Abstract
Background
Adrenergic alpha-1 receptor antagonists (alpha-1 antagonists) are frequently used medications in the management of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) and in the management of therapy-resistant arterial hypertension, two conditions frequently found in older adults. This systematic review aims at presenting a complete overview of evidence over the benefits and risks of alpha-1 antagonist treatment in people ≥ 65 years, and at deriving recommendations for a safe application of alpha-1 antagonists in older adults from the evidence found.
Methods
A comprehensive literature search was performed (last update March 25th 2022) including multiple databases (Medline/Pubmed, Embase, the Cochrane Library) and using the PICOS framework to define search terms. The selection of the studies was done by two independent reviewers in a two-step approach, followed by a systematic data extraction. Quality appraisal was performed for each study included using standardised appraisal tools. The studies retrieved and additional literature were used for the development of recommendations, which were rated for strength and quality according to the GRADE methodology.
Results
Eighteen studies were included: 3 meta-analyses, 6 randomised controlled trials and 9 observational trials. Doxazosin in the management of arterial hypertension was associated with a higher risk of cardiovascular disease, particularly heart failure, than chlorthalidone. Regarding treatment of LUTS suggestive of BPH, alpha-1 antagonists appeared to be effective in the relief of urinary symptoms and improvement of quality of life. They seemed to be less effective in preventing disease progression. Analyses of the risk profile indicated an increase in vasodilation related adverse events and sexual adverse events for some agents. The risk of falls and fractures as well as the effects of long-term treatment remained unclear. All meta-analyses and 5 out of 6 interventional studies were downgraded in the quality appraisal. 7 out of 9 observational studies were of good quality.
Conclusions
It cannot be recommended to use doxazosin as first-line antihypertensive agent neither in older adults nor in younger patients. In the management of BPH alpha-1 antagonists promise to effectively relieve urinary symptoms with uncertainty regarding their efficacy in preventing long-term progression events.
Similar content being viewed by others
Background
Alpha-1 antagonists are widely used agents predominantly in the treatment of arterial hypertension [1] and, since the late 1980s and early 1990s, LUTS suggestive of BPH [2,3,4,5]. Both conditions are very common worldwide [6, 7], especially prominent in the older population and expected to further increase in prevalence [8,9,10].
LUTS suggestive of BPH only concerns male patients, mostly older, and includes a number of symptoms such as frequency, nocturia, urgency, weak stream or interruption during micturition, high post-void residual volume in the urinary bladder or the difficulty initiating micturition [7]. Recent estimates indicate that almost 50% of men aged 50 years or older suffer from LUTS as a consequence of BPH and 80% of males over the age of 80 years [11]. In the US, prevalence numbers have developed substantially over the past years and it is expected that this trend will continue as the population ages [7].
A similar picture can be drawn for hypertension. According to Chow et al. [6] global prevalence figures for hypertension in adults are around 30% to 45% with this number being similar across the world and independent of the country’s income status. Age, however, does play an important role in the prevalence of hypertension as rates increase with progressing age. This results in shares of more than 60% in people with 60 years or more and about 75% in people over the age of 75 years [1]. It is currently forecasted that the number of people with hypertension will rise by 15% to 20% until 2025 resulting in approximately 1.5 billion affected people worldwide [8].
The use of alpha-1 antagonists in the therapy of hypertension is based on the modulation of vessel tone and systemic vascular resistance, which results in an increase in venous capacitance and lowering of blood pressure [12]. As alpha-1 adrenergic antagonists cause a relaxation of smooth muscle both in the vascular system and in the prostate [13], they are also effective in the therapy of LUTS suggestive of BPH reducing the symptoms by up to 50% [14]. Long-term studies showed no reduction in prostate size nor prevention of acute urinary retention events, though [15,16,17]. The most common adverse side effects include dizziness, postural hypotension, asthenia, headache, rhinitis and ejaculatory dysfunction occurring in about 5% to 9% of the patients [18].
The medical treatment of older adults comes with many challenges. On the one hand, it is known that pharmacodynamics as well as pharmacokinetics differ between younger patients and older patients [19] leading to an increased risk of developing ADEs among the elderly [20]. On the other hand, older adults are more frequently affected by multimorbidity, which may result in polypharmacy [21] and this again increases the risk of ADEs, adverse drug interactions, and possibly hospitalisation [22,23,24]. The versions of diverse national PIM (potentially inadequate medication for the elderly) lists are inconclusive on how to categorize alpha-1 antagonists. Doxazosin is included in three PIM lists [25,26,27], two of which also include terazosin [25, 27]. PIM lists of Austria, France and Canada do not include any of the alpha-1 antagonist [28,29,30].
In the light of the above-mentioned it seems overdue to summarize and synthesize the evidence available on the treatment of elderly with alpha-1 antagonists. An ageing population associated with an expected substantial increase of the prevalence of hypertension and LUTS suggestive of BPH in the near future will lead to an increased use of alpha-1 antagonists in patients older than 65 years. The aim of this systematic review is to explore the effectiveness and safety of alpha-1 antagonists in these patients and to develop recommendations on when to discontinue or reduce the dose of alpha-1 antagonists to prevent inappropriate prescribing.
To the best of our knowledge, so far, no systematic review has analysed the specific evidence on the use of alpha-1 antagonists in the aged population.
The aims of this SR are therefore to.
-
systematically review the literature on the risks and benefits of the use of alpha-1 antagonists in older adults (≥ 65 years),
-
critically assess the quality of evidence identified, and
-
develop recommendations for or against the use of alpha-1 antagonists in older adults.
Methods
This systematic review was developed and conducted with reference to the methodology as described in the Cochrane Handbook for Systematic Reviews of Interventions [31].
The study protocol was registered at the international prospective register of systematic reviews (CRD42020183345).
Search method
A thorough review of existing research literature was carried out in a three-stage process. In the first stage, a highly sensitive search was performed in order not to miss out on any relevant studies. In steps two and three papers with irrelevant content according to the inclusion and exclusion criteria were removed.
Development of the search terms
For step one search terms were developed in accordance with the PICOS model for each of the following categories: population, intervention, comparison, outcomes and study design. The terms within each category were connected by “OR” in the search process while the terms of different categories were connected by “AND”. As Medline/Pubmed was used as a search engine, the official MeSH terms or its entry terms were applied as search terms (see Additional file 1 for full list of search terms).
Step 1 – literature search
The search was performed by a data research team at the University of Witten/Herdecke on the 19th of June 2019, and updated on the 25th of March 2022, in Medline/Pubmed, Embase and the Cochrane Database of Systematic Reviews using the OVID interface for each database. The result of the literature search was uploaded to Endnote X8.2 software for data management purposes. Duplicates were removed and step two (selection of studies) was performed with the help of Endnote.
Complementary to the literature search the citations of included studies were examined in order not to miss any important study.
Steps 2 & 3 – selection of studies
For the second step, two reviewers (FM and GK) worked through the list of research papers derived from step one by independently assessing the relevance of each study’s title and abstract. The assessment was based on the inclusion and exclusion criteria defined beforehand (for details, see below). If a paper seemingly met the inclusion criteria the study was included for step three. Research papers evidently not relevant for this systematic review were excluded. Upon conclusion of step two and before starting step three the reviewers compared their results. Studies selected by both were then automatically included in step three. If there were research papers selected by only one of the two, the reviewers had to re-evaluate and discuss to come to a mutual agreement. In case of an unresolvable disagreement a third reviewer (AS) was consulted and asked to decide.
