Abstract
Background
The operational definition of sarcopenia has been updated (EWGSOP2) and apply different cut-off points compared to previous criteria (EWGSOP1). Therefore, we aim to compare the sarcopenia prevalence and the association with mortality and dependence in activities of daily living using the 2010 (EWGSOP1 and 2019 (EWGSOP2 operational definition, applying cut-offs at two levels using T-scores.
Methods
Two birth cohorts, 70 and 85-years-old (n = 884 and n = 157, respectively), were assessed cross-sectionally (57% women). Low grip strength, low muscle mass and slow gait speed were defined below − 2.0 and − 2.5 SD from a young reference population (T-score). Muscle mass was defined as appendicular lean soft tissue index by DXA. The EWGSOP1 and EWGSOP2 were applied and compared with McNemar tests and Cohen’s kappa. All-cause mortality was analyzed with the Cox-proportional hazard model.
Results
Sarcopenia prevalence was 1.4–7.8% in 70-year-olds and 42–62% in 85 years-old’s, depending on diagnostic criteria. Overall, the prevalence of sarcopenia was 0.9–1.0 percentage points lower using the EWGSOP2 compared to EWGSOP1 when applying uniform T-score cut-offs (P < 0.005). The prevalence was doubled (15.0 vs. 7.5%) using the − 2.0 vs. -2.5 T-scores with EWGSOP2 in the whole sample. The increase in prevalence when changing the cut-offs was 5.7% (P < 0.001) in the 70-year-olds and 17.8% (P < 0.001) in the 85-year-olds (EWGSP2). Sarcopenia with cut-offs at − 2.5 T-score was associated with increased mortality (hazard ratio 2.4–2.8, P < 0.05) but not at T-score − 2.0.
Conclusions
The prevalence of sarcopenia was higher in 85-year-olds compared to 70-year-olds. Overall, the differences between the EWGSOP1 and EWGSOP2 classifications are small. Meaningful differences between EWGSOP1 and 2 in the 85-year-olds could not be ruled out. Prevalence was more dependent on cut-offs than on the operational definition.
Similar content being viewed by others
Introduction
According to the European Working Group on Sarcopenia in Older People (EWGSOP), sarcopenia can be defined as the combination of low muscle mass and poor muscle function [1, 2]. The concept was originally introduced by Rosenberg in 1988 [3] and is a geriatric syndrome associated with adverse effects on function, quality of life, and survival [1, 4, 5]. Various definitions have subsequently been proposed [2, 5, 6]. In 2016, the World Health Organization (WHO) launched the International Classification of Disease (ICD) code for sarcopenia [7]. Recently, the EWGSOP published an updated consensus definition that uses poor muscle strength as the key characteristic for the condition rather than low muscle mass (EWGSOP2) [2]. The EWGSOP2 excludes slow gait speed as a diagnostic criterion for sarcopenia, resulting in a lower prevalence [8,9,10]. Several studies comparing EWGSOP1 and 2 have been published. Some studies show good agreement [9, 11,12,13], but others find poor agreement between the two criteria [8, 10, 14,15,16,17,18,19,20,21]. Populations, settings, techniques, testing procedures, and cut-offs have varied, leading to differences in the prevalence of sarcopenia, making comparisons between studies difficult [8, 11,12,13,14,15,16,17,18,19,20,21,22]. Few studies have compared the EWGSOP1 and 2 in a representative and age-standardized sample of community-dwelling older adults [11, 21], and so far, only two (Asian) studies has applied regional cut-offs and also compared with cut-offs suggested by the EWGSOP [9, 10]. There is a lack of knowledge about how EWGSOP1 and 2 impact the prevalence of sarcopenia that is distinct from applying different cut-offs. The objective of this study was, therefore to cross-sectionally compare the difference of the EWGSOP1 and 2 operational definitions on sarcopenia prevalence, applying cut-offs based on T-scores at two levels (− 2.0 and − 2.5) in two population-based samples of 70 and 85-year-olds from Gothenburg, Sweden.
Methods
Study population
This study is part of the Gothenburg H70 Birth Cohort Studies in Sweden (H70 studies) [23]. The studies are multidisciplinary population studies examining systematically recruited birth-cohorts of older populations in Gothenburg, Sweden, via the Swedish Population Register (Statistics Sweden).
The present study includes one cohort born in 1944, examinations started in 2014 at age 70 (n = 1203 response rate 72%), and one cohort born in 1930, examinations started in 2015 at age 85 (n = 491, response rate 64%). In this 1930-cohort, 75 individuals lived in Gothenburg at inclusion in a previous examination in the H70 study but have moved from the city before the present examination. The study has a cross-sectional design except for survival that was checked on June 25, 2020 (Swedish population register). Follow-up time ranged from 2.9 to 6.3 years. The birth cohorts and characteristics of participants and non-participants have been described in detail previously [23,24,25]. All subjects gave informed consent, and the Regional Ethical Review Board in Gothenburg approved the studies.
