Abstract
Background
There is increasing evidence that pre-frailty manifests as early as middle age. Understanding the factors contributing to an early trajectory from good health to pre-frailty in middle aged and older adults is needed to inform timely preventive primary care interventions to mitigate early decline and future frailty.
Methods
A cohort of 656 independent community dwelling adults, aged 40–75 years, living in South Australia, undertook a comprehensive health assessment as part of the Inspiring Health cross-sectional observational study. Secondary analysis was completed using machine learning models to identify factors common amongst participants identified as not frail or pre-frail using the Clinical Frailty Scale (CFS) and Fried Frailty Phenotype (FFP). A correlation-based feature selection was used to identify factors associated with pre-frailty classification. Four machine learning models were used to derive the prediction models for classification of not frail and pre-frail. The class discrimination capability of the machine learning algorithms was evaluated using area under the receiver operating characteristic curve (AUC), sensitivity, specificity, precision, F1-score and accuracy.
Results
Two stages of feature selection were performed. The first stage included 78 physiologic, anthropometric, environmental, social and lifestyle variables. A follow-up analysis with a narrower set of 63 variables was then conducted with physiologic factors associated with the FFP associated features removed, to uncover indirect indicators connected with pre-frailty. In addition to the expected physiologic measures, a range of anthropometric, environmental, social and lifestyle variables were found to be associated with pre-frailty outcomes for the cohort. With FFP variables removed, machine learning (ML) models found higher BMI and lower muscle mass, poorer grip strength and balance, higher levels of distress, poor quality sleep, shortness of breath and incontinence were associated with being classified as pre-frail. The machine learning models achieved an AUC score up to 0.817 and 0.722 for FFP and CFS respectively for predicting pre-frailty. With feature selection, the performance of ML models improved by up to + 7.4% for FFP and up to + 7.9% for CFS.
Conclusions
The results of this study indicate that machine learning methods are well suited for predicting pre-frailty and indicate a range of factors that may be useful to include in targeted health assessments to identify pre-frailty in middle aged and older adults.
Similar content being viewed by others
Background
Frailty is a geriatric syndrome associated with impairments to multiple interrelated physiological systems. It results in decreased resilience, increased vulnerability to stressors [1, 2], poorer health outcomes [3] and increased morbidity and mortality [4]. It is preceded by pre-frailty, a transitional period where individuals are at a greater risk of hospitalization, disability and death, but are more likely to return to good health than those who’ve already progressed to a frail state [4].
In Australia, approximately 50% of people aged 65 years and older are classified as pre-frail [5]. As the average lifespan increases and the population ages, the number of Australians at risk of becoming pre-frail or frail also rises, contributing to significant morbidity and mortality, as well as increased health care costs [6]. This trend is occurring globally. A recent systematic review found there was a high risk of frailty and pre-frailty in community dwelling adults across 28 countries [7].
Although frailty is often associated with older age it is not a natural or inevitable consequence of growing older [8]. Frailty results from cumulative exposures across the life course [9], so while the prevalence of frailty increases exponentially across the adult lifespan, the precursors can manifest before middle age [10]. During the pre-frailty period there is an opportunity to intervene to reduce adverse outcomes, but early indicators of a transition into frailty are often detected too late [11, 12].
Known physiological factors contribute to pre-frailty and frailty, but the impact of environmental, social and modifiable lifestyle factors on ageing trajectories is less established [13, 14]. A better understanding of the interrelated risk factors contributing to pre-frailty will provide insight to develop more effective primary health care interventions to delay or prevent adverse decline and improve the health and independence of people as they age [15]. There is a growing interest amongst policymakers and health care services to recognise determinants associated with becoming frail earlier and provide preventative interventions [16]. There is some evidence to show the effectiveness of programs to reverse pre-frailty [4]. Strategies to reliably identify and intervene in early stages of pre-frailty will assist community dwelling adults to preserve their independence for longer [6].
Broadly, tools used for early identification of frailty indicators include objective performance-based measures of physical function, such as the Fried Frailty Phenotype (FFP) [17], subjective clinical classifications, such as the Clinical Frailty Scale (CFS) [18], and self-report measures, such as the Edmonton Frail Scale [19]. Historically these tools were utilized with older people after admission to hospitals or residential facilities, but more recently, they have been validated for identifying pre-frailty indicators in middle-aged, community dwelling adults [20].
