Abstract
Background
Frailty is a physiological condition characterized by a decreased reserve to stressors. In patients with COVID-19, frailty is a risk factor for in-hospital mortality. The aim of this study was to assess the relationship between clinical presentation, analytical and radiological parameters at admission, and clinical outcomes according to frailty, as defined by the Clinical Frailty Scale (CFS), in old people hospitalized with COVID-19.
Materials and methods
This retrospective cohort study included people aged 65 years and older and admitted with community-acquired COVID-19 from 3 March 2020 to 31 April 2021. Patients were categorized using the CFS. Primary outcomes were symptoms of COVID-19 prior to admission, mortality, readmission, admission in intensive care unit (ICU), and need for invasive mechanical ventilation. Analysis of clinical symptoms, clinical outcomes, and CFS was performed using multivariable logistic regression, and results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Of the 785 included patients, 326 (41.5%, 95% CI 38.1%–45.0%) were defined as frail (CFS ≥ 5 points): 208 (26.5%, 95% CI 23.5%–29.7%) presented mild-moderate frailty (CFS 5–6 points) and 118 (15.0%, 95% CI 12.7%–17.7%), severe frailty (7–9 points). After adjusting for epidemiological variables (age, gender, residence in a nursing home, and Charlson comorbidity index), frail patients were significantly less likely to present dry cough (OR 0.58, 95% CI 0.40–0.83), myalgia-arthralgia (OR 0.46, 95% CI 0.29–0.75), and anosmia-dysgeusia (OR 0.46, 95% CI 0.23-0.94). Confusion was more common in severely frail patients (OR 3.14; 95% CI 1.64-5.97). After adjusting for epidemiological variables, the risk of in-hospital mortality was higher in frail patients (OR 2.79, 95% CI 1.79-4.25), including both those with mild-moderate frailty (OR 1.98, 95% CI 1.23-3.19) and severe frailty (OR 5.44, 95% CI 3.14-9.42). Readmission was higher in frail patients (OR 2.11, 95% CI 1.07–4.16), but only in mild-moderate frailty (OR 2.35, 95% CI 1.17–4.75)..
Conclusion
Frail patients presented atypical symptoms (less dry cough, myalgia-arthralgia, and anosmia-dysgeusia, and more confusion). Frailty was an independent predictor for death, regardless of severity, and mild-moderate frailty was associated with readmission.
Similar content being viewed by others
‘What’s known?’ and ‘What’s new?’
-
The Clinical Frailty Scale is an easy measure of frailty used in COVID-19 and a risk factor for in-hospital mortality.
-
We analyzed the clinical presentation and outcomes of COVID-19 according to frailty.
-
Frailty is associated with atypical clinical presentation.
-
Frailty is an independent predictor for death and readmission.
Introduction
The global COVID-19 pandemic, caused by severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV2), has caused more than 554 million known infections and well over 6.3 million deaths globally as of 9 July 2022 [1]. Over two years into the pandemic, COVID-19 continues to cause high mortality and hospitalization rates in older adults [2, 3].
Frailty is a clinical condition or state characterized by a decreased reserve to stressors [4, 5], associated with healthcare-related outcomes such as disability, hospitalization, and death [4, 6,7,8]. It can be measured with different instruments, the most common of which is the Clinical Frailty Scale (CSF) [9]. Several COVID-19 studies have pointed to frailty as an independent predictor for a fatal outcome [10,11,12,13]. Other authors have proposed screening for frailty to inform early decision-making on resource allocation and clinical management [4].
Chronic diseases and other health deficits tend to be more common in frail people, and these can obscure the recognition of new symptoms [14]. Several studies have reported an association between frailty and atypical clinical presentation of infectious diseases [15]. However, it is unclear whether frailty may also be a predictor for atypical symptoms in older patients with COVID-19 [11, 15].
