Abstract
Objectives
Frailty and sarcopenia have been related with adverse events, including hospitalization. However, its combined effect with hospitalization-related outcomes, including costs, has not been previously investigated. Our purpose was to explore how frailty, sarcopenia and its interaction could impact on healthcare expenditures.
Methods
1358 community-dwelling older adults from the Toledo Study of Healthy Ageing (TSHA) were included. Sarcopenia was measured using the Foundation for the National Institutes of Health criteria fitted to our cohort. Frailty was defined according to Frailty Trait Scale 5 (FTS5) and the Frailty Index fitted to the cut-off points of TSHA population. Hospitalization costs were taken from hospital records and costs were attributed according to Diagnostic-Related Groups, using as the cost base year 2015. Two-part regression models were used to analyze the relationship between frailty and sarcopenia and hospital admission, number of hospitalizations, length of stay and hospitalization costs.
Results
Sarcopenia was associated only with the probability of being admitted to hospital. Frailty was also associated with higher hospital use, regardless of the frailty tool used, but in addition increased hospital admission costs at follow-up by 23.72% per year and by 19.73% in the full model compared with non-frail individuals. The presence of sarcopenia did not increase the costs of frailty but, by opposite, frailty significantly increased the costs in people with sarcopenia, reaching by 46–56%/patient/year at follow-up. Older adults with frailty and sarcopenia had a higher risk of hospitalization, disregarding the tool used to assess frailty, and higher hospitalization costs (FTS5) in the full model, at the cross-sectional and at the follow-up level.
Conclusions
Frailty is associated with increased hospitalization costs and accounts for the potential effects of sarcopenia.
Similar content being viewed by others
Introduction
Healthcare systems are suffering an increasing burden in terms of a heavier demand due to chronic health diseases accompanying the ageing of the population. Since 1980, people aged 65 years and older has almost tripled, and prospective studies expected these population to double by 2050 [1]. Although healthcare expenditures (HCE) increase as people age, age itself is considered a distraction from the true drivers of spending [2, 3], like physical debilitating conditions [4]. This is also true for those who are not heavily disabled: older adults with moderate functional limitations are expected to live for longer periods of time and with greater use of medical care than most older adults with disabilities, increasing in an exponential way as their frailty status worsen [5].
Frailty and sarcopenia, two highly prevalent conditions in older people, are characterized by physical function impairment with a high potential risk of disability [6, 7]. Frailty is defined as an age-associated, biological syndrome characterized by decreased biological reserves of the individual, increasing the risk of adverse events when facing minor stressors [7]. On the other hand, sarcopenia is defined as a loss of lean mass, strength and/or function usually associated with ageing [8]. These entities are closely related to chronic disease, a high prevalence of polypharmacy [9, 10] and adverse events, such as falls and poor quality of life [11,12,13] In addition, it has been proposed that older people with frailty or sarcopenia are more likely to be hospitalized, augmenting health service costs [4, 14,15,16]. Furthermore, frailty and sarcopenia could be reverted [17,18,19,20], and the cost associated to them avoidable, thus preventing imminent future society demands.
Frailty and sarcopenia have been related between them [21], could coexist, but are two different conditions [22]. In addition, the lack of a universal gold standard for frailty assessment has allowed a wide spectrum of frailty assessment tools that focus on different characteristics of subjects with a poor agreement between them [23,24,25]. The Frailty Index is one of the most used frailty tools inside the “cumulative deficit” approach to frailty. It is constructed through a long checklist of symptoms, signs, diseases, disabilities, laboratory, radiographic or electrocardiographic abnormalities and social characteristics [26, 27] and even health utilization [28]. The Frailty Trait Scale 5 (FTS5) is an instrument stemming from the frailty phenotype conceptual framework [13] with a predictive ability of adverse events in community-dwelling older adults that could improve the FI one and aiming to overcome some of its drawbacks [29]. It includes objective functional measures, and it has proven to be highly dynamic and sensitive to changes with different risks of adverse events as mortality, hospitalization and disability associated to its score [30]. Although there are some studies that have already supported the increasing economic burden of frailty and sarcopenia in terms of healthcare costs in older populations [4, 31,32,33,34], to the best of our knowledge, there is no study that has analyzed the economic impact of these two prevalent conditions separately and their interaction on costs related to hospitalizations in older adults. This gap in the literature has motivated the purpose of this study, motivating the purpose of our research.
