Abstract
Background
Uncoordinated interprofessional communication in nursing homes increases the risk of polypharmacy and inappropriate medication use. This may lead to augmented frequency of adverse drug events, hospitalizations and mortality. The aims of this study were (1) to improve interprofessional communication and medication safety using a combined intervention and thus, (2) to improve medication appropriateness and health-related outcomes of the included residents.
Methods
The single-arm interventional study (2014–2017) was conducted in Muenster, Germany and involved healthcare professionals and residents of nursing homes.
The intervention consisted of systematic education of participating healthcare professionals and of a structured interprofessional medication review which was performed via an online communication platform.
The primary endpoint was assessed using the Medication Appropriateness Index MAI. Secondary endpoints were: cognitive performance, delirium, agitation, mobility, number of drugs, number of severe drug-drug interactions and appropriateness of analgesics.
Outcomes were measured before, during and after the intervention. Data were analyzed using descriptive and inference-statistical methods.
Results
Fourteen general practitioners, 11 pharmacists, 9 nursing homes and 120 residents (n = 83 at all testing times) participated.
Overall MAI sum-score decreased significantly over time (mean reduction: -7.1, CI95% -11.4 – − 2.8; median = − 3.0; dCohen = 0.39), especially in cases with baseline sum-score ≥ 24 points (mean reduction: -17.4, CI95% -27.6 – − 7.2; median = − 15.0; dCohen = 0.86).
MAI sum-score of analgesics also decreased (dCohen = 0.45). Mean number of severe drug-drug interactions rose slightly over time (dCohen = 0.17). The proportion of residents showing agitated behavior diminished from 83.9 to 67.8%. Remaining secondary outcomes were without substantial change.
Conclusion
Medication appropriateness increased particularly in residents with high baseline MAI sum-scores. Cognitive decline of participating residents was seemingly decelerated when compared with epidemiologic studies. A controlled trial is required to confirm these effects. Interprofessional interaction was structured and performance of medication reviews was facilitated as the online communication platform provided unlimited and consistent access to all relevant and updated information.
Trial registration
DRKS Data Management, ID: DRKS00007900, date of registration: 2015-09-02 (retrospectively registered i.e. 6 weeks after commencement of the first data collection).
Similar content being viewed by others
Background
Polypharmacy and inappropriate medication use are major health concerns and represent common phenomena in older persons [1], leading to adverse drug events (ADEs) [2], increased hospitalization rates [3, 4] and mortality [5]. Residents of nursing homes (NHs) are at a particularly high risk of polypharmacy and inappropriate medication [5] due to complex and multiple comorbidities.
In Germany, most drug prescriptions for NH residents (NHRs) are performed by general practitioners (GPs), but also by other physicians [6] (e.g. specialists in neurology or psychiatry). Usually several different GPs and pharmacists working in offices or community pharmacies respectively supply one NH. Pharmacists dispense the medication, control drug storage and train the nurses in adequate delivery of drugs. They also perform analyses of drug-drug interactions (DDIs) but are not consulted regularly for complete medication reviews. Frequencies of NH visits performed by GPs, other physicians or pharmacists are variable and sometimes inconsistent. Nurses deliver medications and monitor the residents’ clinical condition [7].
The involvement of numerous different providers in the medication process leads to the demand for a regular exchange of information between all healthcare professionals concerned and for a periodic review and adjustment of the medication; however, previous studies have shown that this fails to occur regularly [8] which entails an increased risk of inappropriate prescribing [9]. Hence, a need for improved interprofessional communication is highlighted [10].
Health information technology (HIT) interventions are considered to be valuable in this regard, however, their impact on interprofessional collaboration is unclear [11]. A US controlled study applied prospective medication reviews performed by pharmacists, HIT-supported patient assessment, formalized pharmaceutical care planning in NHRs at high risk for medication-related problems, and direct communication with prescribers and found a positive effect on medication appropriateness, but no changes in number of medications, hospitalizations and mortality [12]. A current Belgian cluster-RCT with a multi-faceted, complex intervention in NHs applies a web tool for sharing patient-related information between healthcare professionals, preparing medication reviews and generating standardized reports of multi-disciplinary case conferences [13].