Increased screening of environmental, social and lifestyle factors contributing to frailty and pre-frailty amongst middle-aged individuals will assist in developing more accurate modelling of predictors of frailty, more efficient clinical practice guidelines, better targeted health care policy for older people, and improved predictions of health service use patterns. However, further investigation of the validity of pre‐frailty criteria measures is needed to determine the applicability for people younger than 65 years.
This study investigates physiological, anthropomorphic, environmental, social and lifestyle factors that may be associated with pre-frailty outcomes, using machine learning (ML) approaches. ML is a well-known computational technique that offers a means to construct accurate and reliable prediction tools, and to identify the key indicators contributing to the risk of pre-frailty and frailty. ML is a branch of artificial intelligence that is used for data analysis and comprises of numerous algorithms capable of learning from the data, identifying patterns and transforming data into knowledge with minimal or no human intervention [21].
Based on the outcomes of a comprehensive set of health assessments, four of the most popular ML algorithms were used to identify differences amongst individuals classified as not frail and pre-frail using the CFS [18] and FFP [17].
Methods
Study design, participants, and features
The Inspiring Health study population comprised of 656 volunteers aged 40–75 years, living independently in one large Australian metropolitan city. The cohort included more women than men (66.8% women) and the average age was 59.9 years (SD 10.6). Details of the participant demographics, study recruitment, inclusion and exclusion criteria, and methodology for data collection has been published previously [15]. Health assessments were performed in accordance with relevant guidelines and regulations.
Participants completed online surveys assessing mental, social and physical health, medical history and health behaviours; and attended an extensive health assessment session to collect objective measures of anthropometry and physiological measures. A complete list of the health assessments collected as part of the Inspiring Health study, and the management of these assessments have been reported [15].
A summary of the cohort characteristics, health measures and assessments used in the analysis is outlined below (Table 1). A detailed description of how these features were defined is also provided in Additional file 1: Appendix A.
Outcome measures
The outcome measures used to categorise not frail and pre-frailty was the CFS and FFP. As it was possible to collect objective measures of physical function, self-reported measures of frailty were not collected in this project. Used exclusively by trained health professionals, the CFS categorises individuals across a nine-point scale: Very Fit (1), Well (2), Managing well (3), Vulnerable (4), Mildly frail (5), Moderately Frail (6), Severely Frail (7), Very Severely Frail (8), and Terminally Ill (9) [18]. The first six CFS categories were used for the Inspiring Health assessments: very fit, well, managing well, vulnerable, mildly frail, and moderately frail. Due to small numbers in each category and for simple implementation and analysis, individuals identified as ‘very fit’ and ‘well’ were categorised as not frail, and those considered to be ‘managing well’ and ‘vulnerable’ were categorised as pre-frail. Individuals categorised as ‘mildly frail’ and ‘moderately frail’ were categorised as frail and excluded from the subsequent analysis. This classification of pre-frailty was in part determined by a recent guide from the CFS developers which expands on the definitions of the managing well and vulnerable categories. Managing well includes people with controlled medical problems who are inactive, and the vulnerable category (now renamed to be ‘living with very mild frailty’) includes people who experience symptoms that limit their activities [22]. These categories have been interpreted to equate to an indication of pre-frailty.
The second outcome measure, the FFP, assesses individuals on five criteria [17]:
-
1.
Self-reported unintended weight loss of 4.5 kg or 5% of body mass in the last year.
-
2.
Self-reported exhaustion, reporting most or all of the time in response to the question: “About how often did you feel tired out for no good reason?”
-
3.
Self-reported low levels of physical activity (less than the median recommended amount of time spent walking per week, and no moderate or vigorous intensity physical activity each week)
-
4.
Observed slower than standardised values of Australians gait speed, measured using the individual 6MWT
-
5.
Observed sitting dominant handgrip strength below 10.th percentile normative values (derived from British population studies)
Participants who scored 0 on the FFP scale were categorised as not frail. If they met 1 or 2 criteria; they were categorised as pre-frail. Due to low numbers, individuals who scored 3 or greater were excluded as frail.