Participant characteristics
Participants provided information regarding smoking habits, medications, cohabitation, and education level during a semi-structured face-to-face interview. Self-rated health was assessed using the general question; how would you rate your health?”, with response options ranging from very good to very poor. Leisure-time physical activity was classified on four levels with a validated question: sedentary (mainly reading or watching television), moderate (walking outdoors or cycling regularly), regular (sports or strenuous gardening ≥3 h per week), and athletic (regular strenuous physical activity) [26]. Independence in activities of daily living (ADL) was assessed with the Barthel index [27], which includes ten domains of function (bowels, bladder, grooming, toilet use, feeding, transfer, mobility, dressing, stairs, and bathing). Response options are either independent or dependent/unable and summed to a total score ranging from zero (low function, dependent) to 100 (high function, independent). A score < 100 indicates ADL dependence.
Anthropometry
Bodyweight was measured with a calibrated electronic medical scale and body height with a stadiometer. Mid-arm circumference was measured on the right arm, relaxed mid-humerus, with a measuring tape. Body composition was measured by Dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy scanner in the 70-year-olds and Lunar iDXA (GE Health Care) in the 85-year-olds. Whole-body scans were performed, and lean soft tissue was analyzed (enCORE software version 12.30.008 and 14.10.022, respectively). Appendicular lean soft tissue index (ALSTI, kg/m2), defined as the sum of lean soft tissue in arms and legs divided by body height squared, was used as an estimate of muscle mass. Scanners were cross calibrated by a double scan of 33 subjects (12 men, 21 women) on the same day. iDXA ALST (appendicular lean soft tissue) measurements were calibrated with Prodigy using a regression equation with ALST, fat mass, and sex as predictors (R2 = 0.992, RMSE = 0.55 kg).
Strength and performance
Handgrip strength was measured with a Martin Vigorimeter (Gebrüder Martin GmbH & Co, Tuttlingen, Germany) with the shoulder joint in a neutral position. The large bulb was used for men and the medium bulb for women. The test was repeated three times for each hand, and the highest value of the strongest hand was used. Self-selected gait speed was measured over 30-m indoors with standing start (meter/second).
Sarcopenia definitions
Sarcopenia was defined using both the 2010 and the 2019 operational definitions proposed by the EWGSOP, Table 1 [1, 2]. When low muscle strength, low muscle quantity, and low physical performance are all detected, sarcopenia is considered severe [1, 2]. T-score based cut-offs were applied at two levels, − 2.0 and − 2.5 T-score, based on a regional normative reference population, Table 3. Cut-offs as originally published by the EWGSOP 1 and 2 were also applied for comparison (here termed “original”), except for hand grip strength were a cut-off of − 2.5 T-scores were applied, since no cut-offs for the Martin Vigorimeter have been published by the EGWSOP, Table 3 [1, 2]. In this way we studied the impact of the EWGSOP 1 and 2 operational definitions, with the same cut-offs, and the impact of different cut-off points on the prevalence of sarcopenia. T-scores for hand grip strength were estimated from healthy subjects aged 20–45 years (125 men, 125 women) from Sweden using the Martin Vigorimeter [28] (Table 3). T-scores for gait speed was estimated by a meta-analysis (pooling) of studies of healthy adults (30–59 years) from Sweden (Gothenburg area), measured over 30 m in “normal” walking speed with a standing start (67 men, 68 women) [29, 30]. Low muscle mass was defined as ALSTI measured by DXA below a T-score of − 2.0 and − 2.5 in Swedish healthy adults (Gothenburg area) aged 20–40 years (183 men, 239 women) [31] (Table 3).
Statistics
Data are presented as group mean ± standard deviation (SD) and proportions and prevalences as count and percent (%). Differences in means were tested with Student’s t-test and differences in proportions with Pearson’s Chi2-test. Differences in paired proportions (i.e. Sarcopenia classifications) were tested with McNemar’s exact test (binomial test) with confidence intervalls calculated according to Newcombe [32] and expressed as difference in percentge points. Cohen’s kappa were used to show agreement between classifications, rated according to Landis and Koch [33]. Differences in mortality between the cohorts and between participants vs. non-participants were tested with log-rank test. Associations with sarcopenia classifications and mortality were tested with Cox proportional-hazards model and associations with ADL dependence (Barthel index< 100) were tested with logistic regression. Models were adjusted for age (cohort) and sex. Result are presented as Hazard-ratios (HR) and Odds-ratios (OR) with 95% confidence interval. Statistical analyses were performed IBM SPSS statistics 26.0 software program (IBM Corp., Armonk, NY). The tests were considered significant at the level of P < 0.05 in two-tailed analyses.
Result
Population characteristics
Data on all diagnostic criteria measures were available for 1041 participants, 884 70-year old’s and 157 85-year-olds (i.e., 73 and 32% of those participating in the general exams, respectively). Characteristics of the samples are presented in Table 2.
There was a higher proportion who died during the 3 to 6 years of follow-up among non-participants, i.e., in those not taking part in the sarcopenia study compared to the participants, in both cohorts. Among men in both cohorts, and 70-year-old women, there were a higher proportion of smokers among non-participants. Among 70-year-old women there was a higher proportion with poor self-rated health and the same tendency was also shown among 85-year-old women, however not significant. In the 70-year-olds, participants in the sarcopenia study had higher education, lower BMI and faster gait speed compared to non-participants. In the 85-year-olds, non-participants used more medications than participants, see Table S1, Additional file 1.
Sarcopenia prevalence
The prevalence of reduced hand grip strength, gait speed and muscle mass at the T-score cut-offs (T-scores − 2.0 and − 2.5) and at the originally published cut-offs are shown in Table 3. The prevalence of reduced hand grip strength, gait speed and muscle mass were higher among the 85-year-olds compared to the 70-year-olds, both among men and women (P < 0.001).