The aim of this study was to assess patient characteristics on admission (clinical, analytical and radiological features) and clinical outcomes (mortality, readmission, admission in intensive care unit [ICU], and need for invasive mechanical ventilation [IMV]) according to CFS-assessed frailty [9] in hospitalized patients aged 65 years or older with COVID-19. We hypothesized that frailty would be strongly related with atypical symptoms and clinical outcomes.
Materials and methods
Study design and population
This retrospective cohort study took place from 3 March 2020 to 31 April 2021 at the Dr. Balmis General University Hospital (Alicante, Spain). Eligible patients were adults (≥ 65 years) admitted to hospital and diagnosed with COVID-19 pneumonia using the reverse transcriptase polymerase chain reaction (RT-PCR) test for SARS-CoV-2. Patients under 65 years old, those with nosocomial acquisition, and those who had been vaccinated were excluded.
Frailty assessment
Frailty was assessed using the CFS, which is based on the patient’s condition two weeks prior to hospital admission.9 Patients are scored on an ordinal scale from 1 to 9, with the score of 1 indicating that the person is very fit; 2 well; 3 managing well; 4 vulnerable; 5 mildly frail; 6 moderately frail; 7 severely frail; 8 very severely frail; and 9 terminally ill [9, 16]. A single geriatrician retrospectively decided the degree of frailty in all patients by scoring the CFS and combining information from inpatient and outpatient electronic medical records. All borderline cases were adjudicated by another specialist physician in line with our previous study [17] Both physicians were blinded to clinical outcomes.
We did not anticipate an adequate number of events for each score, so we grouped them as 1–4 (no frailty), 5–6 (mild-moderate frailty, with initial signs of frailty but some degree of independence), and 7–9 (severe frailty) for the purposes of the analyses. We also analyzed CFS as a dichotomous variable (no frailty [1–4] versus frailty [5–9]).
Variables and data collection
More in-depth information about the data collection and definition of variables has been provided in papers by the COVID-19 ALC research group [18, 19]. Pre-admission comorbidities were collected from the patient’s electronic medical record. The burden of comorbidities was assessed using the age-adjusted Charlson comorbidity index (CCI), a method of estimating mortality risk from comorbid disease and two-year mortality at a specified time point in longitudinal studies [20].
On admission, laboratory data were collected (total lymphocytes, C-reactive protein, procalcitonin, ferritin, lactate dehydrogenase, D-dimer, interleukin-6, brain natriuretic peptide (BNP), potassium, estimated glomerular filtration rate [eGFR] according to the CKD-EPI equation, troponin). Opacities on chest X-ray were also assessed.
The main outcome variables were: (A) clinical presentation (symptoms), including duration of symptoms prior to admission, fever, dyspnea, dry cough, wet cough, asthenia, diarrhea, myalgia-arthralgia, confusion and anosmia-dysgeusia; and (B) clinical outcomes, including all in-hospital mortality, readmission, admission in ICU, and need for IMV. Confusion was defined according to the Glasgow Coma Scale as a score of less than 14 points. Asthenia was considered present if the patient or their relatives reported weakness or lack of energy and strength. In cases of patients with altered mental status, some conditions, including asthenia, confusion, myalgia-arthralgia, and anosmia-dysgeusia were evaluated using the medical history as related by relatives or caregiver.
Statistical analysis
Categorical variables are expressed as frequencies (percentages) and continuous variables as medians (interquartile range, IQR) or mean (standard deviation). Differences between groups were analyzed using the Mann-Whitney U test for continuous variables and Pearson’s chi-square test for categorical variables. Differences in CFS as dichotomous (no frailty versus frailty) and categorical (no frailty, mild-moderate frailty, and severe frailty) variables were calculated using odds ratios (ORs) with 95% confidence intervals (CIs).