Methods
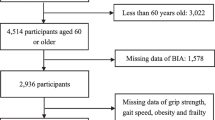
Participant data were taken from the Toledo Study of Healthy Ageing (TSHA). TSHA is a longitudinal cohort that was designed to analyze different models of frailty and ageing and to measure the impact that frailty, comorbidity and disability may have on health system by assessing rural and urban community dwelling older adults aged 65 years or older [35]. TSHA was performed in concordance with the Helsinki Declaration of 1975 and was approved by the Clinical Research Ethics Committee of the Toledo Hospital in Spain. Participants signed an informed consent form. Visits were performed in the second wave of the study (basal visit) between 2011 and 2013 and the outcomes were collected during the third wave (until December 2015).
Measures of frailty
Frailty was measured using Frailty Index (FI) and Frailty Trait Scale 5 (FTS5).
Frailty Index: characterizes frailty as an accumulation of deficits. It is based on the ratio between deficits present in the individual and the total measured deficits [36]. Subjects were considered frail if the score was ≥0.275. The TSHA FI was built following Searle et al., using 40 items with scores of 0 to 1 [37]. TSHA FI scoring tool is displayed in the Supplementary Information Table S1.
Frailty Trait Scale 5 [29]: a Short Form of the 12 items one [38] is a continuous scale that monitor frailty status in a range between 0 to 50. Each domain score range between 0 to 10. Subjects were considered as frail if their score were > 25 and no frail ≤25 [29]:
-
Physical Activity: defined using the PASE scale [39].
-
Gait speed: was defined using the 3-m walking test at their usual pace, according to the standard protocol. Best time of two performances was chosen.
-
Hand grip strength: was measured using JAMAR Hydraulic Hand Dynamometer (Sammons Preston Rolyan, Bolingbrook, IL). Best peak strength of three performances was selected and gathered using international standard procedures [40]. Between performances, at least 1 min of resting was permitted.
-
Body Mass Index: was measured according to the standard recommendation (weight/height2).
-
Balance test: was stablished according to the standing balance feet together, semi-tandem, and tandem position (progressive Romberg balance test) [41].
FTS5 scoring tool is displayed in the Supplementary Information Table S2.
Sarcopenia
Sarcopenia was measured at baseline and was calculated according to the Foundation for the National Institutes of Health (FNIH), but fitted to the cut-points of our population (standardized FNIH [sFNIH]) [22]. According to this definition, sarcopenia is present when the low grip strength, low muscle mass and low gait speed criteria are met.
Muscle mass was determined using Dual-Energy X-ray Absorptiometry (DEXA) (Hologic, Serie Discovery QDR, Bedford, MA, USA). DXA scans were analyzed using the software Physician’s Viewer (apex System Sofware, version 3.1.2: Bedford, USA). BMI- adjusted by Appendicular Lean Mass (ALM/BMI), derived as the sum of the muscle mass of the arms and legs, it was used as marker of low lean mass. According to sFNIH diagnosis algorithm, low muscle mass was considered in men and women when ALM/BMI is below 0.65 and 0.54 respectively.
Gait speed and handgrip strength measurement methodologies were explained above. Gait speed cut-off point was < 0.8 m/s and handgrip strength cut-off points were < 25.51 kg for men and < 19.19 kg for women.
Hospitalization
Hospitalization after recruitment was defined as first admission and was registered reviewing the records of Toledo Hospital Complex. Participants’ hospitalizations were followed-up to December 2015 with a median of 167 weeks (36.43 weeks SD).