The prevalence of sarcopenia and severe sarcopenia was higher in 85-year-olds compared to 70-year-olds irrespective of diagnostic criteria (P < 0.001) (Table 4). There was sex difference in the prevalence of sarcopenia among 70-year-olds when applying the − 2.0 T-score cut-offs for EWGSOP1 and 2 and the EWGSOP 1 with original cut-offs (Table 4). Among the 85-year-olds there was a sex difference when applying the EWGSOP1 T-score − 2.5 (Table 4). The prevalence of low hand grip strength without sarcopenia (EWGSOP2 T-score − 2.5) were 10 and 35% in the 70 and 85-year-olds, respectively. Consequently, 13 and 55% of those who had low hand grip strength (termed probable sarcopenia according to EWGSOP2) in the 70 and 85-year-olds respectively were confirmed sarcopenic according to EWGSOP2 (T-score − 2.5). There were no cases of severe sarcopenia in the 70-year-olds using the original EWGSOP 1 or 2 criteria.
Agreement between the EWGSOP 1 and 2 was “almost perfect” (Cohen’s kappa, range 0.82–1.00), except for 70-year-old men when applying the − 2.5 T-scores (Table 5).
Overall, the prevalence of sarcopenia was 1.0 and 0.9 percentage points lower using the EWGSOP 2 compared to EWGSOP 1 when applying the T-score − 2.0 cut-offs (15.0 vs. 15.9%, P = 0.008) and − 2.5 cut-offs (7.5 vs. 8.4%, P = 0.008) respectively (Table 5). There were significant differences in prevalence using the EWGSOP 2 compared to EWGSOP 1 with T-score − 2.0 in the 70-year-olds. No significant differences were found in the 85-year-olds, but confidence intervals of the difference were wide, including a difference of 3.9 to 8.2% (Table 5).
Agreement between the EWGSOP 1 and 2 when applying original cut-offs was “almost perfect” (Cohen’s kappa, range 0.82–0.92) (Table S2, Additional file 1). There was no significant difference in prevalence between EWGSOP 2 and 1 (Δ -0.3%, 10.5 vs. 10.8%, P = 0. 61) with original cut-offs. Among the 85-year-old men this difference nearly reached significance (Δ -8.8%, 52.6 vs. 61.4%, P = 0.063) (Table S2, Additional file 1).
There were significant differences in sarcopenia prevalence applying the − 2.0 vs. -2.5 T-score cut-offs to the EWGSOP1 and 2 operational definitions (P < 0.001) (Table 6). Overall, the prevalence was doubled (15.0 vs. 7.5%) using the − 2.0 vs. -2.5 T-scores with EWGSOP2. The increase in prevalence when changing the cut-offs was relatively larger in the 70-year-olds from 1.4 to 7.0% (5-fold) than in the 85-year-olds but were larger in absolute terms in the older cohort (Δ + 17.8%, 42 to 60%, P < 0.001) (Tables 4 and 6). The prevalence of severe sarcopenia increased from 0.2 to 1.2% (P = 0.004) in the 70-year-olds and from 19 to 31% (Δ + 12.1%, P < 0.001) in the 85-year-olds when changing cut-offs from T-score − 2.5 to − 2 (see Table S3, Additional file 1).
Mortality and ADL dependence
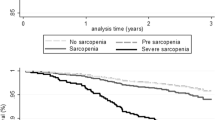
The median follow-up time was 5.2 years (range, 4.1—6.3) in the 70-year-olds and 3.7 years (range, 2.9—4.3) in the 85-year-olds. Mortality rates were 3.3% (n = 29) in the 70-year-olds and 15.3% (n = 24) in the 85-year-olds (Table 2). Sarcopenia according to both EWGSOP1 and 2 with − 2.5 T-score cut-offs were associated with increased mortality in the entire sample (HR 2.8 and 2.4 respectively, P < 0.05), but not when applying the − 2.0 T-score cut-offs (Table 7). In the 70-year-olds the hazard-ratios were 7.4 for EWGSOP1 (P = .001) and 7.6 for EWGSOP2 (P = .006) when applying the − 2.5 T-score cut-offs. Sarcopenia and severe sarcopenia were not associated with mortality in the 85-year-olds, regardless of diagnostic criteria (HR 1.2 to 3.2, P > 0.05) (see Table S4, Additional file 1).
Sarcopenia according to EWGSOP1 (− 2.5 T-scores), EWGSOP1 and 2 with “original” cut-offs and severe sarcopenia were associated with ADL dependence (P < 0.05) (Table 7). Gait speed was the only individual criteria associated with increased mortality and ADL dependence in the whole sample (Table 7). Stratified by cohort, gait speed was associated with ADL dependence in both cohorts, but with mortality only in the 70-year-olds (at T-score − 2.0 and − 2.5) (see Table S4 and S5, Additional file 1).
Discussion
We assessed the prevalence of sarcopenia estimated by the EWGSOP1 and 2 criteria applying T-score cut-offs at two levels in a population-based samples of 70-year-olds born in 1944 and 85-year-olds born in 1930. In this way we studied the impact of the difference in operational definitions as well as the impact of different cut-off points on the prevalence of sarcopenia.