Multivariable logistic regression models were fit to study the association of frailty with initial clinical features and clinical outcomes. In model A, analyses were adjusted for epidemiological variables including age group (65-74 years, 75-84 years, and \(\geq\) 85 years), sex (male/female), residence in nursing home (yes/no), and CCI (0-4 and \(\geq\) 5 points). The analysis of clinical outcomes was additionally adjusted for analytical variables yielding statistically significant results in the bivariable analysis (model B), including: procalcitonin > 0.5 ng/mL, ferritin > 500 mg/L, lactate dehydrogenase > 250 U/L, D-dimers > 1 mg/mL, eGFR < 60 ml/min/m2, troponin T > 14 ng/L, and brain natriuretic peptide > 125 pg/mL
The log-likelihood function was used to determine whether one model fit the data better than another. The log-likelihood ensures that the maximum value of the log probability occurs at the same point as the original probability function. Moreover, to evaluate the goodness-of-fit of the logistic regression model, we used the Cox-Snell R2 and Nagelkerke R2 statistics. The Nagelkerke R2 test is an adjusted version of the Cox-Snell R2 (Supplementary Table 1-3). Both tests measure the proportion of the total variation of the dependent variable that can be explained by independent variables in the current model. The Cox-Snell R2 and Nagelkerke R2 express the explanatory power of the model. All analyses were performed using IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY IBM Corp). Statistical significance was set at p < 0.05.
Ethical aspects
This work was approved by the Institutional Research Ethics of Dr. Balmis General University Hospital – ISABIAL (EXP. 200145). The requirement to obtain informed consent from participants was waived by the Institutional Research Ethics of Dr. Balmis General University Hospital – ISABIAL. The research was conducted according to the principles of the Declaration of Helsinki.
Results
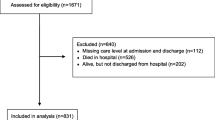
A total of 1745 adults with PCR-confirmed COVID-19 were admitted to Dr. Balmis General University Hospital during the study period. Of these, we excluded 63 patients with a nosocomial infection, 840 who were under 65 years of age, and 55 who were fully vaccinated, leaving a sample of 785.
Table 1 shows the number of patients according to their CSF score. Mean CFS was 4.6 (standard deviation 1.6), and 326 (41.5 %, 95% CI 38.1%–45.0%) patients were defined as frail (CFS ≥ 5 points). Frailty was mild-moderate (5–6 points) in 208 (26.5%, 95% CI 23.5%–29.7%) and severe (7–9 points) in 118 (15.0%, 95% CI 12.7%–17.7%). Prevalence of frailty increased with age: in patients aged 65-74 years, it was 13.8%; 75-84 years, 40.5%; and 85 years or older, 45.7% (p < 0.001). Table 1 shows demographics, comorbidities, laboratory findings, and outcomes according to CFS.
Clinical presentation and frailty
The median duration of symptoms before admission was seven days in non-frail patients, five days in those with mild-moderate frailty, and three days in severely frail patients (p<0.001). In the bivariable analysis, confusion was associated with frailty (no frailty, 7.6%; mild-moderate frailty, 16.7%; and severe frailty, 29.2%; p < 0.001, Table 2). Fever, dry cough, asthenia, diarrhea, myalgias-arthralgia, and anosmia-dysgeusia were significantly less common in patients with frailty, regardless of severity (Table 2). Dyspnea and wet cough were similar between groups.
After adjusting for epidemiological variables, the multivariable analysis (Table 3; supplementary table 1) showed that frail patients had a lower risk of dry cough (OR 0.58, 95% CI 0.40–0.83) and myalgia-arthralgia (OR 0.46, 95% CI 0.29–0.75). This was true for patients with both mild-moderate frailty (OR 0.66, 95% CI 0.45–0.97; OR 0.53, 95% CI 0.32–0.88, respectively) and severe frailty (OR 0.40, 95% CI 0.23–0.67; OR 0.30, 95% CI 0.13–0.70, respectively). Anosmia-dysgeusia were also significantly less common in frail patients (OR 0.46, 95% CI 0.23–0.94), especially in severely frail cases (OR 0.21, 95% CI 0.04–0.95). In contrast, confusion was significantly more common in frail patients (OR 2.01, 95% CI 1.18–3.43), which is also attributable to the severe frailty subgroup (OR 3.14, 95% CI 1.64–5.97). Finally, a duration of symptoms of five days or less prior to admission was more common in patients with severe frailty (OR 1.99, 95% CI 1.17–3.28).