Hospitalization is composed of four measures: having been admitted to hospital which takes value 1 if the respondent has been hospitalized in the previous 12 months and 0 otherwise, number of hospitalizations per year, average length of stay in days per year and hospitalization costs in euros per year. In order to estimate hospitalization costs, we used data from each survey respondent at baseline and during the follow-up period from hospital clinical records and we subsequently estimated the costs per person and per year in €. The unit costs per each Diagnostic Related Group (DRG), which were obtained from national data sources, were multiplied by the number of hospital admissions for each DRG. All costs were updated, if applicable, and expressed in 2015 euros.
Covariates
Age and gender were self-reported. Comorbidities were ascertained by the Charlson Index Score [42]. Polypharmacy was defined as the intake of ≥5 drugs/day [43]. All covariates were measured at the baseline visit (between 2011 and 2013).
Study variable and statistical analysis
Given the substantial proportion of zeros within the number and costs of hospital admission, as well as the length of stay, which led to a skewed distribution of the data, two-part regression models will be run for such outcomes, which assume a first part for modelling the probability of being admitted to hospital and a second part on the positive continuous outcome only on those who have been admitted to hospital.
Two-part models combine a model for the binary response variable, which would take value 1 if the individual has been admitted to hospital at least once and 0 if no hospitalizations, and a model for the outcome variable that is conditioned on the binary response [44], conditional on having been admitted to hospital.
The first stage of the two-part model was performed as a traditional logit regression model, in which the estimated coefficients capture the effects on the log-odds-ratio [45].
The second stage involves a Generalized Linear Model (GLM) with a gamma distribution in case of hospitalization costs with a log link and a zero-truncated Poisson distribution for the number of hospitalizations and length of stay, in days.Footnote 1 GLM models have frequently been used for healthcare costs analysis recently, given the skewed distribution of costs [47]. GLMs are empirical transformations of the classical ordinary least square (OLS) regression model, which specify the conditional mean function directly. Specifically, GLMs do not require transformation scales, but a response distribution of one of the exponential family of distributions (normal, Poisson, gamma, binomial, inverse Gaussian), which relates the mean of the response to a scale on which the model effects combine additively [48]. As previously stated, according to the Modified Park Test, the chosen family was the Gamma distribution for modelling total hospitalization costs and the Poisson distribution for the other two continuous outcomes in our analysis [49].
Several regression models have been run. The first regression model only includes one of the main variables of interest (sarcopenia or frailty). In a second regression model, age and its square, and gender have been included. Model three adds to Model two the other variable of interest (sarcopenia or frailty, as applicable). Models four adds the comorbidity severity of individuals according to the Charlson Index, which is medium-low if the Charlson Index score is 1 or 2; and high if the Charlson Index score is three or higher. Moreover, polypharmacy is also included if the daily number of drugs the subject is taking is 5 or more. We also perform the likelihood ratio test in order to detect overfitting after the inclusion of new variables in the nested models.
Two assessments will be made regarding the association between frailty and sarcopenia and hospitalization outcomes: the first analysis will entail the associations within the same wave (outcome and main variables of interest measured in wave 2), whereas the second analysis will consider outcomes at follow-up, wave 3, and the independent variables evaluated in wave 2. The sample size will be reduced at follow-up assessment from 1358 individuals to 1349 older adults due to mortality, during the hospital admission (n = 9).
Results
Participants´ characteristics classified depending on frailty status according to different frailty tools and sarcopenia are shown in Table 1. Prevalence of frailty are 12.42% (FI) and 10.38% (FTS5). In our sample, 22.31% were diagnosed with sarcopenia.
Regardless of the tool used to assess frailty, in the bivariant analyses frail individuals seem to be older, more likely to have sarcopenia, and to be in worse health status (higher Charlson index value). Moreover, they seem to be more prone towards polypharmacy. Older adults with sarcopenia presented similar characteristics to those with frailty.