EWGSOP 1 vs. 2
Overall, differences in sarcopenia prevalence were small and agreement was high between EWGSOP1 and EWGSOP 2, in both age groups, when applying the same cut-offs. However, in the 85-year-olds, the confidence interval of the difference in prevalence was wide, including a difference up to 8.2% at an overall prevalence of 42–62% (Table 5). Thus, a potential clinically meaningful difference in the older cohort could not be ruled out.
The sarcopenia prevalence will always be higher according to EWGSOP1 than 2 when using uniform cut-offs. The difference between the two operational definitions is the proportion of subjects with low performance but normal muscle strength (Table 1). Only three previous studies have compared EWGSOP 1 and EWGSOP 2 using uniform cut-offs (i.e., comparing operational definitions of sarcopenia) [8,9,10]. Locquet and colleagues reported a prevalence with EWGSOP1 and 2 of 11.6 and 7.4% respectively when applying the EWGSOP2 cut-offs (non-corrected ALSTI < 6.0 in women) in community-dwelling older adults (mean age 74 yrs.) [8]. Similar to us, Yang and colleagues found a slightly smaller difference applying population specific cut-offs in community-dwelling older adults (mean age 72 yrs.), 11.7 vs. 9.9% for EWGSOP1 and 2 respectively [9]. According to our study and these two studies, the EWGSOP2 operational definition decreases the prevalence by 0.8–4.2 percentage points when the overall prevalence is around 10%. However, Shafiee and colleagues found an unexpectedly poor agreement (Cohen’s kappa 0.34) and a large difference in prevalence, 7.9% (16.7 vs. 8.8%), between EWGSOP1 and 2, when applying uniform regional cut-offs in a large Iranian sample (mean age 69). This difference could be both method and population dependent which may need further exploration.
In the EWGSOP2, the cut-offs for low hand grip strength have been reduced for both sexes and for muscle mass in men compared to those suggested in EWGSOP1 [1, 2]. This should result in lower prevalence of sarcopenia, especially in men, that are separate from changes in operational definition. Consequently, most studies report that more individuals are classified as sarcopenic according to EWGSOP1 than according to EWGSOP2 with a difference around 2–6% (range − 2 to 20%) [8, 9, 11,12,13,14, 16,17,18,19,20, 34, 35]. Most studies have also found limited agreement between EWGSOP1 and 2 [8, 10, 14,15,16,17,18,19,20,21], while others have found a fair to good agreement [9, 11,12,13]. We found a substantial to almost perfect agreement between the two diagnostic criteria, especially when applying uniform T-score cut-offs.
Prevalence
We found that the prevalence of sarcopenia was 1.4–7.8% in 70-year-olds and 42–62% in 85 years-old’s, depending on the criteria used.
In a cohort of Swedish community-dwelling men, mean age 86.6 yrs. [11], the prevalence with EWGSOP1 was 20% and with EWGSOP2 22%. In our study, the corresponding prevalence was 61 and 53% in 85-year-old men, respectively. Our high prevalence of sarcopenia in the 85-year-olds is also considerably higher than in most other studies within the similar age range, applying varying diagnostic criteria [36, 37]. The difference might be explained by the choice of method, cut-off, and reference population. We found a higher prevalence of low handgrip strength and low muscle mass than has been reported previously in the same age groups in Sweden and Denmark [11, 38]. The prevalence found in the 70-year-olds is within the range of other studies, though the range among studies is large [36, 37, 39].
Impact of cut-off points
In the present study, a 0.5 T-score difference in cut-offs resulted in relatively large differences in the prevalence between these individual criteria, which translated to large differences in sarcopenia and severe sarcopenia prevalence (Tables 4, 6 and Table S3, Additional file 1). In the 70-year-olds a 5.8% difference (1.4 to 7.0%) and in the 85-year-olds a 17–18% difference (42 to 60%) in sarcopenia were observed. According to our results, the choice of cut-offs in a range suggested by the EWGSOP (i.e. -2.0 or − 2.5 T-scores) results in a difference in sarcopenia prevalence that is approximately 8 times larger than the difference due to the operational definitions of EWGSOP1 and 2 (Table 5, Table 6) [2].
Mortality and ADL dependence
We found that sarcopenia and severe sarcopenia were associated with a more than two-fold higher risk of all-cause mortality in the entire sample of 70 and 85-year-olds when applying the − 2.5 T-scores or the “original” cut-offs (Table 7). In a meta-analysis of studies defining sarcopenia according to EWGSOP1, the pooled HR for all-cause mortality was 1.6 (range 1.25–3.89) [40]. In three studies that applied both EWGSOP1 and 2, HR estimates have ranged from 1.16 to 1.95 in adjusted models. Two of the three community-based studies found that EWGSOP1, but not 2, were significantly associated with mortality [16, 41, 42]. In our study no clear difference between the two diagnostic criteria can be established, however, due to overlapping HR estimates.
According to our survival analysis, sarcopenia at age 70 was associated with an increased relative risk of death, while at age 85, there was no significant association (see Table S4, Additional file 1). This might indicate that sarcopenia is a more serious condition at age 70. Since sarcopenia is closely related to ageing, it might be more normal being classified as sarcopenic at age 85 (i.e., primary sarcopenia) compared to at age 70. At this younger age, sarcopenia could be a sign of an underlying illness (i.e., secondary sarcopenia).