Analytical and radiological parameters and frailty
Regarding analytical and radiological parameters on admission, bivariable analysis showed that frail patients (both mild-moderate and severe frailty) were more likely to show procalcitonin > 0.5 ng, D-dimers > 1 mg/mL, grain natriuretic peptide > 125 pg/mL, eGFR < 60 ml/min/m2, and troponin T > 14 ng/L, along with a lower eGFR. Compared to non-frail patients, a smaller proportion of frail patients had ferritin > 500 mg/L and lactate dehydrogenase > 250 U/L. Parameters showing no relationship with frailty were lymphocytes, C-reactive protein, interleukin 6, potassium, and opacities on more than 50% of lung surface on chest X-ray (Table 1).
After adjusting for epidemiological variables, frailty (both mild-moderate and severe) remained significantly associated with elevated BNP (frail: OR 2.23, 95% 1.23–4.05; mild-moderate frailty: OR 1.97, 95% CI 1.05–3.71; severe frailty: OR 3.51, 95% CI 1.11–11.04) and troponin T (frail: OR 2.70, 95% 1.69–4.32; mild-moderate frailty: OR 2.28, 95% CI 1.39–3.75; severe frailty: OR 5.12, 95% CI 2.04–12.86) on admission. The association with high procalcitonin and D-dimers held only for patients with severe frailty (OR 2.02, 95% CI 1.06–3.84; OR 4.27, 95% CI 2.44–7.84, respectively; Table 4, supplementary Table 2).
Clinical outcomes and frailty
In the bivariable analysis, in-hospital mortality was associated with frailty: 33.6% of all frail patients died (mild-moderate 27.4%, severe 53.4%), compared to 12.6% non-frail patients (p < 0.001, Table 1). Readmission was also higher in frail patients (frail: 10.7% (mild-moderate frailty: 12.1%, severe frailty: 8.0%) compared to the non-frail group (4.8%, p = 0.003, Table 1). On the other hand, admission to the ICU and need for IMV was higher in non-frail patients (21.6% and 14.8%, respectively) compared to those with mild-moderate frailty (6.7% and 5.3%) or severe frailty (0.9% and 0.0%) (p < 0.001, Table 1).
After adjusting for epidemiological and analytical variables (model B), frail patients were more likely than non-frail patients to die during admission (frail: OR 3.74, 95% CI 2.19-6.38; mild-moderate frailty: OR 2.74, 95% CI 1.54-4.49; and severe frailty: OR 9.28, 95% CI 4.52-18.87). Readmission was higher in mild-moderate frailty (model A: OR 2.35, 95% CI 1.17-4.75 and model B: OR 2.35, 95% CI 1.07-5.14) (Table 5, Regardless of the multivariable model used (A or B), admission to the ICU and the need for IMV were similar in non-frail and frail patients (Table 5).
Discussion
This study analyzed the association between frailty according to the CFS and clinical presentation, analytical and radiological parameters, and clinical outcomes in older inpatients with COVID-19. Our results show that frailty is independently associated with an atypical presentation, elevated BNP and troponin T, in-hospital mortality, and readmission.
The prevalence of frailty in people with COVID-19 depends on the scale used and the age of the sample population. Polo et al. [15] reported a prevalence of frailty of 25% in patients aged over 50 years, Tehnari et al. [21] of 50% in patients over 65, and Collins et al. [22] of 66.9% in patients over 65. In our study, 41.5% of the cohort aged 65 or older met CFS criteria for frailty, which is consistent with previous research.