Missing values, final participant numbers, features and outcome measures
Out of the original 656 participants, 25 individuals were removed from the cohort due to missing outcome data (either FFP or CFS). This led to a sample of 631 (656 – 25) participants. In addition, as the frailty numbers were very small (n = 15), individuals categorised as frail according to the FFP (identified as exhibiting 3 or more of the criteria for frailty) were removed that from the analysis. This resulted in 616 (631—15) participants for the analysis. For CFS, the number of individuals in each category were: not frail (n = 446), pre-frail (n = 170), and for FFP: not frail (n = 378), pre-frail (n = 238).
The dataset contains 79 input features in addition to the two outcome measures (CFS and FFP). One input variable (employment status) was excluded from the analysis due to greater than 50% missing data. Missing values for the remaining 78 features were imputed using missRanger algorithm which predicts both continuous and categorical missing values using Random Forest algorithm trained on the observed data [23].
Feature selection
Not all features have significant class discrimination information, and using multivariate high dimensional data is computationally expensive. Feature selection methods can help to remove the irrelevant and redundant features. This could reduce the computational time and improve classification performance. In this study, a popular feature selection method was used: correlation-based feature selection with best fit search method [24]. This feature selection finds the feature subset that shows high correlation with the class label and less correlation with other features. It ignores the irrelevant and redundant features. The selected features were ranked using random forest algorithm according to their contribution to classification.
Machine learning models
There are three forms of ML: supervised, unsupervised and reinforcement. Supervised models utilise training data with inputs and the desired output labels, while unsupervised models use data without output labels. Reinforcement learning is based on the analysis of past outcomes and feedback. In this study, four widely accepted supervised ML models were used: logistic regression (LR) [25], linear discriminant analysis (LDA) [26], support vector machine with Radial Basis Function (SVM) [27], and random forest (RF) [28]. LR and LDA are two linear classification methods while SVM and RF are more sophisticated ML models that support nonlinear relationships. Most modern deep learning models could not be used for this analysis due to the relatively small size of the dataset.
LR uses a simple classification approach that uses a linear equation with one or more independent features to predict a binary dependent variable. The predicted values are mapped to probabilities using the sigmoid function. LDA finds the orientation vector that maximizes the distance between the two classes after standardizing for within class variance. SVM classifiers determine the optimal separating hyperplane that separates the data into 2 classes with maximum distance margin between both classes. Lastly, RF, a popular ensemble classification method, combines multiple learning algorithms to achieve better performance. RF builds large number of individual decision trees and obtain vote from each tree and classifies the data using majority vote.
Software
The experimental codes were implemented using Python, RStudio and Weka. Missing data imputation was done in the R 3.6.1 using the missRanger package. Feature selection done in Weka using weka.attributeSelection method, standarisation of features and ML algorithms (LR, LDA, SVM and RF) were implemented using Scikit-learn library python.
Performance measures
The performance of the ML models was evaluated using area-under-curve (AUC) score, sensitivity, specificity, precision, F1-score and accuracy. The optimal classification threshold was found from the receiver-operating-curve (ROC), which showed the greatest difference in sensitivity and specificity.
Experimental setting
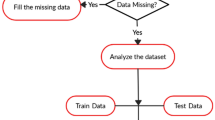
An overview of the ML approach used in this study is reported in Fig. 1. After missing values imputation, the input features values were standardised to ensure each feature had the same influence on the cost function in designing the ML models. Then the data was shuffled and stratified K-fold cross validation approach was used to optimise the models’ robustness and generalisation. The stratified K-fold cross validation approach divides the data randomly into K subsets/folds of roughly equal sizes ensuring each fold has the same proportion of outcome class values. Then the model is trained using K-1 folds and tested with left out Kth fold. This process is repeated K times, with each fold serving as a testing fold at some point. In this study, we have used tenfold cross validation (K = 10) and the reported values are the median of tenfold cross validation. We have used median as they are robust to outlying predictions.
Results
Feature analysis
While analysing 78 features, a combination of 5 features was identified for FFP classification while a combination of 14 features was identified for CFS classification (not frail vs pre-frail).