We found that gait speed was associated with both mortality and ADL dependence, whereas handgrip strength and muscle mass showed no associations. Reduced strength and slowness are well-known predictors of mortality and other adverse health outcomes [2, 43,44,45,46]. Low muscle mass is associated with ADL dependence, but its association with mortality in community-dwelling older adults is less clear [44, 47, 48]. The exclusion of gait speed as a criterion for sarcopenia in EWGSOP2 may reduce the prognostic abilities for adverse outcomes.
Methodological considerations
There are some methodological challenges concerning cut-offs and testing procedures that need to be considered when interpreting and comparing results from different studies using the EWGSOP criteria. Firstly, in the EWGSOP1, specific cut-offs were not advised [1], but in the EWGSOP2, cut-offs were recommended to increase harmonization between studies. However, handgrip strength and gait speed are dependent on stature, and there are variations in muscle mass between populations in different geographical regions. Therefore, the use of regional normative population cut-offs (i.e., T-scores) is recommended [2, 49,50,51].
Secondly, how handgrip strength and gait speed are measured may also vary between studies (i.e., which hand, how many trials, a mean value for three trials or the highest value, walking distance and standing or rolling start) [9, 11]. Regarding gait speed, the cut-off suggested by EWGSOP, 0.8 m/s, is mainly derived from studies over short distances (2.4 to 6 m) and usually with a standing start [43, 46]. This procedure results in slower gait speed and reduced reliability compared to longer distances and a rolling start [52, 53]. For that reason, we derived cut-offs from Swedish studies using the same protocol as in our study (i.e., standing start over 30 m). In some studies, time up and go tests (TUG) are used instead of gait speed, which leads to a large difference in sarcopenia prevalence compared to using gait speed [17, 21, 50, 54]. Regarding handgrip strength, the type of device also affects results. The Jamar dynamometer is validated and widely used for measuring handgrip strength, but other devices, such as the Martin Vigorimeter, are also used [55]. Measures with the Jamar dynamometer and the Vigorimeter are highly correlated, show comparable reliability, and are probably clinically relevant to a similar extent [55,56,57]. However, the prevalence of low handgrip strength of 75–81% (at T-score − 2.5) in the 85-year-olds in the present study is considerably higher than the 42% found in a large British sample measured with the Jamar, from which the EWGSOP2 cut-offs (T-score − 2.5) were derived [58]. In the 70-year-olds, the prevalence was similar to the British sample (10–12% versus 9%) [58]. We speculate that the decline in strength at very old age might be assessed differently with a handle or a bulb dynamometer.
Thirdly, for assessment of muscle mass, several alternatives are available, and DXA is regarded as the most reliable for clinical purposes [59, 60]. Considerable differences among methods have been found, and there is limited agreement between different muscle mass adjustment techniques (i.e., height, BMI, etc.) [36, 61]. Taken together, these issues need consideration when comparing results from different studies.
According to our analysis, as well as others, differences in prevalence are mainly due to different cut-offs rather than the operational definition, and cut-offs, in turn, are dependent on different reference populations, measurement techniques, and procedures [17, 21, 36, 61].
These methodological variations hinder appropriate comparison between studies and populations and may have clinical implications leading to variations in treatment according to how sarcopenia is identified [36, 61].
Study sample
As expected, given the 15-year age difference, there was a significant difference in sarcopenia prevalence between the 1944 and 1930 cohorts. However, for interpretation of results, it is important to consider that primary, as well as secondary non-response, was greater in the 1930 cohort compared to the 1944 cohort, meaning that the 85-year-olds probably is less representative for and in better health compared to the general population in this age group. We therefore suspect that the difference in sarcopenia prevalence between 70- and 85-year-olds is even greater in the general population. Differences between participants and non-participants were found within both age groups indicating that participants were healthier than the general population at the same age. Moreover, the 15-year age difference between cohorts does not only mean an effect of age but also period, and cohort effects should be considered [62], e.g., differences in living conditions during the life course. In the Gothenburg H70 Birth Cohort Studies, a positive trend has been shown through cohorts at the same age with later-born cohorts generally at better health [63,64,65,66,67].
Strength and limitations
A strength in the present study is the use of a population-based and age-standardized sample. However, a considerable proportion of non-responders resulted in a small sample that may limit the generalizability of our findings, especially in the 85-year-olds and particularly among men of this cohort. Although our five and four-year follow-up of mortality is a v strength, the results should be interpreted with caution due to a small number of deaths during this timeframe. Comparing the birth cohorts, from a statistical point of view, the prevalence of sarcopenia, around 50% in 85-year-olds, gives stable values as opposed to the 70-yr-olds with a low prevalence, which leads to greater uncertainty in the true prevalence in the 70-year-olds. However, the smaller sample size of the 85-year-old cohort results in greater uncertainties in the estimates of prevalence and associations with mortality and ADL dependence. The use of DXA is a strength of this study [59, 60]. Handgrip strength was measured by the Martin Vigorimeter and gait speed was measured over 30 m, but no normalized reference cut-offs are present for these methods. We, therefore, constructed T-score cut-offs from a limited sample. On the one hand, this could be questioned and regarded as a limitation. On the other hand, the use of T-score cut-offs derived from a local population and with the exact same methods is a strength of this study. For this reason, the EWGSOP1 and 2 “original” were compared with the same cut-offs for handgrip strength, which is expected to make the difference between them smaller than when using the non-uniform published cut-offs [1, 2].