The scant literature on the topic suggests that frailty is associated with atypical clinical presentation in infectious disease [15, 23, 24]. Typical features of COVID-19, like fever and cough, were less prevalent in frail older patients, as reported elsewhere [15, 23], although in our multivariable analysis fever did not show significant, independent differences according to frailty. Dry cough presented less frequently in frail patients, as reported by Poco et al. [15] and Jachymek et al., [24] and this difference was significant after adjusting for confounders. However, our results contrast with other studies showing that this symptom is more common in older, frail patients with COVID-19 [15, 23, 25]
Myalgia-arthralgia was less common in frail patients in multivariable analysis, contradicting other results showing no association [24]. Anosmia-dysgeusia was likewise less common in frail patients, independently of other variables. It is well known that the prevalence of anosmia-dysgeusia decreases with the age of infected patients, as von Bartheld et al. concluded from their meta-analysis. [23] The reduced presence of this symptom in frail patients has been described in fewer studies [24].
Confusion was more prevalent in frail—especially severely frail—patients.This result is consistent with the reduced level of consciousness and higher rates of delirium and confusion reported by other authors [15, 23]. Finally, asthenia, wet cough, and diarrhea were not significantly different after adjusting for epidemiological and analytical variables in frail versus non-frail patients.
Atypical presentation on emergency admission due to COVID-19 in frail older adults may be attributable to several factors. Frailty and chronic inflammation are linked to immunosenescence and can influence response to infection and subsequent immunity [23, 26]. In frail older patients, changes in innate immunity and a defective activation pathway, possibly due to desensitization and a reduced antibody response to vaccines, have already been linked to atypical symptoms [27]. Moreover, older age and frailty are also associated with a higher CCI, which can impair the ability to recognize and report symptoms of the acute disease [25, 26].
After adjusting for epidemiological variables, frail patients were more likely to have elevated BNP and troponin T levels on admission. Moreover, severely frail patients were more likely than non-frail patients to have high procalcitonin and D-dimers. These associations may be attributable to a higher CCI in frail patients, with more cardiovascular comorbidities and concomitant bacterial infections, not specifically assessed in this study. However, we did not observe an association between frailty and markers of inflammation like C-reactive protein, ferritin, and lactate dehydrogenase.
Numerous studies show that frailty is independently associated with mortality, and several meta-analyses have demonstrated that CFS is positively correlated with mortality [28, 29], especially in people over 65 years of age [15, 16, 21, 30, 31]. In our study, mortality was independently correlated with the level of frailty. In addition, evidence suggests that clinical frailty is determined more by the number of dysfunctional organ systems than by abnormalities in any system in particular [29, 32]
Use of life-sustaining therapies (ICU admission, IMV) was more limited in patients with CFS-defined frailty, but after adjusting for epidemiological and analytical variables, these differences were not significant. These finding are similar to reports from a recent meta-analysis of frailty in COVID-19.30 In our study, readmission was also higher in frail patients, especially in those with mild-moderate frailty. Previous studies have noted a higher risk of readmission in frail individuals with multiple chronic diseases after surgical or medical admission [33]. In our study the patients with severe frailty did not have a higher risk of readmission. This may be because severely frail people are more likely to already be under constant and closely supervised care, whether at home or in a nursing home, and in some cases they die in these settings. However, this question was beyond the scope of this study.
Our results should be interpreted in light of the study’s limitations. Frailty was evaluated retrospectively, so calculation of the CFS relied on information obtained from electronic health records [21], which may have resulted in an underestimation of prevalence. However, several published studies also used retrospective methods for assessing the CFS [15, 21]. Furthermore, this is a single-center study, so caution is warranted when extrapolating our results to other healthcare settings. Finally, we do not have data on the variants of SARS-CoV-2 in patients hospitalized with COVID-19 and cannot rule out a reduction in complications and mortality after the first wave.
Conclusion
In this study of COVID-19 in older inpatients in a university hospital in Spain, CFS-defined frailty was independently associated with atypical clinical presentation on admission, with less dry cough, myalgia-arthralgia, and anosmia-dysgeusia, and more confusion after adjusting for confounders. Frailty was an independent prognostic factor for death, while mild-moderate frailty was related to readmission. Further multicenter and prospective studies are necessary to understand the real relevance of frailty in the clinical presentation in old people with COVID-19.