Lower 6MWT values, poor grip strength, unintentional weight loss, distress, and housing type (for example, living in a house rather than renting a room or living in a low-level care residence) were the selected prominent features for FFP. Having higher body mass index (BMI) scores, lower 6MWT, inactivity, distress, poor balance and functional mobility scores, shortness of breath, lower multiple health conditions, and limited endurance (assessed with stair climbing) were the selected prominent features for CFS. Table 2 shows the most prominent feature subset selected.
The features relating to assessments included as part of the FFP scale were unsurprisingly prominent in the preliminary investigations. However, the identification of health features not included in the FFP assessments suggested pre-frail adults may exhibit a wider array of physical, global health and social indicators of frailty than those included in the FFP. To identify additional, more subtle indicators of pre-frailty, the performance of four ML models for predicting pre-frailty were retested with a refined dataset of variables. The 15 variables used to assess frailty in the FFP tool were removed from the dataset. Nine of these variables related to levels of activity (hours and minutes spent gardening, in vigorous activity, moderate activity and walking, and total minutes of exercise), three related to grip strength (in right and left hands and combined) and one each measured unintentional weight loss, 6MWT scores and a measure of distress (K9).
While analysing the reduced feature set of 63 features, a combination of 20 features were identified for FFP, and a combination of 19 features were identified for CFS. Table 3 outlines the most prominent feature subset selected from the sub analysis of 63 features. With the FFP related features removed, the features associated with a FFP classification of pre-frailty were much broader. Poor balance, lower levels of functional mobility, impaired cognitive function and lung function, pain, low quality sleep, dental problems, higher BMI and consumption of alcohol were new factors identified as predictors for pre-frailty. Higher K10 scores and housing remained as predictors (Table 3).
For the CFS, impaired cognitive function and lung function, low quality sleep, mode of transport, higher fat mass and lower muscle mass, and dental problems were new factors identified as predictors for pre-frailty. Multiple health conditions, poor balance, lower levels of functional mobility, higher K10 scores (indicating higher levels of distress), higher BMI, lower endurance (assessed with stair climbing) and housing remained as predictors. Inactivity was no longer associated with being classified as pre-frail as many of the activity variables were removed.
Prediction accuracy
Table 4 reports the prediction accuracy according to discrimination (AUC score) for each of the four ML models using the selected features and all 63 features for both FFP and CFS. While using all 63 features, random forest achieved the highest AUC scores of 0.701 and 0.776 for FFP and CFS, respectively. All ML models achieved significant improvements in discrimination while using selected features compared to the 63 feature set (from + 2.1% for the random forest model to + 7.4% for support vector machine for FFP and from + 2.4% for random forest to + 7.9% for support vector machine for CFS).
Classification analysis
Using 63 features, the ML models achieved up to 71% accuracy, 82% specificity, 63% sensitivity, 62% precision and an F1-score of 62% for FFP (Table 5). In comparison, the ML models with selected features achieved up to 71% accuracy, 88% specificity, 61% sensitivity, 75% precision, and an F1-score of 61%. The number of subjects correctly classified as not frail (specificity), and the proportion of subjects correctly classified as pre-frail (precision) were significantly better with selected features.
For CFS, using 63 features, the ML models achieved up to 74% accuracy, 81% specificity, 74% sensitivity, 53% precision and an F1-score of 58%. In comparison, the ML models with selected features had a higher accuracy (79%), specificity (82%), sensitivity (79%), precision (61%) and F1-score (62%).
Discussion
The results of this study indicate that machine learning methods are well suited for predicting pre-frailty. The ML analysis identified key health assessment measures that contribute to the shift between not frail and pre-frail, as defined using the FFP and CFS. For the final model with 63 features, random forest achieved the highest AUC score of 0.722 for FFP and logistic regression achieved the highest AUC score of 0.817 for CFS. All ML models achieved significant improvements in discrimination while using selected features compared to the 63 feature set. For classification, comparing the 63 feature set to the selected features, for the FFP, the specificity and precision of the selected feature models were significantly better, and for the CFS, selected features models had higher accuracy, specificity, sensitivity, precision and F1-scores.