Nevertheless, up to now electronic tools have rarely been used as direct interprofessional communication instruments within the medication process in NHs. In general, previous HIT interventions aiming at improving medication safety frequently used computerized order entry and clinical decision support systems (CPOE/CDS) [14] by addressing various steps of the prescription process. Positive effects on quality of prescribing (improved guideline adherence, reduction of medication errors) and partly on surrogate outcomes (reduction of ADEs) were demonstrated [15, 16], but the use of these systems was limited in NHs [14, 17] and patient-related outcomes (e.g. mortality/hospitalizations) were studied only occasionally with inconsistent results [14, 18]. CPOE systems have also shown negative effects on communication, e.g. misconceptions about abrogated needs of face-to-face communication [19]. An American cluster-randomized controlled trial (RCT) used a HIT intervention targeting the monitoring stage of the medication process in NHs by using a software which evaluated medication regimens, identified patients at risk for falls and/or delirium and generated specific monitoring plans; the study found no effect on falls, but positive effects on delirium, hospitalization rates and mortality for newly admitted NHRs of the intervention group [20].
Other interventions addressing medication safety in NHs consisted of medication reviews, educational programs or multi-disciplinary case conferences and seemed to also be effective in reducing inappropriate prescribing [21], but couldn’t show consistent improvements of patient-related outcomes: some studies found a reduction of falls [22, 23], delirium [24] and hospital days [25], while no changes regarding mortality [10, 22, 25, 26], residents’ behavior [27], hospitalizations [22, 23, 26], quality of life [23], functional status or cognitive skills [22] were noted. Nevertheless, it seems plausible that enhanced interprofessional communication and consequent medication surveillance impacts patients’ outcomes favorably.
The aim of the InTherAKT study (“Initiative zur Therapiesicherheit in der Altenhilfe durch Kooperation und Teamwork”- Initiative for medication safety in long-term care via cooperation and teamwork) was to improve interprofessional collaboration within the medication process in NHs by a combined intervention consisting of systematic education of participating healthcare professionals and a structured interprofessional medication review via an online communication platform. The study evaluated changes in medication appropriateness as well as patient-related outcomes and changes in interprofessional communication.
Study hypothesis
-
1.
The intervention improves appropriateness of drug prescriptions for included NHRs (primary outcome).
-
2.
The intervention has effects on patient-related outcomes (cognitive skills, mobility, agitation, delirium), number of drugs, severe DDIs and on appropriateness of analgesics (secondary outcomes).
The impact on interprofessional communication was investigated using a qualitative approach; these results will be reported in a separate publication.
Methods
Details of methodology and study protocol have been published previously [7] and are summarized in the present article. The study adheres to CONSORT guidelines.
Study design and population
The single-arm interventional study (2014–2017) involved healthcare professionals operating in NH care (GPs, nurses, pharmacists) and NHRs in Muenster, Germany.
Recruitment and sample size
Recruitment (January 2015 – July 2015) was started with GPs as participation of all other healthcare professionals depended from GPs’ disposition to attend. GPs were invited by written information (newsletter of the GP association of Muenster to all its 120 members), an on-site information event and personal visits. Additionally, to achieve the aspired number of NHRs, physicians of the GP association of Muenster holding contracts for intensified treatment [7] of NHRs were addressed specifically. Participating GPs recruited their patients living in NHs and fulfilling the inclusion criteria. Corresponding NHs and pharmacists were recruited by the project team.
Inclusion criteria (residents): written informed consent (resident/legal representative), age ≥ 65 years, ≥1 drug prescription/s.
Exclusion criteria (residents): missing consent, insufficient cognitive performance and no legal representative, acute life-threatening situation, quarantine (isolation due to infectious diseases).
Inclusion criteria (professional groups): GPs treating NHRs, pharmacists supplying participating NHs, nurses with ≥3 year state-approved training.
Power calculation was performed with a power of 0.8 (1-β = 80%). According to comparable trials [28], an effect size of at least dCohen = 0.33 was assumed, leading to a necessary sample size of 58 NHRs for the primary endpoint.
Combined intervention
The intervention was developed and executed by the multi-professional project team (nurses, GPs, specialist in internal medicine, clinical pharmacists) by integrating current evidence, expertise and experiences regarding geriatric pharmacotherapy, e-learning and challenges arising during interprofessional cooperation in NHs.