Conclusion
In this cross-sectional study we found that the prevalence of sarcopenia was significantly higher in the 85-year-olds compared to 70-year-olds, irrespective of diagnostic criteria. Overall, the differences between the EWGSOP1 and 2 classifications were small within the same age group. The prevalence of sarcopenia was more dependent on cut-offs than on the operational definition. Meaningful differences in sarcopenia prevalence between EWGSOP1 and 2 in the 85-year-olds could not be ruled out. More research is needed to fully understand the predictive value of sarcopenia defined by EWGSOP1 and 2 in relation to morbidity and mortality. With this study, we hope to contribute to the emerging data on EWGSOP2 diagnostic criteria of sarcopenia and to the understanding of strategies leading to the implementation of standard measurements of sarcopenia in older adults.
Availability of data and materials
An anonymized dataset is available to collaborative partners of the H70 Birth Cohort Studies study group after approved request. The senior author, Ingmar Skoog, can be contacted for further information (ingmar.skoog@neuro.gu.se).
Abbreviations
- ADL:
-
Activities of daily living
- ALSTI:
-
Appendicular lean soft tissue index
- BMI:
-
Body mass index
- DXA:
-
Dual-energy X-ray absorptiometry
- EWGSOP:
-
European working group on sarcopenia in older people
- H70:
-
Gothenburg H70 Birth Cohort Studies
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- TUG:
-
Timed up and go
- Yrs.:
-
Years
- WHO:
-
World Health Organization
- ICD:
-
International Classification of Disease
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990s–1s.
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548.
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol: Ser A. 2014;69(5):547–58.
World Health Organization. The international classification of diseases, 10th revision. 10th ed. Geneva: World Health Organization (WHO); 2016.
Locquet M, Beaudart C, Petermans J, Reginster JY, Bruyere O. EWGSOP2 versus EWGSOP1: impact on the prevalence of sarcopenia and its major health consequences. J Am Med Dir Assoc. 2019;20(3):384–5.
Yang M, Liu Y, Zuo Y, Tang H. Sarcopenia for predicting falls and hospitalization in community-dwelling older adults: EWGSOP versus EWGSOP2. Sci Rep. 2019;9(1):17636.
Shafiee G, Heshmat R, Ostovar A, Khatami F, Fahimfar N, Arzaghi SM, et al. Comparison of EWGSOP-1and EWGSOP-2 diagnostic criteria on prevalence of and risk factors for sarcopenia among Iranian older people: the Bushehr Elderly Health (BEH) program. J Diabetes Metab Dis. 2020;19(2):727–34.
Franzon K, Zethelius B, Cederholm T, Kilander L. The impact of muscle function, muscle mass and sarcopenia on independent ageing in very old Swedish men. BMC Geriatr. 2019;19(1):153.
Krzymińska-Siemaszko R, Tobis S, Lewandowicz M, Wieczorowska-Tobis K. Comparison of four sarcopenia screening questionnaires in community-dwelling older adults from Poland using six sets of international diagnostic criteria of sarcopenia. PLoS One. 2020;15(4):e0231847.
Zhuang CL, Shen X, Zou HB, Dong QT, Cai HY, Chen XL, et al. EWGSOP2 versus EWGSOP1 for sarcopenia to predict prognosis in patients with gastric cancer after radical gastrectomy: analysis from a large-scale prospective study. Clin Nutr. 2020;39:2301-10.
de Freitas MM, de Oliveira VLP, Grassi T, Valduga K, Miller MEP, Schuchmann RA, et al. Difference in sarcopenia prevalence and associated factors according to 2010 and 2018 European consensus (EWGSOP) in elderly patients with type 2 diabetes mellitus. Exp Gerontol. 2020;132:110835.
Menna Barreto APM, Barreto Silva MI, Pontes K, Costa MSD, Rosina KTC, Souza E, et al. Sarcopenia and its components in adult renal transplant recipients: prevalence and association with body adiposity. Br J Nutr. 2019;122(12):1386–97.
Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Morales C. New versus old guidelines for sarcopenia classification: what is the impact on prevalence and health outcomes? Age Ageing. 2020;49(2):300–4.
Phu S, Vogrin S, Zanker J, Bani Hassan E, Al Saedi A, Duque G. Agreement between initial and revised European working group on sarcopenia in older people definitions. J Am Med Dir Assoc. 2019;20(3):382–383.e381.
Reiss J, Iglseder B, Alzner R, Mayr-Pirker B, Pirich C, Kässmann H, et al. Consequences of applying the new EWGSOP2 guideline instead of the former EWGSOP guideline for sarcopenia case finding in older patients. Age Ageing. 2019;48(5):719–24.
Warzecha M, Amarowicz J, Berwecka M, Czerwiński E, Kumorek A. Relation between risk of falls, sarcopenia and parameters assessing quality of skeletal muscles in a group of postmenopausal women. Przeglad mEnopauzalny = Menopause Rev. 2020;19(3):123–9.
Villani A, McClure R, Barrett M, Scott D. Diagnostic differences and agreement between the original and revised European Working Group (EWGSOP) consensus definition for sarcopenia in community-dwelling older adults with type 2 diabetes mellitus. Arch Gerontol Geriatr. 2020;89:104081.