Table 2 reports the differences in hospitalization-related outcomes, showing a higher number of hospital admissions, longer stays, and higher hospitalization costs among older adults with frailty and with sarcopenia, compared to their counterparts.
Results from the regression analyses
Table 3 shows that sarcopenia, when it is the only independent variable, is associated with an increased risk of being admitted to hospital both at the cross-sectional level (OR 1.56) and at follow-up (OR 1.47). However, it is not significantly related to the other hospital-related outcomes. Such associations were observed in the second regression model, when age and gender were considered (OR 1.48 in the cross-sectional analysis and OR 1.76 at follow-up), but only remained at follow-up when frailty entered the analysis (Model 3). In the fourth regression model, sarcopenia was only significantly associated with higher odds of being admitted to hospital at follow-up (OR 1.46) when frailty is evaluated by the Frailty Index.
As it is shown in the Table 4, in the raw model, frailty was cross-sectionally associated with higher risk of hospitalization, mean length of stay (the log-count of average length of stay increases by 0.74 and 0.53 days in case of being frail according to the FI and the FTS5, respectively, compared to non-frail older adults), disregarding the tools used, and with an increase in hospital costs by 22.85%Footnote 2 when FTS-5 was used to assess the condition, but not when FI was used. In the fully adjusted model, only FI showed a significantly higher risk of hospitalization. When the association in the follow-up was estimated, frailty was associated with almost all the outcomes. Frailty was associated with an increase in costs, ranging from an increase by 13.98% (FTS-5, Model 2) to a maximum increase by 38.69% (FI, Model 1). When fully adjusted, some of the figures and associations changed. When we assessed the relationship between frailty and the outcomes in the long-term, we observed an association between frailty and the probability of being admitted to hospital (FTS5), the number of hospital admissions (FTS5 and FI) and length of hospitalization (FI, which increased the log count of average length of stay by 0.611 days). The increase in costs was only significant for the FTS5, which were higher by 19.73% among frail individuals than the non-frail. The differences in the significant associations between frailty and hospital admission and sarcopenia and hospital admission observed before were confirmed in Table 5, when the different combinations of sarcopenia and frailty status entered the analysis. Older adults with sarcopenia but without frailty showed a significantly higher probability of hospitalization at follow-up regardless of the tool used in Models 2 and 3, but not for the other outcomes either cross-sectional or longitudinal. On the other hand, older adults with frailty but without sarcopenia were significantly more likely to have most of the hospitalization-related outcomes in the raw model, especially when frailty was assessed by the Frailty Index, although only the length of hospital stay was significantly longer in the fully adjusted cross-sectionally model, and number and length of hospitalization for the FI at follow-up. However, older adults that have frailty and sarcopenia presented a significant association with the likelihood of being admitted to hospital, both at the cross-sectional and the follow-up levels, regardless of the frailty tool when the interaction was the only variable. In the fully adjusted model, both tools showed significant association with the probability of being admitted to hospital, in both cross-sectional and longitudinal analysis, but only FTS5 showed higher hospitalization costs, which increased by 11.18 and 4.74% among frail individuals with sarcopenia against the reference category, at the cross-sectional and at follow-up level, respectively.Footnote 3 Additionally, we assessed the likelihood ratio test for each regression model in order to estimate if the sequence of models suffer from overfitting (Table 6).
Moreover, when performing the analyses only on frail older adults (Supplementary Information Table S4), presence of sarcopenia significantly reduced length of hospitalization in the FI cross-sectional raw model but does not significantly modify any other outcome. This result would confirm the limited significance of sarcopenia as a determinant of hospital use. In case of running the analyses on older adults with sarcopenia (Supplementary Information Table S5), frailty is, in fact, significantly associated with higher hospital use, especially at follow-up, when in the full model (adjusted for age, gender, comorbidity severity and polypharmacy), being frail was associated with more number of hospitalization (FTS5), higher length of stay (FI) and hospital admission costs, which increased by 55.63% per year when the FI was used and by 45.50% in case of the FTS5, compared with non-frail individuals.