The same procedure was applied to step three but now the assessment was based on the full manuscript of all studies selected in step two. The research papers selected in step three met all inclusion criteria and were therefore incorporated in this systematic review.
Inclusion and exclusion criteria
Types of studies
The following types of studies were included: systematic reviews, meta-analyses, interventional studies and observational studies. Other study types were excluded, e.g. narrative reviews, expert opinions, case reports or letters.
Types of participants
Studies were only eligible if they reported results for older adults. The term older adults was defined as people with the age of 65 years or older. We included studies if the mean age minus 1.8 times the standard deviation was 65 years or older or if there was separate reporting for age subgroups equal to or greater than 65 years. In addition to the age criterion, we also defined patient conditions of interest for this systematic review (e.g. LUTS suggestive of benign prostatic hyperplasia and resistant arterial hypertension).
Types of interventions
Studies were only included if the intervention lasted for at least 4 weeks. Analyses on acute care and short-term treatment were thus excluded. In addition, the intervention had to include an alpha-1 antagonist (e.g. tamsulosin, doxazosin, alfuzosin) and a comparator. Such comparator could be no treatment, placebo, other drug (also different alpha-1 antagonists), phytotherapy or other non-pharmacological interventions. Studies without a comparator were excluded.
Types of outcomes
Studies were deemed eligible if they investigated direct patient relevant outcomes such as efficacy or effectiveness (e.g. change in the International Prostate Symptom Score [IPSS]) and/or ADEs (e.g. dizziness, asthenia, falls) and/or long-term drug safety (e.g. cognitive decline, cardiovascular events) as well as QoL, mortality, and/or hospitalisations. It was irrelevant for the inclusion of a study whether these outcomes were defined as primary or secondary outcomes. Studies only investigating surrogate parameters (e.g. asymptomatic changes in blood pressure as a proxy for orthostatic hypotension) were excluded.
Publication dates
No limit was set regarding publication dates.
Language
Studies were only included if they were written in English or German.
Data extraction
All relevant data from included studies was extracted. Data was deemed relevant if it met all criteria to answer the research questions of this systematic review. It is therefore possible that only parts of the results of an entire study were extracted, e.g. subgroup analysis for study participants with the age of 65 years or older.
Standardised data collection forms were used for the data extraction. Results include tables for.
-
the summary of characteristics of included studies,
-
the summary of patient characteristics of included studies, and
-
the summary of study findings of included studies.
Each of them is specific to the study designs included (i.e. meta-analyses, interventional studies, and observational studies). Data extraction was reviewed by a second researcher and checked for completeness, accuracy, and relevance.
Data synthesis/Meta-analyses
Due to high heterogeneity between the studies regarding interventions, comparators and outcomes meta-analyses were only performed with respect to six different outcomes: change in IPSS, change in QoL-score, the occurrence of ADEs, and incidence of dementia, falls and fractures. The meta-analyses on the incidence of dementia, falls and fractures are based on data on events per person (derived from events/1,000 person-years for two studies [32, 33]). The former two meta-analyses include data derived from three interventional studies [34,35,36]. While one of the studies provided values for mean and the 95% confidence interval (CI) for change figures [35], the other two studies reported mean scores and standard deviation (SD) for the baseline and final measurements but did not provide values for SD or CI of the changes [34, 36]. These figures were therefore imputed with reference to the suggestions described in the Cochrane Handbook for Systematic Reviews of Interventions [31]. For one study the p-value for change figures was used to obtain the t-values in order to calculate the standard error and finally the SD as the p-values were reported [36]. For the second study [34], the change-from-baseline SD from the study conducted by Gotoh et al. (2005) [35] was used as the study design for both studies was very similar and the p-value was not published. For the calculation of the meta-analyses the mean difference random effect model was used as all the studies used the same outcome scales (IPSS and QoL-score). Three interventional studies reported ADEs for tamsulosin and naftopidil [34,35,36], respectively, out of which one study conducted by Nishino et al. (2006) did not report any ADEs for neither of the interventions [36]. It was therefore decided to exclude this study from the meta-analysis as suggested in the Cochrane Handbook for Systematic Reviews of Interventions [31]. The Mantel–Haenszel method was used due to the low number of events. Review Manager, version 5.3, was used for computations and the creation of figures [37].
Quality appraisal
A critical assessment of the methodological quality was performed for each included study in duplicate following the same logic as for the selection of studies. Established and validated appraisal tools were therefore used depending on the respective study type. Interventional studies were evaluated with the Revised Cochrane Risk-of-Bias Tool for Randomised Trials (RoB 2) [38]. Observational studies were assessed by using the checklists offered by Critical Appraisal Skills Programme (CASP) on the critical appraisal of case–control studies [39] and cohort studies [40]. Systematic reviews including meta-analyses were assessed with AMSTAR 2, an instrument developed for the measurement and assessment of systematic reviews [41].
Development of recommendations
Recommendations on the use of alpha-1 antagonists in patients aged ≥ 65 years were developed based on the findings of this systematic review supplemented with additional references. A specific non-systematic literature search was therefore performed in the Cochrane Database of Systematic Reviews and Medline/Pubmed including papers found during steps two and three of the search for this systematic review and studies found by snowballing. In addition, the most recent evidence based guidelines for the treatment of hypertension by the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) [42] and for the treatment of non-neurogenic male LUTS by the European Association of Urology (EAU) [43] were consulted and its references screened. Each recommendation was rated with respect to strength (weak or strong) and quality (high, moderate, low, very low) [44,45,46] according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
Results
Results of the search
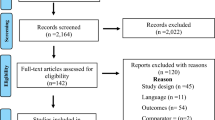
Two thousand nine hundred forty-five records could be identified through the first step of the systematic review process, out of which 36 duplicates were immediately removed. No additional records were identified through other sources. 2909 research papers were screened during the second step, out of which 2521 could be excluded based on title and abstract. The remaining 388 records were assessed for eligibility based on full texts. 370 full texts were excluded in step three. We included 15 primary studies (6 RCTs [34,35,36, 47,48,49], 9 observational studies [32, 33, 50,51,52,53,54,55,56]) and three meta-analyses [57,58,59]. As opposed to the original studies [60,61,62,63,64], the meta-analyses reported the results for relevant age-related subgroups by using unpublished data. Their references were thus excluded throughout the search process. Additional file 2 lists all research papers excluded in the last step individually with the respective reason for exclusion. Figure 1 shows the PRISMA flow diagram [65].
Characteristics of included studies
Eighteen studies were included in this systematic review, out of which six are interventional studies [34,35,36, 47,48,49], nine are observational studies, three cohort studies [32, 33, 50, 51, 54,55,56] and two case–control studies [52, 53], and three are meta-analyses, two including two [57, 59] and one including six [58] randomised controlled trials. The trials’ geographical focus was mainly on the United States of America (US), Canada, European countries and Japan. Follow-up was minimum 4 weeks [36, 57] and the longest lasting trial had a maximum follow-up period of 13 years [55]. For a detailed summary of the characteristics of included studies please refer to Table 1.