-
(1)
A three-step education of participating healthcare professionals was conducted (August 2015 – November 2015) addressing medication safety in older adults [7]. The blended-learning concept (combining face-to-face and online training) [29] covered the following topics: drug therapy in older and multi-morbid adults, drug-drug and drug-disease interactions, medication errors, ADE risk groups and monitoring, pharmacovigilance, polypharmacy, potentially inappropriate prescriptions (PIP), over- and under-prescribing, prioritizing and deprescribing of drugs (evaluation of patient’s drug regimens according to current diagnoses and therapy goals and subsequent dose reduction or stopping of medications with lack of benefit or causing potential harm), medication review, legal aspects of drug therapy, strategies to enhance interprofessional cooperation [7].
The training consisted of:
-
a kick-off interprofessional face-to-face workshop (3 h)
-
three profession-specific online sessions (each 20–45 min) with audio-visual presentations and autonomous processing of case files addressing medication-related problems
-
a final interprofessional face-to-face event with discussion of the cases and instructions for the second part of the intervention (1.5 h).
-
(2)
Upon conclusion of the training, the therapy check process was conducted (January 2016 – September 2016) with the aim of structuring interprofessional communication within the medication process. An electronic communication tool, the InTherAKT-online Platform (I-oP), was designed for this purpose by the software-engineering company smart-Q (Germany) in collaboration with the project team. The tool was accessible online (https://www.intherakt.de/app) via double-secured access keys. Each participant was granted access solely to the respective residents in treatment.
The system prompted users to enter patient-related data (age, gender, corresponding NH/GP/pharmacist, diagnoses, allergies/drug intolerances, height/weight, creatinine, other relevant laboratory values, physician visits/hospitalizations) and medication-related data (brand name, International Nonproprietary Name, indication, dosage, form/time of administration, indications for administration/monitoring, regular/as-needed medication, prescriber, date of first prescription). The medication plan was based on the German Medication Plan 2.0 [30] (standardized, nationally introduced schedule to improve patient safety by enhancing the information flow between concerning healthcare professionals). Furthermore, the I-oP allowed documentation of patients’ symptoms and to convene and document case conferences (Fig. 1).
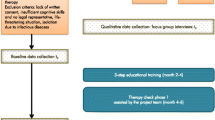
After an intensive testing and revising phase, the tool was authorized for use in the therapy check process, which consisted of the following steps (Fig. 2):
-
Nurses/study assistants (see below) entered data in the I-oP and informed the responsible GP
-
The GP completed the medication plan and performed a comprehensive medical review of the drug regimens based on updated and complete drug/patient information (clinical and laboratory parameters) and on the knowledge acquired during the training. The GP released the reviewed medication plan to the responsible nurses and pharmacist. GPs also had the possibility to ask the pharmacists specific questions relating to medication use.
-
The pharmacist performed a medication review type 1 [31] (Fig. 3), informed the GP about suggestions for improvement if applicable
-
GP reviewed pharmacist’s suggestions, released the revised medication plan to the nurses and communicated with the NHR and/or legal representative verbally during onsite visits in the NH
-
Nurses performed clinical monitoring and weekly documentation of residents’ symptoms (section “Therapiebeobachtung”/therapy monitoring, Fig. 1) [32] and informed the GP in case of unexpected changes
-
On demand: organization of multi-disciplinary case conferences if interprofessional decision-making was required.
Communication between healthcare professionals was conducted via a standardized messaging system. Messages were generated automatically whenever the next therapy check step was released and were sent to the email address of the relevant contact person. Due to data security requirements messages contained no patient data, but included a link to the start page of the I-oP (“To-Do” section), accessible via the individual access keys, where all current actions to perform were listed.
Participants used the platform whenever they were advised there was a step to perform and additionally for inputting documentation. In case of an urgent need for action (e.g. acute or severe symptoms), healthcare professionals communicated by phone and retrospectively documented events, decisions and therapeutic implications in the online platform.
All participants received profession-specific instruction manuals. After the first intervention period, some functions were revised and optimized, e.g., when clicking “InTherAKT connect”, a dichotomization between “invite for action” and “notice” was introduced (Fig. 4).