Availability of data and materials
The datasets generated during the current study are not publicly available due data are not publicly available due to privacy or ethical restrictions, but are available from the corresponding author on reasonable request.
Abbreviations
- BNP:
-
Brain natriuretic peptide
- CCI:
-
Charlson comorbidity index
- CFS:
-
Clinical frailty scale
- COVID-19:
-
Coronavirus disease2019
- eGFR:
-
Estimated glomerular filtration rate
- ICU:
-
Intensive care unit
- IMV:
-
Intensive mechanical ventilation
- IQR:
-
Interquartile range
- SARS.CoV-2:
-
Severe acute respiratory distress syndrome coronavirus 2
References
COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Accedido abril 23, 2022. https://doi.org/https://coronavirus.jhu.edu/map.html.
Ramos-Rincon J-M, Buonaiuto V, Ricci M, et al. Clinical characteristics and risk factors for mortality in very old patients hospitalized with COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. 2021;76(3):e28–37. https://doi.org/10.1093/gerona/glaa243.
Andrés M, Leon-Ramirez JM, Moreno-Perez O, et al. Fatality and risk features for prognosis in COVID-19 according to the care approach - a retrospective cohort study. PLoS ONE. 2021;16(3):e0248869. https://doi.org/10.1371/journal.pone.0248869.
Covino M, Russo A, Salini S, et al. Frailty Assessment in the Emergency Department for Risk Stratification of COVID-19 patients aged ≥ 80 years. J Am Med Dir Assoc. 2021;22(9):1845–52.e1. https://doi.org/10.1016/J.JAMDA.2021.07.005.
Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7. https://doi.org/10.1016/j.jamda.2013.03.022.
Kundi H, Wadhera RK, Strom JB, et al. Association of Frailty with 30-Day outcomes for Acute Myocardial Infarction, Heart failure, and Pneumonia among Elderly adults. JAMA Cardiol. 2019;4(11):1084–91. https://doi.org/10.1001/jamacardio.2019.3511.
Hewitt J, Carter B, McCarthy K, et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. 2019;48(3):388–94. https://doi.org/10.1093/ageing/afy217.
Kundi H, Popma JJ, Reynolds MR, et al. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40(27):2231–9. https://doi.org/10.1093/eurheartj/ehz187.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. https://doi.org/10.1503/cmaj.050051.
Moug S, Carter B, Myint PK, Hewitt J, McCarthy K, Pearce L. Decision-making in COVID-19 and frailty. Geriatr. 2020;5(2):30. https://doi.org/10.3390/GERIATRICS5020030.
Boreskie KF, Boreskie PE, Melady D. Age is just a number - and so is frailty: strategies to inform resource allocation during the COVID-19 pandemic. Can J Emerg Med. 2020;22(4):411–3. https://doi.org/10.1017/cem.2020.358.
Zazzara MB, Penfold RS, Roberts AL, et al. Probable delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. Age Ageing. 2021;50(1):40–8. https://doi.org/10.1093/AGEING/AFAA223.
Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J Am Geriatr Soc. 2021;69(9):2419–29. https://doi.org/10.1111/JGS.17299.
Limpawattana P, Phungoen P, Mitsungnern T, Laosuangkoon W, Tansangworn N. Atypical presentations of older adults at the emergency department and associated factors. Arch Gerontol Geriatr. 2016;62:97–102. https://doi.org/10.1016/j.archger.2015.08.016.
Poco PCE, Aliberti MJR, Dias MB, et al. Divergent: age, Frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci. 2021;75(3):e46–51. https://doi.org/10.1093/gerona/glaa280.
Hewitt J, Carter B, Vilches-Moraga A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, european, observational cohort study. Lancet Public Heal. 2020;5(8):e444–51. https://doi.org/10.1016/S2468-2667(20)30146-8.