Characteristics of study participants
The age structure of participants varied as inclusion criteria were defined differently between studies. All three meta-analyses [57,58,59] and three interventional studies [47,48,49] had a broad age distribution but offered age related subgroup analyses. Two interventional studies [34, 35] set minimum age below 65 years but were included entirely as they met the age eligibility criteria as defined above and one interventional study [36] enrolled only patients aged 66 years or older. Eight observational trials only included older adults [32, 33, 50, 51, 53,54,55,56], one looked at younger patients also but presented relevant age-related content [52]. See Additional file 3 for a summary of the patient characteristics of included studies. Refer to Additional file 4 for further details on patient characteristics for each study used in the included meta-analyses.
Interventions and outcomes
Doxazosin
Two studies focused particularly on doxazosin [47, 52]. The ALLHAT study (2003) compared the efficacy of doxazosin and chlorthalidone in reducing cardiovascular events (e.g. fatal coronary heart diseas or combined cardiovascular disease) in hypertensive patients [47]. Hall and McMahon (2007) performed a retrospective observational study and investigated the relation of exposure to doxazosin and the incidence of fractures (e.g. hip, femur) [52].
Tamsulosin, naftopidil and silodosin
Four interventional studies examined the efficacy of tamsulosin [34,35,36, 48], whereas two observational studies [33, 51] and one meta-analysis [59] explored particular side effects. Oelke et al. (2014) compared treatment satisfaction between tamsulosin and placebo or tadalafil in patients with urinary symptoms related to BPH [48]. Three further interventional studies assessed the efficacy of tamsulosin against naftopidil [34,35,36] and silodosin [34] with regard to the reduction of urinary symptoms according to the IPSS, the increase of QoL and, with the exception of Yokoyama et al. (2011) [34], the occurrence of ADEs. Two meta-analyses could be performed comparing pre to post drug administration data for tamsulosin with regard to change in IPSS and QoL [34,35,36]. One additional meta-analysis was performed to compare the occurrence of ADEs between tamsulosin and naftopidil [34, 35]. Chapple et al. (1997) compared the safety and tolerability of tamsulosin with placebo in older patients with LUTS suggestive of BPH [59]. Duan et al. (2018) and Tae et al. (2019) explored the association of tamsulosin use and the risk of dementia by comparing to no BPH medication and alternative treatment options [33, 56]. Welk et al. (2015) analysed the occurrence of falls in tamsulosin users [51].
Alfuzosin
Roehrborn (2006) explored the occurrence of progression events due to BPH (i.e. worsening of IPSS, BPH related surgery and acute urinary retention events) in patients taking alfuzosin 10 mg per day controlled against placebo over a period of 24 months [49]. A meta-analysis by Buzelin et al. (1997) analysed the incidence of ADEs from two randomised controlled trials [57].
Terazosin
Lowe et al. (1994) performed a meta-analysis and therefore amalgamated the data of six randomised controlled trials to assess the safety of terazosin use in the treatment of BPH related symptoms [58].
Any alpha-1 antagonist
Six observational studies included several alpha-1 antagonists in their analysis partly without differentiating between single agents. Chrischilles et al. (2001) and Hiremath et al. (2019) examined the effect of treatment initiation with terazosin, doxazosin or prazosin on hypotension related adverse events [50, 54]. Hundemer et al. (2021) examined the association of alpha-1 antagonist use and adverse kindey or cardiac events, mortality and safety-related outcomes [32]. Testa et al. (2018) analysed the role of antihypertensive drugs including alpha-1 antagonists in the occurrence of orthostatic hypotension related syncopes in people with dementia [53]. Welk et al. (2015) elaborated on the effect of treatment initiation with tamsulosin, alfuzosin or silodosin on the risk for fractures and falls [51], and Siemens et al. (2021) investigated the association of alpha-1 antagonist treatment and cardiac failure [55].
Main findings
The results of the included studies are structured and summarised by outcome below. Please refer to Table 2 for a summary of study findings and to Additional file 5 for a detailed display of the study results.
Cardiovascular events
Results from the ALLHAT study (2003) showed an increased risk of doxazosin compared to chlorthalidone for combined cardiovascular events (i.e. death from coronary heart disease, nonfatal myocardial infarction, stroke, coronary revascularisation, hospitalised or treated angina, treated or hospitalised congestive heart failure, and peripheral artery disease and heart failure). The combined cardiovascular risk was elevated among patients < 65 years (relative risk RR 1.15, 95% confidence interval CI 1.04 – 1.27) and more pronounced for patients ≥ 65 years (RR 1.23, 95% CI 1.14 – 1.32). The results for heart failure for participants < 65 years of age (RR 1.76, 95% CI 1.40 – 2.22) and participants ≥ 65 years (RR 1.89, 95% CI 1.65 – 2.17) are even clearer [47]. Siemens et al. (2021) analysed the incidence of heart failure in patients with BPH and found an elevated risk in patients treated with alpha-1 antagonists vs. no medication (HR 1.22, 95% CI 1.18 – 1.26) with a slightly higher risk with non-selective vs. selective alpha-1 antagonists (HR 1.08, 95% CI 1.00 – 1.17) [55]. In contrast to above stated findings, Hundemer et al. (2021) showed a decrease in cardiac events (composite of MI, coronary revascularisation, congestive heart failure or atrial fibrillation) when comparing alpha-1 antagonist treatment with other BP lowering drug treatment regimes (HR 0.92, 95% CI 0.89 – 0.95) [32] whereas Hiremath et al. (2019) demonstrated a slight but insignificant increase focusing on women only (HR 1.06, 95% CI 0.99 – 1.13) [54].
Efficacy in reducing BPH related symptoms
Significant improvement of IPSS for tamsulosin in a pre-post comparison was demonstrated by Gotoh et al. (2005) (mean reduction -8.4, 95% CI -10 – (-6.8); p < 0.001) [35], Nishino et al. (2005) (mean score [standard deviation (SD)] 20.4 [3.5] vs. 9.3 [3.0]; p < 0.001) [36] and Yokoyama et al. (2011) (mean score [SD] 18.0 [1.1] vs. 10.7 [1.4]; p < 0.001) [34]. Similar results were reported for naftopidil in the same studies. Silodosin was only investigated in one study conducted by Yokoyama et al. (2011) with comparable results [34]. Significance in superiority of one agent over another could not be demonstrated with intergroup p-values at 0.060 [35], 0.265 [36] and > 0.05 [34].
A meta-analysis was performed to combine the results of these three studies including a total of 303 study participants and assessing the efficacy of tamsulosin and naftopidil in reducing BPH related urinary symptoms expressed as change of mean IPSS pre to post intervention [34,35,36]. The result shows non-superiority of one drug over the other (mean difference -0.89, 95% CI -2.87 – 1.08; see Fig. 2). A second meta-analysis consolidated the results of the same three studies for tamsulosin 0.2 mg (total n = 154), comparing results pre vs. post administration. Tamsulosin was chosen due to its importance on the European market as compared with naftopidil. The result favours the intervention with tamsulosin 0.2 mg (post administration) over no intervention (pre administration) showing a significant reduction in urinary symptoms (mean difference -8.86, 95% CI -11.14 – -6.58; see Fig. 3).
Progression events from BPH associated LUTS
In a two-year follow up, the results of Roehrborn (2006) showed that alfuzosin has a non-significant positive effect on slowing down the progression of the IPSS (RR 0.84, 95% CI 0.62 – 1.15) and the need for BPH related surgery (RR 0.64, 95% CI 0.36 – 1.12) and no effect on the reduction of acute urinary retention events (RR 0.98, 95% CI 0.39 – 2.44) in patients over the age of 65 years [49].