Documentation in the I-oP had to be conducted in addition to routine documentation in the respective electronic health records (EHRs) as the tool has not yet been embedded into primary documentation. Thus, study assistants were recruited to aid nurses entering data and to support the therapy check in the NHs in close collaboration with the nurses. The project team supervised all participants; in the second intervention period (between data collection t1 and t2, see below) this supervision was restricted to technical support and the project team intervened only if activities in the I-oP had ceased for 1 week. Study assistants were supervised continuously during the whole study period by the project team.
The therapy check procedure was planned to be completed at least once for every resident until t1 and was intended to re-start at any relevant change of the medication (e.g. new prescription, modification of dosage etc.).
Outcome measurements
Primary endpoint: Appropriateness of prescribed medications, measured via the Medication Appropriateness Index (MAI) [33]. MAI ratings were conducted by two different clinical pharmacists (one at t0, one at t1/t2) who were not study participants. The pharmacists who conducted the MAI ratings were instructed and supervised by the same expert clinical pharmacist throughout the study period. They followed an instruction regarding the use of the MAI which was provided on request by the originators of the MAI (unpublished document, last updated on 2014 Nov 01).
Data used for the MAI rating were collected from the NH documentation (complete list of medications with brand names and International Nonproprietary Names of drugs, indication, dosage, mode of application, duration of therapy, instructions for application; patient-related data: height, weight, age, current ICD-10 coded diagnoses, laboratory values e.g. creatinine, potassium, blood sugar; known drug intolerances, allergies).
The MAI consists of 10 items which are assessed for each prescribed drug. Each item is rated as “appropriate” (0 points) or “inappropriate”; in the latter case, a weighted score is assigned [34] (Table 1). MAI scores per drug are summated to get the sum-score (MAI-Sum) per NHR [28]. Higher sum-scores indicate higher inappropriateness; however, the absolute MAI-Sum is not a direct parameter for medication appropriateness because it depends on the number of drugs. Thus, MAI-Sum shows high variability and therefore we calculated also a MAI-Mean. Mean difference of MAI-Sum (mean MAI change) is used [28] to interpret changes within a sample and to compare results between different studies.
Secondary endpoints are listed in Table 1.
Data collection
Outcome parameters were collected before (t0, July – October 2015), during (t1, May – July 2016) and after the intervention (t2, October – December 2016). Due to delays in the recruitment phase (caused by summer vacations and absence of participants), baseline data collection was concluded 2 months later than scheduled and t0 concerning 17 NHRs was completed after the first training session. Data collections were performed by trained study assistants in direct contact with NHRs (e.g. MMSE), as proxy-tools with nurses (e.g. DSS) or using the EHR (e.g. medication-related data). Residents’ diagnoses derived from EHRs were compared with the diagnoses registered in the I-oP (filled in by nurses and GPs) and completed when required. After pseudonymization by the study assistants, data were exported for analysis.
Furthermore, structural data of participating NHs, pharmacies and GP offices (e.g. number of residents/employees, monthly NH visits per GP) and variables describing the intervention period were collected (level of care, number of hospitalizations/physician consultations, falls, performed case conferences, mortality, physical function via Barthel Scale [42], presence of pain via Verbal Rating Scale (VRS) [43] and Pain Assessment in Advanced Dementia Scale (PAINAD) [44]).
Data analysis
Statistical analysis was performed via IBM®SPSS Statistics®23 using descriptive methods, calculations of effect sizes and Pearson’s correlations. For the primary endpoint, change between t0 and t2 was analyzed using one-sided T-tests for dependent samples (95%CI, significance level: 0.05). Due to the study’s descriptive nature hypothesis-testing procedures aimed primarily to generate hypotheses.
Based on a recent RCT determining 24 MAI-Sum points as potential cut-off to initiate a medication review [45], NHRs were dichotomized according to their baseline sum-score (< 24/≥24 points). MAI scores were calculated for all NHRs and for these two subgroups.
Results
Study participants
Fourteen of 120 invited GPs, 9 of 15 invited NHs, 11 of 12 invited pharmacists and 120 of 233 invited NHRs (Fig. 5, Table 2) participated.
83 NHRs completed the intervention. No difference was found between drop-outs and remaining NHRs regarding MAI-sum (Tdf = 118 = 0.83, p = .409) or MAI-mean (T118df = 118 = 0.98, p = .329) at baseline
Intervention
All institutions were represented at the first onsite training with the exception of one GP. As the first training session was recorded, footage of this event could be provided to all participants.