For feature analysis, when considering all assessment of health (78 features), pre-frailty was most prominently predicted with a range of physical assessments, including 6MWT, grip strength assessments, BMI, mental health, inactivity and functional mobility. Balance, weight loss, shortness of breath and endurance were also identified as factors. For the FFP, the strength of the 6MWT, accounting for 50% of the predictive power, demonstrates the importance of walking speed as a measure of pre-frailty. This finding is supported by the wider literature, walking speed has been shown to be a strong predictor of future health and frailty [29, 30]. Housing was the only nonphysical assessment and that feature accounted for less than 1% of the predictive value. The ML models were able to achieve significantly high predictive accuracy (AUC scores of 0.916 and 0.861 (Additional file 2: Appendix B) for FFP and CFS, respectively), but the findings of these models can only confirm what is already understood about the correlation between physical criteria and frailty. Thus, these preliminary models were superseded by the subsequent analysis with 63 features.
When the 15 FFP assessment features were removed, a broader range of physical factors were associated with pre-frailty, including impaired cognitive function, pain, poor sleep quality, lung function, dental health, pelvic floor distress, higher fat mass and lower muscle mass. As well social indicators associated with pre-frailty were identified, including alcohol consumption, type of housing, source of income and mode of transportation. No single feature accounted for more than 16% of the predictive value.
A function of using the correlation-based feature selection algorithm is that when features with similar correlation with the outcome variable are identified, one variable is dropped from the analysis. In this dataset, the measures of the functional mobility scale were highly correlated, and it is likely that when a variable measuring functional mobility was selected for a model, another functional mobility variable was excluded [24]. This has had no meaningful real impact on the model, but we advise the reader that the left and right leg or arm differences are not likely to be important for interpreting the reported results.
Indicators of balance, stability and strength, and selected anthropomorphic measures (muscle mass and waist, hip circumference) appeared across all models, suggesting greater relative importance of physiological measures in predicting pre-frailty. The K10 scale was the only assessment to be identified across all models to identify a pre-frail classification for the ML analysis. Previous studies have reported similar associations between mental health and pre-frailty in younger people. A Switzerland study found depression to have prognostic value when identifying pre-frailty in people defined as the “youngest old” (aged 65–70 years) [31]. The connection between depression or depressive symptoms in pre-frail individuals has only recently been investigated. Studies report a significant association between depression and pre-frailty and depressive symptoms are associated with an increased risk of becoming pre-frail [32].
The appearance of social and psychological factors associated with pre-frailty suggest the factors contributing to pre-frailty for this cohort are more holistic and complex than age related health decline alone. The relationship between physical frailty and sociodemographic factors has been established, highlighting the importance of collecting social and quality of life measures in investigations of frailty in older adults [33]. Our analysis found age was not an important predictor of classification as not frail and pre-frail. This can be attributed to the nature of the fitted models. Age is likely highly correlated with many of the other variables and, as a result of the feature selection process, was not selected as a feature.
A previous publication reported the results of a factor analysis utilising the Inspiring Health data set. Gordon et al. (2020) investigated factors associated pre-frail and frail independent community-dwelling adults, as determined by the FFP assessments [15]. The outcomes of the factor analysis, based on 25 binary frailty measures, were compared to the outcomes of the ML models derived from 63 variables (Fig. 2). Variables identified to be significantly associated with pre-frailty using a factor analysis approach included poor balance, stability and strength, incontinence, and poor nutrition. In comparison, the ML model identified anthropometric, environmental, social and lifestyle variables in addition to the physiologic ones. This may suggest ML approaches can expose more subtle causal mechanisms that could not been identified with factor analysis.
There is currently no standard screening test for frailty or pre-frailty. Various tools have been developed for a variety of settings and populations [34]. ML enables the development of a more complex modelling strategy, as well as automation and adaptation. It offers an alternative to traditional forms of analysis that allow for a greater number of features to be considered in investigations. ML has increasingly been used in recent years to predict frailty risk in older populations as this approach is ideal for complex, nonlinear clinical problems [35]. Our ML application to detect not frail and pre-frail populations provides greater information about functional decline and the pre-frailty trajectory.