Treatment satisfaction and QoL
The results of Oelke et al. (2014) showed no significantly better scores in treatment satisfaction of patients > 65 years with LUTS suggestive of BPH when treated with tamsulosin than with placebo (mean score [SD] 32.4 [15.8] vs. 32.2 [17.9]; p-value = 0.759) [48].
In a pre-post comparison, significant improvement in QoL was demonstrated for tamsulosin, naftopidil and silodosin in three trials [34,35,36]. Significant differences in the improvement of QoL-scores between tamsulosin, naftopidil and silodosin could not be demonstrated with intergroup p-values at 0.801 [35], 0.201 [36] and > 0.05 [34].
A meta-analysis was performed to combine the results of three interventional studies including a total of 303 study participants assessing the efficacy of tamsulosin and naftopidil in improving QoL expressed as reduction of QoL-score pre to post administration [34,35,36]. The result shows non-superiority of one drug over the other (mean difference -0.01, 95% CI -0.25 – 0.24; see Fig. 4). A second meta-analysis consolidated the results of the same three studies for tamsulosin 0.2 mg only (total n = 154), comparing results pre vs. post administration. The result favours the intervention with tamsulosin 0.2 mg (post administration) over no intervention (pre administration) showing a significant improvement in QoL of patients with LUTS (mean difference -1.77, 95% CI -2.11 – -1.43; see Fig. 5).
ADEs
Chapple et al. (1997) conducted a safety analysis for tamsulosin compared with placebo in 291 older patients and found no significant difference in the occurrence of any adverse events between groups (tamsulosin: 70/191, 37%; placebo: 31/100, 31%; p = 0.330) or adverse events, which were considered drug-related (tamsulosin: 23/191, 12%; placebo: 9/100, 9%; p = 0.459) or adverse events possibly associated with vasodilation (tamsulosin: 8/191, 4.2%; placebo: 4/100, 4%; p = 0.523) [59]. Buzelin et al. (1997) reported an almost equal distribution of ADEs comparing alfuzosin with placebo (alfuzosin: 12/149, 8.1%; placebo: 12/153, 7.8%). Also, adverse events related to vasodilation (alfuzosin: 2/149, 1.3%; placebo: 2/153, 1.3%) occurred evenly between both groups [57]. Lowe et al. (1994) provided a more detailed analysis on different specific ADEs (see Additional file 5) [58].Three interventional studies reported on ADEs and found no significant differences between agents [34,35,36].
A meta-analysis was undertaken to combine the results of two interventional studies (total n = 266) assessing the occurrence of ADEs for treatment with tamsulosin or naftopidil [34, 35]. The result shows no significant differences between these two agents (odds ratio 0.96, 95% CI 0.38 – 2.40; see Fig. 6).
Syncope and hypotension
Testa et al. (2018) presented results indicative of the fact that alpha-1 antagonist use may play a role in orthostatic hypotension related syncopes in adults with dementia especially when taken concomitantly with diuretics (adjusted for age and sex RR 1.83, 95% CI 0.85 – 3.96) [53].
The effect of treatment initiation with alpha-1 antagonists on hypotension and hypotension related adverse events was examined by four observational studies. The numbers reported by Chrischilles et al. (2001) showed a significant difference between the alpha-1 antagonist cohort and no alpha-1 antagonist cohort when comparing incidence rates of hypotension related ADEs four months before treatment initiation with the four months after (p-value = 0.001) [50]. Welk et al. (2015) demonstrated an increased risk for hospitalisation or emergency room assessment due to hypotension (Odds ratio [OR] 1.80, 95% CI 1.59 – 2.03) within 90 days after treatment initiation with alpha-1 antagonists tamsulosin, alfuzosin or silodosin [51]. Hiremath et al. (2019) also showed an association of alpha-1 antagonist treatment and the incidence of hypotension related events when compared to other BP-lowering medication (HR 1.10, 95% CI 1.01 – 1.20) [54], whereas Hundemer et al. (2021) reported non-significant results in hypotension/1,000 person-years (HR 1.08, 95% CI 0.96 – 1.21) [32].
Falls and fractures
Welk et al. (2015) demonstrated a correlation of initiation of alpha-1 antagonist treatment and new fracture (OR 1.16, 95% CI 1.07 – 1.21), head trauma (OR 1.15, 95% CI 1.04 – 1.27) and falls (OR 1.14, 95% CI 1.07 – 1.21). Agent specific results for falls differed between tamsulosin, alfuzosin and silodosin [51]. In contrast, Hall and McMahon (2007) could not show an association between fractures commonly due to falls (i.e. hip/femur, wrist and humerus) and initiation of doxazosin use (OR 0.57, 95% CI 0.17 – 1.92), current doxazosin use (adjusted OR 0.90, 95% CI 0.68 – 1.19) or any previous doxazosin use (adjusted OR 0.92, 95% CI 0.69 – 1.23) [52]. Hiremath et al. (2019) also did not find a significant association with the occurrence of falls (HR 1.02, 95% CI 0.92 – 1.13) or fractures (HR 0.94, 95% CI 0.82 – 1.08) when comparing alpha-1 antagonist treatment vs. other BP-lowering drugs [54]. Similar results were presented by Hundemer et al. (2021): falls/1,000 person-years (HR 1.00, 95% CI 0.94 – 1.06) and fractures/1,000 person-years (HR 1.03, 95% CI 0.95 – 1.12).
Meta-analyses were performed to synthesize the data presented above based on three retrospective cohort trials [32, 51, 54]. No significant association of initiation of alpha-1 antagonist treatment with falls (RR 1.08, 95% CI 0.99 – 1.17) or fractures (RR 1.07, 95% CI 0.99 – 1.15) could be demonstrated. The results of these meta-analyses are depicted in Figs. 7 and 8.
Ejaculation disorders
Participants receiving silodosin showed a high percentage of ejaculation disorders after 4 weeks of treatment (10/11, 90,9%) and after 12 weeks of treatment (8/10, 80%) whereas tamsulosin (4 weeks: 1/12, 8,3%; 12 weeks: 1/5, 20%) and naftopidil (4 weeks: 1/15, 6,7%; 12 weeks: 1/14, 7,1%) did not [34]. Chapple et al. (1997) reported on elevated numbers of abnormal ejaculations during treatment with tamsulosin compared to placebo (tamsulosin: 5/191, 2.6%; placebo: 1/100, 1%; p = 0.668) [59].
Dementia
Duan et al. (2018) found significant increases in the incidence (number of cases/1.000 person-years) of dementia when tamsulosin was compared to no BPH medication (hazard ratio (HR) 1.17, 95% CI 1.14 – 1.21), to doxazosin (HR 1.20, 95% CI 1.12 – 1.28), to terazosin (HR 1.11, 95% CI 1.04 – 1.19), and to alfuzosin (HR 1.12, 95% CI 1.03 – 1.22) as well as dutasteride (HR 1.26, 95% CI 1.19 – 1.34) and finasteride (HR 1.13, 95% CI 1.07 – 1.19) [33]. Tae et al. (2019) could not reproduce these findings and published results showing a decreased risk of dementia (number of cases/number of patients) when comparing tamsulosin to no BPH medication (HR 0.705, 95% CI 0.635 – 0.782). Similar results were presented for doxazosin (HR 0.710, 95% CI 0.637 – 0.792), terazosin (HR 0.831, 95% CI 0.749 – 0.921) and alfuzosin (HR 0.682, 95% CI 0.607 – 0.766). There was no significant difference between substances when comparing medium dose levels [56].