Sui SX, Holloway-Kew KL, Hyde NK, Williams LJ, Tembo MC, Leach S, et al. Definition-specific prevalence estimates for sarcopenia in an Australian population: the Geelong Osteoporosis Study. JCSM Clin Rep. 2020;5(4):89–98.
Yang L, Yao X, Shen J, Sun G, Sun Q, Tian X, et al. Comparison of revised EWGSOP criteria and four other diagnostic criteria of sarcopenia in Chinese community-dwelling elderly residents. Exp Gerontol. 2020;130:110798.
Rydberg Sterner T, Ahlner F, Blennow K, Dahlin-Ivanoff S, Falk H, Havstam Johansson L, et al. The Gothenburg H70 birth cohort study 2014-16: design, methods and study population. Eur J Epidemiol. 2019;34(2):191–209.
Lissner L, Skoog I, Andersson K, Beckman N, Sundh V, Waern M, et al. Participation bias in longitudinal studies: experience from the population study of women in Gothenburg, Sweden. Scand J Prim Health Care. 2003;21(4):242–7.
Eiben G, Dey DK, Rothenberg E, Steen B, Björkelund C, Bengtsson C, et al. Obesity in 70-year-old swedes: secular changes over 30 years. Int J Obesity (2005). 2005;29(7):810–7.
Grimby G, Börjesson M, Jonsdottir IH, Schnohr P, Thelle DS, Saltin B. The “Saltin-Grimby physical activity level scale” and its application to health research. Scand J Med Sci Sports. 2015;25(Suppl 4):119–25.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland State Med J. 1965;14:61–5.
Thorngren KG, Werner CO. Normal grip strength. Acta Orthop Scand. 1979;50(3):255–9.
Sunnerhagen KS, Hedberg M, Henning GB, Cider A, Svantesson U. Muscle performance in an urban population sample of 40- to 79-year-old men and women. Scand J Rehabil Med. 2000;32(4):159–67.
Willén C, Lehmann K, Sunnerhagen K. Walking speed indoors and outdoors in healthy persons and in persons with late effects of polio. J Neurol Res. 2013;3:62–7.
Larsson I, Lissner L, Samuelson G, Fors H, Lantz H, Näslund I, et al. Body composition through adult life: Swedish reference data on body composition. Eur J Clin Nutr. 2015;69(7):837–42.
Altman D, Machin D, Bryant T. Statistics with confidence: confidence intervals and statistical guidelines. Hoboken: Wiley; 2000.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Harvey NC, Orwoll E, Kwok T, Karlsson MK, Rosengren BE, Ribom E, et al. Sarcopenia definitions as predictors of fracture risk independent of FRAX(®), falls, and BMD in the osteoporotic fractures in men (MrOS) study: a meta-analysis. J Bone Mineral Res. 2021;36(7):1235–44.
Van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: a clinical perspective. Arch Gerontol Geriatr. 2020;90:104125.
Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, De Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56.
Purcell SA, MacKenzie M, Barbosa-Silva TG, Dionne IJ, Ghosh S, Olobatuyi OV, et al. Sarcopenia prevalence using different definitions in older community-dwelling Canadians. J Nutr Health Aging. 2020;24(7):783–90.
Suetta C, Haddock B, Alcazar J, Noerst T, Hansen OM, Ludvig H, et al. The Copenhagen sarcopenia study: lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J Cachexia Sarcopenia Muscle. 2019;10(6):1316–29.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90.
Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas. 2017;103:16–22.
Bachettini NP, Bielemann RM, Barbosa-Silva TG, Menezes AMB, Tomasi E, Gonzalez MC. Sarcopenia as a mortality predictor in community-dwelling older adults: a comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur J Clin Nutr. 2020;74(4):573–80.
Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85-89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318.
Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J Nutr Health Aging. 2009;13(10):881–9.
Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020;68(7):1410–8.
Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. 2016;16(1):5–20.
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–8.
Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68(7):1429–37.
Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(1):3–25.
Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016;17(8):767.e761–7.
Sim M, Prince RL, Scott D, Daly RM, Duque G, Inderjeeth CA, et al. Sarcopenia definitions and their associations with mortality in older Australian women. J Am Med Dir Assoc. 2019;20(1):76–82.e72.
Zanker J, Scott D, Reijnierse EM, Brennan-Olsen SL, Daly RM, Girgis CM, et al. Establishing an operational definition of sarcopenia in Australia and New Zealand: Delphi method based consensus statement. J Nutr Health Aging. 2019;23(1):105–10.
Bohannon RW, Wang YC. Four-meter gait speed: normative values and reliability determined for adults participating in the NIH toolbox study. Arch Phys Med Rehabil. 2019;100(3):509–13.
Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182–9.
Savas S, Taskiran E, Sarac FZ, Akcicek F. A cross-sectional study on sarcopenia using EWGSOP1 and EWGSOP2 criteria with regional thresholds and different adjustments in a specific geriatric outpatient clinic. Eur Geriatr Med. 2020;11(2):239–46.
Sipers WM, Verdijk LB, Sipers SJ, Schols JM, van Loon LJ. The Martin Vigorimeter represents a reliable and more practical tool than the Jamar dynamometer to assess handgrip strength in the geriatric patient. J Am Med Dir Assoc. 2016;17(5):466.e461–7.
Merkies IS, Schmitz PI, Samijn JP, Meché FG, Toyka KV, van Doorn PA. Assessing grip strength in healthy individuals and patients with immune-mediated polyneuropathies. Muscle Nerve. 2000;23(9):1393–401.