Discussion
To our knowledge, this is the first study to demonstrate in community-dwelling older adults the costs of hospitalization to frailty and sarcopenia taking into account their interaction.
While sarcopenia increases only the likelihood of being hospitalised, frailty was also related to other outcomes such as an increase in the number of hospitalizations, in hospital stay and in healthcare costs. In addition, the interaction of having frailty and sarcopenia had an increased likelihood of hospitalization at both cross-sectional and follow-up, and increased length of stay and hospital-related costs (FTS5) at follow-up. Further segmentation of the sample revealed that could be frailty and not sarcopenia what increased healthcare expenditures. Among older adults with sarcopenia, the presence of frailty, even when adjusted for potential confounders such age, gender, polypharmacy or comorbidity, increased both the number of admissions and hospital stay and exponentially increased costs. In this line, it has been reported that those older adults with sarcopenia have two-fold risk for hospitalization and a significative higher hospital stays that those without sarcopenia, increasing in $375 the cost per person and had an annual marginal increase of $2315 [34], but in that study frailty was not assessed. However, our results might indicate that probably the frailty construct, and not the presence of sarcopenia, is what increases health economic costs in the medium/long term but more studies showing the combination of these entities are needed.
Traditionally, the allocation of healthcare resources has been made based on chronic conditions profiles. However, such approach might have been wrongly understood [13], since more recent existing evidence has pointed towards a greater burden of disability and dependency, as well as their previous stages [50]. Functional decline explains health care utilization with chronic conditions and disability [4] and hospitalization represents a major driver of total HCE [32, 51] and long-term care expenditure [52]. According to these new burdensome conditions, health economic resources should be destinated to policies or interventions in which greatest health patient benefits can be obtained [32] contributing with valuable information improving decision-making processes. Our results could certify this hypothesis, although some differences have also been observed depending on the tool with which we assess frailty.
Frailty status, and not age, has been proposed as a criterion for risk interventions in older adults [53]. As an example, frail subjects spend three times more resources than robust older adults in costs derived from surgical hospitalization and total post-operative costs at six-months [54]. Although prevalence of sarcopenia and frailty are estimated at around 10% in community-dwelling older adults [55, 56], which could be interpreted as minor rates, its estimated relationship with adverse outcomes and high associated expenditures may lead to a costly public health problem [57, 58], as our findings have also supported. Moreover, our results could confirm that in addition to the contribution of sarcopenia and frailty to the healthcare costs, different frailty construct can identify different individuals at risk of suffering adverse events. The use of frailty scales may be inclusive and not substitutable [59]. Furthermore, our results enhance the necessity of identifying frailty in people with sarcopenia [22] which may help policy makers in the efficient allocation of health care resources, being an opportunity for healthcare professionals to revert these ageing associated conditions.
Strengths
This study has many strengths, including the large population included and the use of two relevant frailty tools previously adjusted and validated in our cohort to assess frailty. Another strength is the excellent ascertainment of the hospitalization related outcomes which are not self-reported, but extracted from clinical records, which enabled us to estimate healthcare cost expenditures associated with each available GRD.
Muscle mass has been determined using DEXA, which is the gold-standard. Moreover, the inclusion of relevant cofounders in adjusted models contributes to the increase the strength of our findings.
Limitation
Although hospitalization has been proposed as the main health costs, we could not include other resources as physiotherapy, drugs, specialist or general practitioner visits, or diagnostic tests which are not included within the corresponding DRG.
Despite our sample size, due to the segmentation according to frailty and sarcopenia status (Supplementary Information Table S6), and the low number of hospitalized events, problems of low power to detect differences could be occurring, and subsequently, more studies should confirm our results. The results could be extended to a longer period of time in order to check whether the results obtained in the current study are consistent through a longer follow-up. Moreover, having only two points of analysis might not allow us to establish causality and, hence, the results should be interpreted with caution, suggesting associations rather than causal relationships.
