing. 2020;49:966–73.
Ma L, Tang Z, Zhang L, Sun F, Li Y, Chan P. Prevalence of frailty and associated factors in the community-dwelling population of China. J Am Geriatr Soc. 2018;66:559–64. https://doi.org/10.1111/jgs.15214.
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. https://doi.org/10.1016/S0140-6736(12)62167-9.
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. https://doi.org/10.1093/gerona/59.3.m255.
Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–51. https://doi.org/10.1093/gerona/60.8.1046.
Gu D. General Data Quality Assessment of the CLHLS. In: Yi Z, Poston DL, Vlosky DA, Gu D. (eds) Healthy Longevity in China. The Springer Series on Demographic Methods and Population Analysis, vol 20. Springer, Dordrecht; 2008. https://doi.org/10.1007/978-1-4020-6752-5_3.
Dupre ME, Gu D, Warner DF, Yi Z. Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009;338:b1175. doi: https://doi.org/10.1136/bmj.b1175.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5:e650–60.
Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing. 2013;42:372–7. https://doi.org/10.1093/ageing/afs180.
Muggeo VM. Estimating regression models with unknown break-points. Stat Med. 2003;22:3055–71.
Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–32. https://doi.org/10.1016/S2468-2667(18)30091-4.
Davies K, Maharani A, Chandola T, Todd C, Pendleton N. The longitudinal relationship between loneliness, social isolation, and frailty in older adults in England: a prospective analysis. Lancet Healthy Longev. 2021;2:e70–7.
Su D, Wu XN, Zhang YX, Li HP, Wang WL, Zhang JP, et al. Depression and social support between China’ rural and urban empty-nest elderly. Arch Gerontol Geriatr. 2012;55:564–9. https://doi.org/10.1016/j.archger.2012.06.006.
Shi GP, Ma T, Zhu YS, Wang ZD, Chu XF, Wang Y, et al. Frailty phenotype, frailty index and risk of mortality in Chinese elderly population- Rugao longevity and ageing study. Arch Gerontol Geriatr. 2019;80:115–9. https://doi.org/10.1016/j.archger.2018.11.001.
Ekram ARMS, Woods RL, Britt C, Espinoza S, Ernst ME, Ryan J. Erratum: The Association Between Frailty and All-Cause Mortality in Community-Dwelling Older Individuals: An Umbrella Review. J Frailty Aging. 2022;11:247.
Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200. https://doi.org/10.1093/ageing/afx162.
Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–7.
Zeng Y. Towards deeper research and better policy for healthy aging—using the unique data of Chinese longitudinal healthy longevity survey. China Econ J. 2012;5:131–49. https://doi.org/10.1080/17538963.2013.764677.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
National Key R&D Program of China (No. 2020YFC2008703). National Nature Science Foundation of China (No. 82073671).
Author information
Authors and Affiliations
Contributions
QC and RZ were involved in the research design and funding support. XZ drafted the manuscript and performed the computational analysis. JH and QC acted as corresponding authors, responsible for reviewing the content of the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Medical Ethics Committee of Tongji University, Shanghai, People’s Republic of China, approved the present study (approval number: 2022tjdxsy041). Written informed consent was obtained from all participants prior to the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, X., Zhu, R., Chen, Q. et al. Effect of frailty status on mortality risk among Chinese community-dwelling older adults: a prospective cohort study. BMC Geriatr 23, 150 (2023). https://doi.org/10.1186/s12877-023-03759-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-023-03759-8