Ramos-Rincon JM, Moreno-Perez O, Pinargote-Celorio H, et al. Clinical Frailty score vs Hospital Frailty Risk score for predicting mortality and other adverse outcome in hospitalised patients with COVID-19: spanish case series. Int J Clin Pract. 2021;75(10):e14599. https://doi.org/10.1111/IJCP.14599.
Moreno-Pérez O, Andres M, Leon-Ramirez JM, et al. Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: a retrospective cohort study. J Autoimmun. 2020;114:102523. https://doi.org/10.1016/j.jaut.2020.102523.
Moreno -PO, Leon-Ramirez JM, Fuertes-Kenneally L, et al. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 Mediterranean patients. Int J Infect Dis. 2020;100:449–54. https://doi.org/10.1016/j.ijid.2020.09.033.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Tehrani S, Killander A, Åstrand P, Jakobsson J, Gille-Johnson P. Risk factors for death in adult COVID-19 patients: Frailty predicts fatal outcome in older patients. Int J Infect Dis. 2021;102:415–21. https://doi.org/10.1016/j.ijid.2020.10.071.
Collins JT, Short R, Carter B, et al. The clinical Frailty Scale: estimating the prevalence of Frailty in older patients hospitalised with COVID-19. The COPE Study. Geriatr (Basel Switzerland). 2020;5(3). https://doi.org/10.3390/GERIATRICS5030058.
Von Bartheld CS, Butowt R, Hagen MM. Prevalence of Chemosensory Dysfunction in COVID-19 patients: a systematic review and Meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020;11(19):2944–61. https://doi.org/10.1021/ACSCHEMNEURO.0C00460.
Jachymek M, Cader A, Ptak M, et al. The value of clinical Frailty Scale (CFS) as a Prognostic Tool in Predicting Mortality in COVID-19-A Retrospective Cohort Study. Int J Environ Res Public Health. 2022;19(3):1104. https://doi.org/10.3390/IJERPH19031104.
Ruiz LA, España PP, Gómez A, et al. Age-related differences in management and outcomes in hospitalized healthy and well-functioning bacteremic pneumococcal pneumonia patients: a cohort study. BMC Geriatr. 2017;17(1):130. https://doi.org/10.1186/S12877-017-0518-0.
Nidadavolu LS, Walston JD. Underlying vulnerabilities to the Cytokine storm and adverse COVID-19 outcomes in the Aging Immune System. J Gerontol A Biol Sci Med Sci. 2021;76(3):E13–8. https://doi.org/10.1093/GERONA/GLAA209.
Wang GC, Casolaro V. Immunologic changes in Frail older adults. Transl Med @ UniSa. 2014;9:1.
Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis: clinical Frailty Scale in COVID-19. Arch Gerontol Geriatr. 2021;93:1043224. https://doi.org/10.1016/j.archger.2020.104324.
Subramaniam A, Shekar K, Afroz A, et al. Frailty and mortality associations in patients with COVID-19: a systematic review and meta-analysis. Intern Med J. 2022;52(5):724–39. https://doi.org/10.1111/IMJ.15698.
De Smet R, Mellaerts B, Vandewinckele H, Lybeert P, Frans E, Ombelet S, Lemahieu W, Symons R, Ho E, Frans J, Smismans ALM. Frailty and Mortality in hospitalized older adults with COVID-19: Retrospective Observational Study. J Am Med Dir Assoc. 2020;21(7):928–32. https://doi.org/10.1016/J.JAMDA.2020.06.008.
Moledina SM, Maini AA, Gargan A, et al. Clinical characteristics and predictors of mortality in patients with COVID-19 infection outside intensive care. Int J Gen Med. 2020;13:1157–65. https://doi.org/10.2147/IJGM.S271432.
Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. Journals Gerontol - Ser A Biol Sci Med Sci. 2009;64(10):1049–57. https://doi.org/10.1093/gerona/glp076.
Lujic S, Randall DA, Simpson JM, Falster MO, Jorm LR. Interaction effects of multimorbidity and frailty on adverse health outcomes in elderly hospitalised patients. Sci Rep. 2022;12(1):14139. https://doi.org/10.1038/S41598-022-18346-X.