Conclusions
Demographic ageing could burden health care services. Interventions to improve frailty and sarcopenia must be promoted to prevent disability and health care pressure. Strategies for early detection and integrated multidisciplinary interventions should be promoted. Our results should be used to predict future health economic trends according to patient screening and assessment.
Availability of data and materials
Data will be available upon request to the corresponding author.
Notes
Since the number of hospital admissions and the average length of stay do not allow for zero-count data, a zero-truncated regression model was run for the second part of the two-part model for these outcomes. The data are truncated because there are no observations on individuals who stayed for zero days nor for negative values [46]. Additionally, we used robust standard errors for the parameter estimates.
The coefficients on hospitalization costs in the text do not match the coefficients reported in the tables, due to the corresponding exponential transformation associated with the log-link. For example, the 22.85% comes from the calculation of exp. (0.2058307)-1.
When the interaction between frailty and sarcopenia was included in addition to each of these terms individually, the results showed that none of the interactions between frailty and sarcopenia were statistically significant, but for its interaction in the full model when assessing hospitalization costs and frailty was measured through the FTS5 (Table S3, Supplementary Material).
References
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019: Highlights. https://www.un.org/development/desa/pd/news/world-population-ageing-2019-0.
Werblow A, Felder S, Zweifel P. Population ageing and health care expenditure: a school of “red herrings”? Health Econ. 2007;16:1109–26.
Zweifel P, Felder S, Meiers M. Ageing of population and health care expenditure: a red herring? Health Econ. 1999;8:485–96.
Sirven N, Rapp T. The dynamics of hospital use among older people evidence for Europe using SHARE data. Health Serv Res. 2017;52:1168–84.
Chernew ME, Goldman DP, Pan F, Shang B. Disability and health care spending among medicare beneficiaries. Health Aff (Millwood). 2005;24(Suppl 2 Suppl):2.
Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6(192):1–4.
Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition-consensus conference project. J Gerontol. 2013;68:62–7.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56.
Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Cumming RG, Handelsman DJ, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin Pharmacol Ther. 2012;91:521–8.
König M, Spira D, Demuth I, Steinhagen-Thiessen E, Norman K. Polypharmacy as a risk factor for clinically relevant sarcopenia: results from the Berlin aging study II. J Gerontol A Biol Sci Med Sci. 2017;73:117–22.
Öztürk ZA, Türkbeyler İH, Abiyev A, Kul S, Edizer B, Yakaryılmaz FD, et al. Health-related quality of life and fall risk associated with age-related body composition changes; sarcopenia, obesity and sarcopenic obesity. Intern Med J. 2018;48:973–81.
Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70:716–21.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Antunes AC, Araújo DA, Veríssimo MT, Amaral TF. Sarcopenia and hospitalisation costs in older adults: a cross-sectional study. Nutr Diet. 2017;74:46–50.
Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res. 2015;50:305–20.
Bentur N, Sternberg SA, Shuldiner J. Frailty transitions in community dwelling older people. Israel Med Assoc J. 2016;18:449–53.
Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017. https://doi.org/10.1093/ageing/afw247.
Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–23.
Dent E, Morley J, Cruz-Jentoft A, Woodhouse L, Rodríguez-Mañas L, Fried L, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019. https://doi.org/10.1007/s12603-019-1273-z.
Mareschal J, Genton L, Collet TH, Graf C. Nutritional intervention to prevent the functional decline in community-dwelling older adults: a systematic review. Nutrients. 2020;12:1–21.
Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, et al. Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med. 2015;31:367–74.
Davies B, García F, Ara I, Artalejo FR, Rodriguez-Mañas L, Walter S. Relationship between sarcopenia and frailty in the Toledo study of healthy aging: a population based cross-sectional study. J Am Med Dir Assoc. 2018;19:282–6.