A meta-analysis was performed to synthesize the results (number of cases/number of patients) of these two studies showing a significantly lower incidence of dementia in patients treated with tamsulosin vs. no medication (RR 0.95, 95% CI 0.90 – 0.99). The results of this meta-analysis are shown in Fig. 9.
Quality appraisal of included studies
Meta-analyses
None of the included meta-analyses [57,58,59] met any of the criteria defined in the AMSTAR 2 assessment tool [41] with the exception of statement of funding. Neither of the publications provide detailed information on the methodology used. All meta-analyses used unpublished information and presumably based their analysis on raw data. Due to a lack of transparency on the calculations performed the figures published cannot be reconstructed neither is it possible to assess homogeneity of the data or likelihood of publication bias of included studies. A comprehensible reproduction of the results is therefore not possible. See Table 3 for details on quality appraisal.
Interventional studies
The result of the assessment for risk of bias based on the appraisal of five different categories according to the RoB 2 tool [38] is shown for each study in Table 4.
The overall risk-of-bias judgement was “low risk” for one study [47], one study was rated with “some concerns” [36] and four trials were graded “high risk” [34, 35, 48, 49]. Selection bias arising from randomization of the patients raised some concerns in the trial by Gotoh et al. (2005) as baseline characteristics in the categories total IPSS (p-value = 0.088) and prostate volume (p-value = 0,06) were imbalanced between the two interventional groups [35]. Four studies were classified as “high risk” for attrition bias due to a high share of dropouts ranging from 11,2% [48] to 33,7% [49], either in large part attributable to the intervention [34, 48, 49] or without delivering comprehensible data [35]. Some concerns were raised for three trials [34,35,36] due to missing information on blinding of participants and potential influence on the self-assessment in the IPSS. The same studies were also downgraded to some concerns for reporting bias as none of them provided a study protocol. It must be considered that the doxazosin component of the ALLHAT study was stopped prematurely due to higher rates of heart failure and combined cardiovascular events [47].
Observational studies
Nine observational studies were included and their risk of bias assessed in accordance to their study type, three retrospective cohort studies [32, 33, 50, 51, 54,55,56] and two case–control studies [52, 53]. Except for two [50, 55] all studies were well rated on the majority of CASP items and can therefore be considered good quality. For details, refer to Tables 5 and 6.
Sponsoring and conflict of interest of included studies
Fifteen of eighteen included studies reported about conflict of interest and study sponsors [32, 33, 36, 47,48,49,50,51,52,53,54,55,56, 58, 59], seven of which reported direct or indirect funding by pharmaceutical companies [47,48,49,50, 52, 58, 59]. Six studies reported support from national institutes [32, 33, 47, 51, 54, 55], two were university funded [36, 56] and one study was endorsed by a local society [53]. Three studies did not give any information on potential sources of conflict of interest [34, 35, 57].
Additional references of interest for the development of recommendations
Two evidence based guidelines [42, 43], two Cochrane reviews [66, 67], one network meta-analysis [68] and four systematic reviews [69,70,71,72], three of them including a meta-analysis, were identified and incorporated into the recommendations in addition to four randomised controlled trials included in this systematic review [34,35,36, 47]. All the additional literature was not included in the systematic review due to their missing focus on the age subgroup of people ≥ 65 years but was deemed relevant as additional information was retrieved concerning efficacy and risk profile of alpha-1 antagonists.
Nickel et al. (2008) reported about significant improvements in IPSS for alfuzosin, terazosin, doxazosin and tamsulosin with no statistically significant differences between substances [71]. Similar findings were reported by Djavan et al. (2004) [72] and Yuan et al. (2015) [68]. Fusco performed two meta-analyses in 2016 and 2018 indirectly confirming these results as significant improvements in the Bladder Outlet Obstruction Index (BOOI) could be shown for alfuzosin, terazosin, doxazosin, tamsulosin, naftopidil and silodosin [69, 70]. Jung et al. (2017) conducted a Cochrane review on silodosin and reported significant improvement in IPSS and QoL versus placebo but no substantial differences when compared to tamsulosin or alfuzosin [66]. Hwang et al. (2018) focused on naftopidil in their Cochrane review with no significant differences in IPSS and QoL when compared with tamsulosin or silodosin [67]. Regarding drug safety, two main areas of interest were covered by the additional references being vasodilation related ADEs (e.g. dizziness, hypotension, syncope) and sexual ADEs. A significant increase in vasodilation related ADEs was reported by Nickel et al. (2008) for alfuzosin, terazosin and doxazosin, a clear tendency but not significant (p = 0.053) for tamsulosin [71]. Similar results have been mentioned by Djavan et al. (2004) [72]. Yuan et al. (2015) demonstrated significantly increased total ADEs for doxazosin, terazosin and silodosin and a tendency, but insignificant, towards increased severe adverse events for doxazosin and terazosin [68]. Silodosin significantly increased rates of sexual adverse events in all comparisons [66]. Treatment with naftopidil compared with tamsulosin and silodosin showed no difference in cardiovascular risk profile or withdrawal rates but significantly less sexual adverse events than for silodosin [67].
Recommendations
Two recommendations were developed based on the findings of this systematic review and additional references of interest as stated above (see Table 7 for recommendations). One recommendation is related to the management of bothersome LUTS suggestive of BPH. Alpha-1 antagonists prove to be effective in the reduction of the IPSS, an internationally used and validated score to measure urinary symptoms, while having an acceptable risk profile. The ADEs differ between agents and therefore have to be considered on a patient-oriented basis. The recommendation was based on three randomised controlled trials included in this systematic review [34,35,36], the most recent version of the guideline for the management of non-neurogenic male LUTS by the EAU [43], two Cochrane reviews [66, 67], and four systematic reviews including meta-analysis [69,70,71,72]. The recommendation was given the following ratings: strong recommendation and low in quality. The quality was downgraded from high to low due to study limitations in the randomised controlled trials and indirectness of additional references. Based on the findings of the ALLHAT study [47] and the 2018 ESC/ESH guidelines for the management of arterial hypertension [42] the second recommendation is to replace doxazosin with another antihypertensive drug in the treatment of hypertension. The recommendation was rated as strong recommendation and of high quality.
Discussion
This systematic review was performed to summarize the current body of knowledge about the efficacy/effectiveness as well as the safety profile of alpha-1 antagonists in the management of arterial hypertension and LUTS suggestive of BPH in patients ≥ 65 years and to derive recommendations on the use of alpha-1 antagonists in the subgroup of older adults.
Summary of main results
Eighteen studies were included in this systematic review: three meta-analyses, six randomised controlled trials, seven cohort studies and two case–control studies. The studies varied in terms of geographical focus, length of follow-up (shortest 4 weeks; longest 13 years), characteristics of participants, interventions, and outcomes. One study analysed the efficacy of doxazosin to reduce cardiovascular events in the management of arterial hypertension, two more looked at potential side-effects. The other trials studied alpha-1 antagonists in the management of LUTS suggestive of BPH. The included studies reported on cardiovascular outcomes, change in IPSS, QoL, treatment satisfaction, disease progression and typical ADEs such as vasodilatory adverse events (e.g. dizziness, syncope, falls, fractures) and sexual adverse events as well as dementia. Three studies reported on mortality. Based on the results of included studies it was possible to perform meta-analyses on six different outcomes: occurrence of ADEs, change in IPSS, change in QoL-score, and incidence of dementia, falls and fractures.