To ensure that participants could watch all profession-specific online presentations, access keys were not personalized; thus, distinct individualized assignment of accesses was not available.
Participants not attending the final event (6 GPs, 3 pharmacists, 3 NHs) were instructed personally about the therapy check.
At t1, one full therapy check was completed for 35 NHRs (42.2%). 15 (18.1%) of the cycles were at the point where the first GP’s review had been sent to the pharmacist, 30 (36.1%) where at the point after the conduction of the pharmacist’s review (Fig. 2).
At t2, at least one therapy check was completed for all NHRs except for one who was consequently excluded from analysis. A second full cycle was completed in 7 cases (8.4%). For further 9 NHRs (10.8%) a second pharmacist’s review was performed without completing the cycle. Communication steps without medication review were performed for further 15 NHRs (18.1%).
No case conferences took place.
Primary endpoint
Sixty-two point seven percent of NHRs showed a reduction in MAI-Score over time. Distribution of MAI change is shown in Fig. 6. MAI-Sum and MAI-Mean decreased significantly. Change in MAI-Sum showed a small effect (d = 0.39) with a mean reduction of 7.1 ± 19.6 and a median reduction of 3.0 between t0 and t2. As mean variation of MAI-Sum was high due to different numbers of drugs, change in MAI-Mean showed a higher effect (d = 0.61).
Mean MAI change and effect size were high for NHRs with baseline MAI-Sum ≥24 and showed no effect for those with MAI-Sum < 24. A strong association between baseline MAI-Sum and mean MAI change was found: the higher MAI-Sum at baseline, the higher the mean change (thus, the stronger the improvement). MAI-Sum at baseline explained 32.8% of the variance of MAI change (Table 3).
Table 4 shows detailed descriptive results of MAI-Sum and mean MAI change by the single MAI items for the whole sample (n = 83). The greatest mean MAI changes of > 1 point were noted in item 1 (indication), item 10 (costs) and item 3 (dosage). The MAI-Sum of item 6 (clinically significant drug-drug interactions) increased over time. After exclusion of two outliers with extraordinary high baseline sum-scores (Table 3), results did not change substantially. The subgroup with MAI-Sum ≥24 at baseline showed stronger MAI changes in all items while MAI changes were small in the subgroup with MAI-Sum < 24.
Table 5 shows detailed descriptive results of MAI-Sum by drug classes according to ATC level 2.
The strongest decreases of MAI-Sum were found for anti-asthmatics, antifungals for dermatological use, drugs for acid-related disorders, cardiac therapy (glycosides, antiarrhythmics, cardiac stimulants, vasodilators) and psycholeptics (antipsychotics, anxiolytics, hypnotics and sedatives). Despite of the notable decreases of MAI-Sum, in most of these drug classes, the number of prescriptions did not change substantially over time. An exception was the use of antifungals for dermatological use which dropped nearly by half.
Analgesics and psycholeptics were the most frequently prescribed drug classes. Other commonly used drug classes were: laxatives, antithrombotic agents, diuretics, psychoanaleptics (antidepressants, psychostimulants, anti-dementia drugs, nootropics etc.) and drugs for acid-related disorders. All these frequently used drug classes showed a reduction of MAI-Sum except psychoanaleptics where MAI-Sum slightly increased. An increase of MAI-Sum was noted also regarding antiepileptics.
Secondary endpoints
At t0, 67.9% of NHRs were cognitively impaired (45.7% moderately or severely). Mean MMSE-score decreased slightly over time but showing no relevant effect. The DSS-score showed a small effect for declining cognitive function.
All NHRs had impaired mobility, 50% were unable to walk. TUG-time remained constant over time. Nearly 60% displayed signs of delirium. Percentage of cognitively impaired NHRs with agitated behavior decreased between t1 and t2, apathetic behavior increased.
Polypharmacy was frequent and increased slightly over time, number of drugs remained constant as did frequency of hyper-polypharmacy (≥10 prescriptions). Mean number of severe DDIs increased slightly. 52 NHRs (62.7%) were treated with analgesics throughout the duration of the study period. MAI scores of analgesics decreased over time (Table 6).