Our ML models were derived from a dataset of 616 individuals. This sample is sufficiently powered for ML modelling approaches, but additional analysis with larger, external data set is required to support our findings and test the validity and generalizability of the models. Due to the small number individuals categorised as frail by the FFP scoring, frail people were removed from the ML analysis. Few frail volunteers are to be expected, given the cohort sample considered of community-dwelling individuals who were fit enough to participate in extensive health assessments. However, the researchers who recruited the Inspiring Health cohort have indicated the sample is generalisable to other urban Australians in terms of age, gender, socioeconomic index, and proportion of people classified as pre-frail [15].
The Inspiring Health study was limited in the number of social determinants that could be reasonably collected. Hence the outcomes may have been biased by the limited psychological testing included in the assessments. A greater range of environmental, social and lifestyle risk indicators should be considered in future Australian ageing cohorts.
Conclusion
ML has identified sets of indicators that contribute to a classification of not frail or pre-frail. The indicators that performed highly (AUC: 0.722) based on the FFP classification included higher BMI, lower dexterity, greater distress, poorer functional mobility and balance, pelvic floor bother and shortness of breath. Self-reported CFS classification performed highly (AUC: 0.817) in cases characterised by a higher BMI and fat mass, decline in muscle mass and functional mobility, greater distress, poorer balance, shortness of breath and pelvic floor bother. The use of ML has identified different categorisations between not frail and pre-frail participants than previous factor analysis which may suggest ML approaches can expose more subtle casual mechanisms that were not identified with factor analysis.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FFP:
-
Fried Frailty Phenotype
- CFS:
-
Clinical Frailty Scale
- ML:
-
Machine Learning
- 6MWT:
-
Six Minute Walk Test
- LR:
-
Logistic regression
- LDA:
-
Linear discriminant analysis
- SVM:
-
Support vector machine
- RF:
-
Random forest
- AUC:
-
Area-under-curve
- ROC:
-
Receiver-operating-curve
- FMS:
-
Functional mobility score
- BMI:
-
Body mass index
- RBF:
-
Radial basis function
- PDT:
-
Purdue Dexterity Test
- PSQI:
-
Pittsburgh Sleep Quality Index
References
Fried LP. Interventions for human frailty: physical activity as a model. Cold Spring Harb Perspect Med. 2016;6(6):a025916.
Hogan DB. Models, definitions, and criteria for frailty. Conn’s handbook of models for human aging. 2018. p. 35–44.
Darvall JN, Bellomo R, Paul E, Subramaniam A, Santamaria JD, Bagshaw SM, Rai S, Hubbard RE, Pilcher D. Frailty in very old critically ill patients in Australia and New Zealand: a population-based cohort study. Med J Aust. 2019;211(7):318–23.
Frost R, Belk C, Jovicic A, Ricciardi F, Kharicha K, Gardner B, Iliffe S, Goodman C, Manthorpe J, Drennan VM, Walters K. Health promotion interventions for community-dwelling older people with mild or pre-frailty: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):1–3.
Thompson MQ, Theou O, Karnon J, Adams RJ, Visvanathan R. Frailty prevalence in Australia: findings from four pooled Australian cohort studies. Australas J Ageing. 2018;37(2):155–8.
Burgess MS, Hercus C. Frailty in community dwelling older people–using frailty screening as the canary in the coal mine. 2017.
Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, Gasevic D, Ademi Z, Korhonen MJ, LoGiudice D, Bell JS. Global incidence of frailty and pre-frailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198398.
Taylor D, Barrie H, Lange J, Thompson MQ, Theou O, Visvanathan R. Geospatial modelling of the prevalence and changing distribution of frailty in Australia–2011 to 2027. Exp Gerontol. 2019;123:57–65.
Kojima G, Liljas AE, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23.
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94.
de Vos AJ, Asmus-Szepesi KJ, Bakker TJ, de Vreede PL, van Wijngaarden JD, Steyerberg EW, Mackenbach JP, Nieboer AP. Integrated approach to prevent functional decline in hospitalized elderly: the Prevention and Reactivation Care Program (PReCaP). BMC Geriatr. 2012;12(1):7.