Acknowledgements
We acknowledge the members of the COVID19-ALC Research Group: Esperanza Merino, Joan Gil, Vicente Boix, Ximo Portilla, Oscar Moreno-Pérez, Mariano Andrés, Jose-Manuel Leon-Ramirez, Santos Asensio, Cleofé Fernandez, Alfredo Candela, Mª del Mar García, Rosario Sánchez, Diego Torrus, Sergio Reus, Pilar González, Silvia Otero, Jose M Ramos, Beatriz Valero, Alex Scholz, Antonio Amo, Héctor Pinargote, Paloma Ruiz, Raquel García-Sevila, Ignacio Gayá, Violeta Esteban, Isabel Ribes, Julia Portilla, Cristina Herreras, Alejando Cintas, Alicia Ferradas, Ana Martí, Blanca Figueres, Marcelo Giménez, María-Ángeles Martínez, María-Mar García-Mullor, María Angeles Martínez, Irene Calabuig, Marisa Peral, Ernesto Tovar, M Carmen López, Paloma Vela, Pilar Bernabeú, Ana Yuste, José Ponce, Bertomeu Massuti, Vicente Climent, Vicente Arrarte, Fernando Torres, Laura Valverde, Laura Delegido, Cristina Cambra, Miriam Sandín, Teresa Lozano, Amaya García-Fernández, Alejandro Do Campo, Eduardo Vergara, Nicolás López, Elena Elvira, Fátima López, Fernando Dahl, Blanca Serrano, Sarai Moliner , Carmina Díaz, Dolores Castaño, Beatriz López; Antonio Picó, Joaquín Serrano, Sol Serrano, María Marín-Barnuevo, María Díaz, Cristina Gilabert, Estela Martínez, Elena Vivó, Noelia Balibrea, Miguel Perdiguero, Carolina Mangas, Lucía Medina, Oscar Murcia, María Rodríguez, Rodrigo Jover, Javier López, Marina Morillas, Mercedes Khartabil, Cristina Gil, Carlos Salazar, Eva Vera, Helena López, Vanesa Rodríguez, Sandra Baile, Norma Guerra, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabil, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, Mari-Carmen López-González, Blanca Figueres.
Funding
This work was supported by the Alicante Institute for Health and Biomedical Research (ISABIAL), [grant number 2021-0390].
Author information
Authors and Affiliations
Contributions
J-M.R.-R., and R.S.-M. designed the study, had full access to all data in the study, and takes responsibility for the integrity and accuracy of the data analysis. A.M.-P., O.M.-P, E.L.-M, F.V.-.M., A.A.-l., M.-A.M.-G., E.M., J-M.R.-R., and R.S.-M. contributed to data acquisition and data interpretation. J.-M.R.-R., A.M.-P. O.M.-P, and were responsible for literature search, manuscript writing, and data analysis. J-M.R.-R., and R.S.-M. were responsible for supervision of data collection and data management. All authors reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. J.-M.R.-R. are the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was approved by the Institutional Research Ethics of Dr. Balmis General University Hospital – ISABIAL (EXP. 200145). The requirement to obtain informed consent from participants was waived by the Institutional Research Ethics of Dr. Balmis General University Hospital – ISABIAL.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1
. Summary of the models used in the multivariable logistic regression analysis of symptoms according to Clinical Frailty Scale. Supplementary Table 2. Summary of the models of the multivariable logistic regression analysis of analytical and radiological parameters on admission according to Clinical Frailty Scale. Supplementary Table 3. Summary of the models of the multivariable logistic regression analysis of outcome according to Clinical Frail.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Martí-Pastor, A., Moreno-Perez, O., Lobato-Martínez, E. et al. Association between Clinical Frailty Scale (CFS) and clinical presentation and outcomes in older inpatients with COVID-19. BMC Geriatr 23, 1 (2023). https://doi.org/10.1186/s12877-022-03642-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03642-y