Neumann S, Kwisda S, Krettek C, Gaulke R. Comparison of the grip strength using the Martin-Vigorimeter and the JAMAR-dynamometer: establishment of normal values. In vivo (Athens, Greece). 2017;31(5):917–24.
Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–43.
Sanchez-Rodriguez D, Marco E, Cruz-Jentoft AJ. Defining sarcopenia: some caveats and challenges. Curr Opin Clin Nutr Metab Care. 2020;23(2):127–32.
Mayhew AJ, Phillips SM, Sohel N, Thabane L, McNicholas PD, de Souza RJ, et al. Do different ascertainment techniques identify the same individuals as sarcopenic in the Canadian longitudinal study on aging? J Am Geriatr Soc. 2020;11(6):1603–13. Published online 2020 Sep 17. https://doi.org/10.1002/jcsm.12622.
Blanchard RD, Bunker JB, Wachs M. Distinguishing aging, period and cohort effects in longitudinal studies of elderly populations. Socio Econ Plan Sci. 1977;11(3):137–46.
Skoog I, Borjesson-Hanson A, Kern S, Johansson L, Falk H, Sigstrom R, et al. Decreasing prevalence of dementia in 85-year olds examined 22 years apart: the influence of education and stroke. Sci Rep. 2017;7(1):6136.
Zhi X, Joas E, Waern M, Östling S, Börjesson-Hanson A, Skoog I. Prevalence of cardiovascular disorders and risk factors in two 75-year-old birth cohorts examined in 1976-1977 and 2005-2006. Aging Clin Exp Res. 2013;25(4):377–83.
Wilhelmson K, Allebeck P, Steen B. Improved health among 70-year olds: comparison of health indicators in three different birth cohorts. Aging Clin Exp Res. 2002;14(5):361–70.
Horder H, Skoog I, Johansson L, Falk H, Frandin K. Secular trends in frailty: a comparative study of 75-year olds born in 1911-12 and 1930. Age Ageing. 2015;44(5):817–22.
Falk H, Johansson L, Ostling S, Thogersen Agerholm K, Staun M, Host Dorfinger L, et al. Functional disability and ability 75-year-olds: a comparison of two Swedish cohorts born 30 years apart. Age Ageing. 2014;43(5):636–41.
Acknowledgements
The authors thank the participants of the Gothenburg H70 birth cohort studies.
Funding
The 1944 study on 70-year-olds was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALF 716681), the Swedish Research Council (2012-5041, 2015-02830, 2019-01096, 2013-8717, 2017-00639), Swedish Research Council for Health, Working Life and Wellfare (2013-1202, 2018-00471,,AGECAP 2013-2300, 2013-2496), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Hjärnfonden (FO2014-0207, FO2016-0214, FO2018-0214, FO2019-0163, FO2020-0235), Alzheimerfonden (AF-554461, AF-647651, AF-743701, AF-844671, AF-930868, AF-940139), Eivind och Elsa K:son Sylvans stiftelse.
The 1930 study on 85-year-olds was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALF 716681, 722441), The Swedish Research Council (2007-7462, 2012-5041, 2015-02830, 2019-01096, 2013-8717, 2017-00639), Swedish Research Council for Health, Working Life and Wellfare (2006-0596, 2008-1111, 2010-0870, 2013-1202, AGECAP 2013-2300, 2013-2496, 2013-0475), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Hjärnfonden (FO2014-0207, FO2016-0214, FO2018-0214, FO2019-0163, FO2020-0235), Alzheimerfonden (AF-554461, AF-647651, AF-743701, AF-844671, AF-930868, AF-940139), Eivind och Elsa K:son Sylvans stiftelse, The Alzheimer’s Association Zenith Award (ZEN-01-3151), The Alzheimer’s Association (IIRG-09-131338).
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
OW, ER, IB: Conception and design of the study, OW, ER: Drafting and writing the article, IS, KF, HFE, HW, LL, LR, TRS OW, ER, IB,: Acquisition of data, OW, ER,: Analysis and interpretation of data, OW, ER, IB, IS, KF, HFE, HW, LL, LR, TRS: Revising the article critically for important intellectual content. All authors read and approved the final manuscript. ER and IS are joint senior authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The H70 study was approved by the Regional Ethical Review Board in Gothenburg (Approval Numbers: 869-13, T166-14, S377-99, T45-304) and all subjects provided written informed consent. The study was performed in accordance with the principles of the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
None of the authors had any conflict of interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Characteristics of participants and non-participants, Table S2. Difference in sarcopenia prevalence between EWGSOP1 and 2 using cut-offs as published by EWGSOP [1, 2], Table S3. Difference in the prevalence of severe sarcopenia when applying T-scores cut-offs at − 2.0 vs. -2.5, Table S4. Associations between mortality and sarcopenia critera stratified by cohort, Table S5. Associations between ADL dependence and sarcopenia critera stratified by cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wallengren, O., Bosaeus, I., Frändin, K. et al. Comparison of the 2010 and 2019 diagnostic criteria for sarcopenia by the European Working Group on Sarcopenia in Older People (EWGSOP) in two cohorts of Swedish older adults. BMC Geriatr 21, 600 (2021). https://doi.org/10.1186/s12877-021-02533-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-021-02533-y