Doxazosin, used as an antihypertensive medication in older patients, seems to be inferior to chlorthalidone in reducing cardiovascular risk and especially heart failure. Alpha-1 antagonists seem to be effective in patients ≥ 65 years in reducing lower urinary tract symptoms due to BPH reflected by a substantial decrease of the IPSS and an increase in QoL. The study results point out certain ADEs but are inconclusive in defining a reliable risk profile for any of the alpha-1 antagonists. Alpha-1 antagonists may produce certain ADEs due to vasodilation and ejaculatory disorders with differences between substances. There is inconclusive data on the effect of alpha-1 antagonists on fractures, falls and the association of tamsulosin use and occurrence of dementia.
Two recommendations could be derived from the results for older adults. One concerning the role of doxazosin in the management of arterial hypertension and the other regarding the role of alpha-1 antagonists in the treatment of LUTS suggestive of BPH.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, the findings of the ALLHAT study have never been reproduced since. The Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) trial [73] examined the effect of doxazosin as third-line antihypertensive therapy in resistant hypertension and found no excess of heart failure over a median follow-up of 12 months. Wolak et al. (2014) found a significant increase in the composite of cardiac death and acute myocardial infarction in patients being treated with doxazosin for hypertension (as opposed to treatment for LUTS) with a moderate-to-severe ischemia on myocardial perfusion imaging. There was no focus on heart failure [74]. Neither of the studies published subgroup analyses for patients ≥ 65 years. Siemens et al. (2021) found an increased incidence of heart failure in patients treated with alpha-1 antagonists for LUTS due to BPH in their retrospective cohort study.
Three randomised controlled trials [34,35,36] approved of the efficacy of tamsulosin and other alpha-1 antagonists in the reduction of urinary symptoms in older adults and improvement of QoL, which had previously been shown in several trials without focus on the specific age subgroup [66,67,68,69,70,71,72]. As regards the agents’ risk profiles comparable results were reported for tamsulosin, silodosin and naftopidil with a higher occurrence of sexual adverse events for silodosin [34,35,36, 66, 67].
Nickel et al. (2008) show significantly increased vasodilation related adverse events (i.e. dizziness, hypotension, syncope) for alfuzosin, terazosin, doxazosin and almost significant for tamsulosin [71]. Similar results were calculated by Yuan et al. (2015) in their network meta-analysis [68]. This corresponds to the findings on a rise in hypotension related adverse events [50] and fractures and falls [51] upon treatment initiation with doxazosin/terazosin/prazosin and tamsulosin/silodosin/alfuzosin, respectively. These results, however, could not be reproduced by Hall and McMahon (2007), Hiremath et al. (2019) and Hundemer et al. (2021) and our meta-analyses [32, 52, 54]. As opposed to the results of most of the literature reporting significant improvement in symptom scores and QoL scores, Oelke et al. (2014) could not show considerably better treatment satisfaction scores in older adults for tamsulosin than for placebo [48].
Applicability of results
Most findings of this systematic review confirm the important standing of alpha-1 antagnonists in the management of patients with LUTS suggestive of BPH and the minor role of doxazosin in the management of arterial hypertension. Nevertheless, some issues must be addressed concerning the applicability of the results.
The short follow-up time limits the the ability to appraise the effects in long-term treatment with alpha-1 antagonists in LUTS suggestive of BPH [34,35,36]. The applicability of the results is additionally impaired as the dose regime used for tamsulosin was 0.2 mg once daily in all three trials, which is lower than the recommended daily dose of tamsulosin 0.4 mg in Western countries. It also has to be considered that the trials were not placebo controlled. Whether alpha-1 antagonists can be recommended for long-term treatment remains doubtful as the results by Roehrborn et al. (2006) demonstrate similar progression event rates for treatment with alfuzosin as for placebo [49] and results about a possible relationship between tamsulosin and the incidence of dementia remain doubtful [33, 56]. Other classes of drugs such as 5alpha-reductase inhibitors (e.g. dutasteride, finasteride) or phosphodiesterase type 5 inhibitors (e.g. tadalafil) are currently being used in the management of LUTS suggestive of BPH alone or in combination with alpha-1 antagonists [43]. Only one study compared treatment satisfaction between management with tamsulosin 0.4 mg, tadalafil 5 mg and placebo but did not offer separate reporting for older adults [48]. Therefore, no valuable additive information regarding the comparison or combination of treatments in older adults could be delivered by this systematic review.
Limitations and potential biases
A thorough search process was carried out including the application of the PICOS scheme and a two-step approach in the selection of eligible studies thereafter. Nevertheless, it is possible that relevant publications might have been missed as the detection of studies was limited to the databases used.
The results of the meta-analyses must be interpreted with caution. Only a minor fraction of studies included in this SR is represented in the meta-analyses. The results of only three studies in the case of effect on change of IPSS, QoL-score, and incidence of falls and fractures and two in the case of ADEs and incidence of dementia could be included. This is due to high heterogeneity between the studies regarding interventions, comparators and outcomes. The low number of studies, study participants and imputation of SD values due to non-reporting in the original studies reduce the validity of results considerably.
Several sources not focusing particularly on people ≥ 65 years were included for the formulation of the recommendations. Although the additional sources demonstrated similar effects for all age classes the information base may be regarded as diluted. Publication bias has to be regarded as a potential source of bias and could not be assessed due to methodological inconsistencies and the heterogeneity of outcomes. The quality of evidence was evaluated using established and validated quality appraisal tools. Except for one interventional trial [47] all randomised controlled trials were downgraded mostly due to unclear dealing with missing outcome data (attrition bias) and missing study protocol (reporting bias). The authors with missing study protocols were contacted via e-mail but none responded to the request. The quality of included meta-analyses was labelled low quality and most observational studies were rated good quality.
Conclusion
Implications for practice
The use of doxazosin should not be considered as first-line medication for the management of arterial hypertension. The use of alpha-1 antagonists in the management of LUTS suggestive of BPH, however, appears to be promising in reducing urinary symptoms. Thereby, the safety profile of different agents has to be carefully assessed in a patient-oriented manner. Long-term safety and efficacy remain questionable and an assessment of efficacy and safety profile in comparison with other classes of drugs could not be performed.
Implications for research
Even though many older adults suffer from hypertension and the majority of older men experience LUTS from BPH, only eighteen eligible studies could be identified, primarily due to the age restriction, only two of which are placebo controlled randomized trials. This highlights the lack of evidence for older adults although the largest part of medical interventions is performed in this age class. Additionally, randomised controlled trials with extended follow-up periods are needed to assess the benefits and risks of alpha-1 antagonist treatment in long-term use, providing an enhanced understanding of the real-world use of these medications. To complete the picture of management of LUTS suggestive of BPH in people ≥ 65 years it would be also desirable if future research would focus on comparisons and combinations of different classes of drugs.