Oviedo-Briones M, Laso ÁR, Carnicero JA, Cesari M, Grodzicki T, Gryglewska B, et al. A comparison of frailty assessment instruments in different clinical and social care settings: the Frailtools project. J Am Med Dir Assoc. 2021;22:607.e7–607.e12.
Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186:420–34.
Wilke Fallerid J, Do D, Pereira N, de Souza S, Nampo FK, de Souza OF, et al. Instruments for the detection of frailty syndrome in older adults: a systematic review. 2019. https://doi.org/10.1371/journal.pone.0216166.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95.
Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47:193–200.
Li G, Thabane L, Ioannidis G, Kennedy C, Papaioannou A, Adachi JD. Comparison between frailty index of deficit accumulation and phenotypic model to predict risk of falls: data from the global longitudinal study of osteoporosis in women (GLOW) Hamilton cohort. Plos One. 2015;10:e0120144.
García-García FJ, Carnicero JA, Losa-Reyna J, Alfaro-Acha A, Castillo-Gallego C, Rosado-Artalejo C, et al. Frailty trait scale-short form: a frailty instrument for clinical practice. J Am Med Dir Assoc. 2020. https://doi.org/10.1016/j.jamda.2019.12.008.
Álvarez-Bustos A, Carnicero-Carreño JA, Sanchez-Sanchez JL, Garcia-Garcia FJ, Alonso-Bouzón C, Rodríguez-Mañas L. Associations between frailty trajectories and frailty status and adverse outcomes in community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2022;13:230–9.
Peters LL, Burgerhof JGM, Boter H, Wild B, Buskens E, Slaets JPJ. Predictive validity of a frailty measure (GFI) and a case complexity measure (IM-E-SA) on healthcare costs in an elderly population. J Psychosom Res. 2015;79:404–11.
García-Nogueras I, Aranda-Reneo I, Peña-Longobardo LM, Oliva-Moreno J, Abizanda P. Use of health resources and healthcare costs associated with frailty: the FRADEA study. J Nutr Health Aging. 2017;21:207–14.
Hajek A, Bock JO, Saum KU, Matschinger H, Brenner H, Holleczek B, et al. Frailty and healthcare costs-longitudinal results of a prospective cohort study. Age Ageing. 2018;47:233–41.
Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging. 2019;8:93–9.
Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, Amor Andres MS, de La Torre LMDLA, Escribano Aparicio MV, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo study for healthy aging. J Nutr Health Aging. 2011;15:852–6.
Ambagtsheer RC, Visvanathan R, Dent E, Yu S, Schultz TJ, Beilby J. Commonly used screening instruments to identify frailty among community-dwelling older people in a general practice (primary care) setting: a study of diagnostic test accuracy. J Gerontol A Biol Sci Med Sci. 2020;75:1134–42.
Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24.
García-García FJ, Carcaillon L, Fernandez-Tresguerres J, Alfaro A, Larrion JL, Castillo C, et al. A new operational definition of frailty: the frailty trait scale. J Am Med Dir Assoc. 2014;15:371.e7–371.e13.
Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62.
Ottenbacher KJ, Branch LG, Ray L, Gonzales VA, Peek MK, Hinman MR. The reliability of upper- and lower-extremity strength testing in a community survey of older adults. Arch Phys Med Rehabil. 2002;83:1423–7.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65:989–95.
Farewell VT, Long DL, Tom BDM, Yiu S, Su L. Two-part and related regression models for longitudinal data. Annu Rev Stat Appl. 2017;4:283–315.
Heij C, de Boer P, Franses PH, Kloek T, van Dijk HK. Econometric methods with applications in business and economics. Oxford: Oxford University Press; 2004.
Cameron AC, Trivedi PK. Microeconometrics using stata. College Station: Stata press; 2010. p. 2.