Beaton K, McEvoy C, Grimmer K. Identifying indicators of early functional decline in community-dwelling older people: a review. Geriatr Gerontol Int. 2015;15(2):133–40.
The Academy of Medical Sciences. Influencing the trajectories of ageing: summary of a FORUM symposium held on 16 September 2016. https://acmedsci.ac.uk/file-download/42128-58521f0caf63a.pdf
Freer K, Wallington SL. Social frailty: the importance of social and environmental factors in predicting frailty in older adults. Br J Community Nurs. 2019;24(10):486–92.
Gordon SJ, Baker N, Kidd M, Maeder A, Grimmer KA. Pre-frailty factors in community-dwelling 40–75 year olds: opportunities for successful ageing. BMC Geriatr. 2020;20(1):1–3.
Gwyther H, Shaw R, Dauden EA, D’Avanzo B, Kurpas D, Bujnowska-Fedak M, Kujawa T, Marcucci M, Cano A, Holland C. Understanding frailty: a qualitative study of European healthcare policy-makers’ approaches to frailty screening and management. BMJ Open. 2018;8(1):e018653.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–57.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95.
Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, Matthews S, Rolfson DB. The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28(4):182–8.
Gordon S, Grimmer K, Baker N. Do two measures of frailty identify the same people? An age-gender comparison. J Eval Clin Pract. 2020;26(3):879–88.
Alpaydin E. Introduction to machine learning. Cambridge: MIT press; 2009.
Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23(3):210.
Mayer M. “Package ‘missRanger’.” 2019.
Hall MA. Correlation-based feature selection for machine learning. 1999.
Hosmer Jr DW, Lemeshow S, Sturdivant RX. Applied logistic regression. Hoboken: Wiley; 2013;398.
Mika S, Ratsch G, Weston J, et al. Fisher discriminant analysis with kernels. In: Neural networks for signal processing IX: proceedings of the 1999 IEEE signal processing society workshop. 1999. p. 41–8.
Suykens JA, Vandewalle J. Least squares support vector machine classifiers. Neural Process Lett. 1999;9(3):293–300.
Breiman L. Random forests. Mach Learn. 2001;45(1):5–32.
Fritz S, Lusardi M. White paper:“walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):2–5.
Woo J. Walking speed: a summary indicator of frailty? J Am Med Dir Assoc. 2015;16(8):635–7.
Danon-Hersch N, Rodondi N, Spagnoli J, Santos-Eggimann B. Prefrailty and chronic morbidity in the youngest old: an insight from the Lausanne cohort Lc65+. J Am Geriatr Soc. 2012;60(9):1687–94.
Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–72.
de Labra C, Maseda A, Lorenzo-López L, López-López R, Buján A, Rodríguez-Villamil JL, Millán-Calenti JC. Social factors and quality of life aspects on frailty syndrome in community-dwelling older adults: the VERISAÚDE study. BMC Geriatr. 2018;18(1):1–9.
Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark CA. The Identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–38.
Babič F, Majnarić LT, Bekić S, Holzinger A. Machine learning for family doctors: a case of cluster analysis for studying aging associated comorbidities and frailty. In: International cross-domain conference for machine learning and knowledge extraction. Cham: Springer; 2019. p. 178–94.
Acknowledgements
Not applicable.
Funding
This study was supported by internal grant funding from Flinders University and Aged Care Housing Group, South Australia, and who co-fund the Chair of Restorative Care in Ageing, occupied by Professor Susan Gordon.
Author information
Authors and Affiliations
Contributions
SS, SC, AM and SG contributed to the conception and design of the investigation. SS conducted the machine learning analysis. SS and SC drafted the original manuscript. SS, SC, AM and DG were involved in the review and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained in 2017 from the Southern Adelaide Local Health Network (South Australia; 391.16 and 407.16). This paper conforms to the principles embodied in the Declaration of Helsinki. Written informed consent was obtained from all Inspiring Health participants before the objective assessment components of the study and the return of online surveys was considered implied consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sajeev, S., Champion, S., Maeder, A. et al. Machine learning models for identifying pre-frailty in community dwelling older adults. BMC Geriatr 22, 794 (2022). https://doi.org/10.1186/s12877-022-03475-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03475-9