Given that most included studies revealed considerable methodological limitations a stronger emphasis should be laid on the application of appropriate methodology. This would produce higher quality results yielding more reliable evidence helping us all to provide the best possible patient care.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- 5-ARI:
-
5-Alpha reductase inhibitor
- ACE-inhibitor:
-
Angiotensin converting enzyme inhibitor
- ADEs:
-
Adverse drug events
- ALLHAT:
-
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
- Alpha-1 antagonists:
-
Adrenergic alpha-1 receptor antagonists
- ASCOT:
-
Anglo-Scandinavian Cardiac Outcomes Trial
- AUASS:
-
American Urological Association Symptom Score
- AUR:
-
Acute urinary retention
- BOOI:
-
Bladder Outlet Obstruction Index
- BP:
-
Blood pressure
- BPH:
-
Benign prostatic hyperplasia
- CAD:
-
Coronary artery disease
- CASP:
-
Critical Appraisal Skills Programme
- CeVD:
-
Cerebrovascular disease
- CHD:
-
Coronary heart disease
- CHF:
-
Chronic heart failure
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CV:
-
Cardiovascular
- CVD:
-
Cardiovascular disease
- DDD:
-
Defined daily dose
- EAU:
-
European Association of Urology
- ESC:
-
European Society of Cardiology
- ESH:
-
European Society of Hypertension
- ED:
-
Erectile dysfunction
- FORTA:
-
Fit fOR The Aged
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HRQL:
-
Health related quality of life
- IIEF-5:
-
International Index of Erectile Function
- IPSS:
-
International Prostate Symptom Score
- LUTS:
-
Lower urinary tract symptoms
- MI:
-
Myocardial infarction
- mo:
-
Months
- N.a.:
-
Not available
- NSAID:
-
Non-steroidal anti-inflammatory drug
- OR:
-
Odds ratio
- PIM:
-
Potentially inappropriate medication
- PPI:
-
Proton pump inhibitor
- PVD:
-
Peripheral vascular disease
- PVR:
-
Post void residual volume
- Qmax:
-
Maximum urinary flow rate
- QoL:
-
Quality of Life
- RCTs:
-
Randomized controlled trials
- RR:
-
Relative Risk
- RRdia:
-
Diastolic blood pressure
- RRsys:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SR:
-
Systematic review
- SSRI:
-
Selective serotonin reuptake inhibitor
- TIA:
-
Transient ischemic attack
- TSS-BPH:
-
Treatment Satisfaction Scale for BPH
- Vprostate:
-
Prostatic volume
- y:
-
Years
References
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021–104.
Lepor H. Medical treatment of benign prostatic hyperplasia. Reviews in urology. 2011;13(1):20–33.
Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64(1):118–40.
Schwinn DA, Roehrborn CG. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. International journal of urology : official journal of the Japanese Urological Association. 2008;15(3):193–9.
Michel MC. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: alpha-blockers in the treatment of male voiding dysfunction - how do they work and why do they differ in tolerability? J Pharmacol Sci. 2010;112(2):151–7.
Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. Prevalence, Awareness, Treatment, and Control of Hypertension in Rural and Urban Communities in High-, Middle-, and Low-Income Countries. JAMA. 2013;310(9):959–68.
Parsons JK. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Current bladder dysfunction reports. 2010;5(4):212–8.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365(9455):217–23.
Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol. 2013;189(1 Suppl):S102–6.
Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian journal of urology : IJU : journal of the Urological Society of India. 2014;30(2):170–6.
Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clinics N Am. 2016;43(3):289–97.
Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100(23):2336–43.
Michel MC, Vrydag W. α1-, α2-and β-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(S2):S88–119.
Michel MC, Mehlburger L, Bressel HU, Goepel M. Comparison of tamsulosin efficacy in subgroups of patients with lower urinary tract symptoms. Prostate Cancer Prostatic Dis. 1998;1(6):332–5.
Roehrborn CG. Three months’ treatment with the alpha1-blocker alfuzosin does not affect total or transition zone volume of the prostate. Prostate Cancer Prostatic Dis. 2006;9(2):121–5.
Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Morrill B, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. The Journal of urology. 2008;179(2):616–21; discussion 621.
Roehrborn CG, Siami P, Barkin J, Damiao R, Major-Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123–31.
Debruyne FMJ. Alpha blockers: are all created equal? Urology. 2000;56(5, Supplement 1):20–2.
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6–14.
Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Therapeutic advances in drug safety. 2016;7(1):11–22.
van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity. European Journal of General Practice. 1996;2(2):65–70.
Jano E, Aparasu RR. Healthcare outcomes associated with beers’ criteria: a systematic review. Ann Pharmacother. 2007;41(3):438–47.
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc. 2002;50(12):1962–8.
Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS ONE. 2009;4(2): e4439.
American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2019;67(4):674–94.
Renom-Guiteras A, Meyer G, Thurmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015;71(7):861–75.
Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Deutsches Arzteblatt international. 2010;107(31–32):543–51.
Mann E, Böhmdorfer B, Frühwald T, Roller-Wirnsberger RE, Dovjak P, Dückelmann-Hofer C, et al. Potentially inappropriate medication in geriatric patients: the Austrian consensus panel list. Wien Klin Wochenschr. 2012;124(5):160–9.
Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–31.
McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 1997;156(3):385–91.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. www.training.cochrane.org/handbook. Accessed 03 June 2020.
Hundemer GL, Knoll GA, Petrcich W, Hirematch S, et al. Kidney, Cardiac, and Safety Outcomes Associated With α-Blockers in Patients With CKD: A Population-Based Cohort Study. Am J Kidney Dis. 2021;77(2):178-89.e1.
Duan Y, Grady JJ, Albertsen PC, Helen WuZ. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340–8.
Yokoyama T, Hara R, Fukumoto K, Fujii T, Jo Y, Miyaji Y, et al. Effects of three types of alpha-1 adrenoceptor blocker on lower urinary tract symptoms and sexual function in males with benign prostatic hyperplasia. International journal of urology : official journal of the Japanese Urological Association. 2011;18(3):225–30.
Gotoh M, Kamihira O, Kinukawa T, Ono Y, Ohshima S, Origasa H. Comparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trial. BJU Int. 2005;96(4):581–6.
Nishino Y, Masue T, Miwa K, Takahashi Y, Ishihara S, Deguchi T. Comparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover study. BJU international. 2006;97(4):747–51, discussion 751.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366: l4898.
Critical Appraisal Skills Programme. CASP (Case Control Study) Checklist. 2018. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Case-Control-Study-Checklist-2018.pdf. Accessed 15 June 2020.
Critical Appraisal Skills Programme. CASP (Cohort Study) Checklist. 2018. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Cohort-Study-Checklist_2018.pdf. Accessed 15 June 2020.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed). 2017;358: j4008.
ESC/ESH Guidelines for the management of arterial hypertension. Revista espanola de cardiologia (English ed). 2019;72(2):160.
European Association U. European Association of Urology Guidelines. 2020 Edition. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2020.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ (Clinical research ed). 2008;336(7651):995–8.
Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ (Clinical research ed). 2008;336(7652):1049–51.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed). 2008;336(7650):924–6.
Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension (Dallas, Tex : 1979). 2003;42(3):239–46.
Oelke M, Giuliano F, Baygani SK, Melby T, Sontag A. Treatment satisfaction with tadalafil or tamsulosin vs placebo in men with lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH): results from a randomised, placebo-controlled study. BJU Int. 2014;114(4):568–75.
Roehrborn CG, Group AS. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97(4):734–41.