Moran JL, Solomon PJ, Peisach AR, Martin J. New models for old questions: generalized linear models for cost prediction. J Eval Clin Pract. 2007;13:381–9.
Myers RH, Montgomery DC. A tutorial on generalized linear models. J Qual Technol. 1997;29:274–91.
Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–94.
Oliva-Moreno J, López-Bastida J, Montejo-González AL, Osuna-Guerrero R, Duque-González B. The socioeconomic costs of mental illness in Spain. Eur J Health Econ. 2009;10:361–9.
Kohn JL, Liu JS. The dynamics of medical care use in the British household panel survey. Health Econ (United Kingdom). 2013;22:687–710.
Forder J. Long-term care and hospital utilisation by older people: an analysis of substitution rates. Health Econ. 2009;18:1322–38.
Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JPJ. Old or frail: what tells us more? J Gerontol. 2004;59:M962–5.
Robinson TN, Wu DS, Stiegmann G, v., Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202:511–4.
Papadopoulou SK, Tsintavis P, Potsaki G, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24:83–90.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–92.
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5.
Sicsic J, Ravesteijn B, Rapp T. Are frail elderly people in Europe high-need subjects? First evidence from the SPRINTT data. Health Policy. 2020. https://doi.org/10.1016/j.healthpol.2020.05.009.
Cesari M, Gambassi G, Abellan van Kan G, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–2.
Acknowledgements
We would like to thank the participants, cohort members and team researcher members.
Funding
The present work was funded by grants from the Spanish Ministry of Economy, Industry and Competitiveness, cofinanced by the European Regional Development Funds (RD120001/0043) and the Centro de Investigación Biomédica en Red en Fragilidad y Envejecimiento Saludable (CB16/10/00464), and the Papel de la disfunción MITOcondrial en la relación entre multimorbilidad crónica y deterioro FUNcional en ancianos. El Proyecto MITOFUN, Fundación Francisco Soria Melguizo (Section 2/2020). The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n°115621, resources of which are composed of financial contribution from the European Union’ Seventh Framework Programme (FP7/2007–2013) and EFPIA companies’ in kind contribution.
Author information
Authors and Affiliations
Contributions
All authors contributed to editing of the manuscript. AAB and BRS drafted the manuscript for authors to contribute and comment on. All authors contributed to interpretation of the study findings. AAB, BRS and LRM designed the study. BRS analysed the data. The corresponding author (LRM) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted and is the guarantor. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participant data were taken from the Toledo Study of Healthy Ageing (TSHA). TSHA is a longitudinal cohort that was designed to analyze different models of frailty and ageing and to measure the impact that frailty, comorbidity and disability may have on health system by assessing rural and urban community dwelling older adults aged 65 years or older. TSHA was performed in concordance with the Helsinki Declaration of 1975 and was approved by the Clinical Research Ethics Committee of the Toledo Hospital in Spain. All the participants signed the Informed Consent. Ethics approval and Consent to participate are provided in the related files.
Consent for publication
Not Applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Toledo Study of Health Ageing Frailty Index. Table S2. Frailty Trait Scale 5 (FTS5) scoring. Table S3. Results on the association between the interaction term between frailty and sarcopenia and the hospitalization-related outcomes, both at the cross-sectional level and at-follow up. Table S4. Results on the association between sarcopenia and the hospitalization-related outcomes, both at the cross-sectional level and at-follow up, only on the frail older adults. Table S5. Results on the association between frailty and the hospitalization-related outcomes, both at the cross-sectional level and at-follow up, only on the older adults with sarcopenia. Table S6. Cross-tabulations of sarcopenia and frailty according to the FTS5 and the FI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Álvarez-Bustos, A., Rodríguez-Sánchez, B., Carnicero-Carreño, J.A. et al. Healthcare cost expenditures associated to frailty and sarcopenia. BMC Geriatr 22, 747 (2022). https://doi.org/10.1186/s12877-022-03439-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03439-z