Chrischilles E, Rubenstein L, Chao J, Kreder KJ, Gilden D, Shah H. Initiation of nonselective alpha1-antagonist therapy and occurrence of hypotension-related adverse events among men with benign prostatic hyperplasia: a retrospective cohort study. Clin Ther. 2001;23(5):727–43.
Welk B, McArthur E, Fraser LA, Hayward J, Dixon S, Hwang YJ, et al. The risk of fall and fracture with the initiation of a prostate-selective alpha antagonist: a population based cohort study. BMJ (Clinical research ed). 2015;351: h5398.
Hall GC, McMahon AD. Comparative study of modified release alpha-blocker exposure in elderly patients with fractures. Pharmacoepidemiol Drug Saf. 2007;16(8):901–7.
Testa G, Ceccofiglio A, Mussi C, Bellelli G, Nicosia F, Bo M, et al. Hypotensive Drugs and Syncope Due to Orthostatic Hypotension in Older Adults with Dementia (Syncope and Dementia Study). J Am Geriatr Soc. 2018;66(8):1532–7.
Hiremath S, Ruzicka M, Petrcich W, McCallum MK, et al. Alpha-Blocker Use and the Risk of Hypotension and Hypotension-Related Clinical Events in Women of Advanced Age. Hypertension. 2019;74(3):645–51.
Siemens RD, Lusty A, Tohidi M, Whitehead M, et al. Cardiac Failure Associated with Medical Therapy of Benign Prostatic Hyperplasia: A Population Based Study. J Urol. 2021;205(5):1430–7.
Tae BS, Jeon BJ, Choi H, Cheon J, et al. α-Blocker and Risk of Dementia in Patients with Benign Prostatic Hyperplasia: A Nationwide Population Based Study Using the National Health Insurance Service Database. J Urol. 2019;202(2):362–8.
Buzelin JM, Delauche-Cavallier MC, Roth S, Geffriaud-Ricouard C, Santoni JP. Clinical uroselectivity: evidence from patients treated with slow-release alfuzosin for symptomatic benign prostatic obstruction. British journal of urology. 1997;79(6):898–904; discussion 904–6.
Lowe FC. Safety assessment of terazosin in the treatment of patients with symptomatic benign prostatic hyperplasia: a combined analysis. Urology. 1994;44(1):46–51.
Chapple CR, Baert L, Thind P, Höfner K, Khoe GS, Spångberg A. Tamsulosin 0.4 mg once daily: tolerability in older and younger patients with lower urinary tract symptoms suggestive of benign prostatic obstruction (symptomatic BPH). The European Tamsulosin Study Group. Eur Urol. 1997;32(4):462–70.
Buzelin JM, Roth S, Geffriaud-Ricouard C, Delàuche-Cavailier MC, and the ASG. Efficacy and Safety of Sustained- Release Alfuzosin 5 mg in Patients with Benign Prostatic Hyperplasia. European Urology. 1997;31:190–8.
Lepor H, Auerbach S, Puras-Baez A, Narayan P, Soloway M, Lowe F, et al. A Randomized, Placebo-Controlled Multicenter Study of the Efficacy and Safety of Terazosin in the Treatment of Benign Prostatic Hyperplasia. The Journal of urology. 1992;148(5, Part 1):1467–74.
Lloyd SN, Buckley JF, Chilton CP, Ibrahim I, Kaisary AV, Kirk D. Terazosin in the Treatment of Benign Prostatic Hyperplasia: A Multicentre. Placebo-Controlled Trial British journal of urology. 1992;70(s1):17–21.
Di Silverio F. Use of Terazosin in the Medical Treatment of Benign Prostatic Hyperplasia: Experience in Italy. Br J Urol. 1992;70(s1):22–6.
Brawer MK, Adams G, Epstein H. Terazosin in the treatment of benign prostatic hyperplasia. Terazosin Benign Prostatic Hyperplasia Study Group. Arch Fam Med. 1993;2(9):929–35.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339: b2700.
Jung JH, Kim J, MacDonald R, Reddy B, Kim MH, Dahm P. Silodosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. The Cochrane database of systematic reviews. 2017;11:Cd012615.
Hwang EC, Gandhi S, Jung JH, Imamura M, Kim MH, Pang R, et al. Naftopidil for the treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia. The Cochrane database of systematic reviews. 2018;10:Cd007360.
Yuan JQ, Mao C, Wong SY, Yang ZY, Fu XH, Dai XY, et al. Comparative Effectiveness and Safety of Monodrug Therapies for Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Network Meta-analysis. Medicine. 2015;94(27): e974.
Fusco F, Creta M, De Nunzio C, Gacci M, Li Marzi V, Finazzi AE. Alpha-1 adrenergic antagonists, 5-alpha reductase inhibitors, phosphodiesterase type 5 inhibitors, and phytotherapic compounds in men with lower urinary tract symptoms suggestive of benign prostatic obstruction: A systematic review and meta-analysis of urodynamic studies. Neurourol Urodyn. 2018;37(6):1865–74.
Fusco F, Palmieri A, Ficarra V, Giannarini G, Novara G, Longo N, et al. α1-Blockers Improve Benign Prostatic Obstruction in Men with Lower Urinary Tract Symptoms: A Systematic Review and Meta-analysis of Urodynamic Studies. Eur Urol. 2016;69(6):1091–101.
Nickel JC, Sander S, Moon TD. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract. 2008;62(10):1547–59.
Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2004;64(6):1081–8.
Chapman N, Chang CL, Dahlof B, Sever PS, Wedel H, Poulter NR. Effect of doxazosin gastrointestinal therapeutic system as third-line antihypertensive therapy on blood pressure and lipids in the Anglo-Scandinavian Cardiac Outcomes Trial. Circulation. 2008;118(1):42–8.
Wolak T, Toledano R, Novack V, Sharon A, Shalev A, Wolak A. Doxazosin to treat hypertension: it’s time to take it personally – a retrospective analysis of 19 495 patients. J Hypertens. 2014;32(5):1132–7.
Acknowledgements
We want to thank Dr. Tim Mathes and Simone Heß of the University Witten/Herdecke for their assistance in the systematic databank search.
Funding
The Austrian Principal Association of Social Insurances (Hauptverband der österreichischen Sozialversicherungsträger) acted as sponsor of the PIM-Austria project and provided prescription data used in this systematic review.
Author information
Authors and Affiliations
Contributions
FM performed the data extraction, analysis and interpretation of data, and quality appraisal. GK also performed the literature research and data extraction. FM and EM prepared the manuscript. AS took part in the design/conception of the work and, together with EM, the revision of it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics commission of the Medical University of Vienna issued a waiver for this systematic review. Therefore, no separate ethics approval is necessary.
Consent for publication
Not needed for this systematic review.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search terms used in the literature database search.
Additional file 2.
Reasons for exclusion in full text analysis.
Additional file 3.
Summary of characteristics of included studies.
Additional file 4.
Summary of patient characteristics of included studies.
Additional file 5.
Additional information on patient characteristics for each study used in the included meta-analyses.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mansbart, F., Kienberger, G., Sönnichsen, A. et al. Efficacy and safety of adrenergic alpha-1 receptor antagonists in older adults: a systematic review and meta-analysis supporting the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr 22, 771 (2022). https://doi.org/10.1186/s12877-022-03415-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-022-03415-7