Discussion
Summary and interpretation of findings
Investigated NHRs were old-aged and multi-morbid with tendencies to deterioration of physical functions. Medication appropriateness increased significantly during the study period showing a moderate effect similar to previous studies [21, 28]. The effect found for the change in MAI-Sum slightly exceeded the lowest effect size expected in a-priori power analysis. Since pain treatment is common and relevant in NHRs [44] we analyzed the appropriateness of analgesics specifically noting an increase through the study period.
Defined thresholds for clinically relevant MAI changes are not yet in existence [46]. A German RCT (Rose et al.) [45] used a mean reduction of 3.88 found in a Cochrane Review [21] as cut-off-value for a “major benefit” from the intervention. Our findings were above this threshold, the median was beneath. As mean variation of MAI-Sum was high, the median seems to be a more adequate measure of MAI change. Congruently to the findings of Rose et al. [45], our subgroup analysis demonstrated that NHRs with high baseline MAI-Sum derived a “major benefit” from the intervention in terms of enhanced medication appropriateness, while lower baseline scores barely left room for improvement. However, it remains unclear which extent of improved medication appropriateness results in clinically significant benefits for patients.
The strongest improvements were shown regarding the MAI items indication and costs. This could be related to potential educational effects of the training on prescribers and to the fact that the lists of diagnoses and medication plans were revised and updated during the therapy check. Thus, the improvements regarding indication may reflect a more complete documentation of diagnoses.
The item drug-disease interaction was scored relatively low in our sample. The inconsistent documentation of falls throughout the study period (Table 2) may have contributed to this finding in terms of low detection of a risk condition for drug-disease interactions.
Patient-related outcomes did not change substantially aside from a decreased level of agitation. Similarly, current systematic reviews assessing comparable interventions and settings demonstrated improvements of medication appropriateness, but no significant changes of mortality, hospitalization or quality of life [21, 47]. Though, improvements are probably difficult to achieve given the physio-pathologic preconditions in NHRs. An epidemiologic study [48] described a mean cognitive decline of 0.6 MMSE-points/year in populations without dementia and higher declines (2.3/year) in demented persons. In our sample, a decline of 0.6/year was found in a mixed population (45.7% had moderate or severe cognitive impairments). Another study [49] showed a mean decline of 0.5–0.9/year; baseline MMSE-mean in this population was higher than in our study (24.5 vs. 17.4). Thus, cognitive decline in our sample may cautiously be interpreted as positively affected in terms of deceleration compared to epidemiologic data. Frequency of apathetic behavior increased during the study period which could be related to physical deterioration.
Although medication appropriateness increased, mean number of drugs was high and did not change substantially. This phenomenon was also found in other research [50]. There might have been missed opportunities to deprescribing for different reasons, e.g. GPs not identifying overtreatment or low perceived self-efficacy to deprescribe. However, the MAI items indication and duration of drug therapy showed improvements despite of the high number of drugs; moreover, the analysis according to ATC-classes showed that medication appropriateness improved in nearly all of the most frequently used drug classes, and that the drug classes with the highest improvements in medication appropriateness did not show substantial variations regarding numbers of prescriptions. This may underpin the hypothesis that polymedication is not necessarily synonymous with inappropriateness [51, 52].
Despite enhanced medication appropriateness and the intervention with medication review and DDI check, mean number of severe DDIs increased. Previous studies found similar results [53, 54]. This may be related to multi-morbidity and polypharmacy which are associated with an elevated risk of DDIs [52, 55]. In daily routine, if the expected benefit outweighs the risk, DDIs are sometimes accepted under appropriate patient monitoring [56].
Mortality rates in our sample were similar to observational [49] and other interventional NH studies [10, 22, 23, 25] and correspond to presupposed mortality in this population due to age and morbidity.
Strengths and limitations
A strength is the innovative and multi-faceted approach [57] combining blended-learning education and HIT-based structuring of interprofessional communication. As previously described, implementation of HIT requires considering socio-organizational factors [58] besides providing technical preconditions. We addressed this by involving participants in the design process and by adapting the online platform to suit the participants’ organizational settings. As verbally reported by the study participants, onsite trainings had positive effects on the social relationships between healthcare professionals (details of qualitative results will be published separately). Herewith, communication was not only standardized, but also individualized [59].
The online platform enabled healthcare professionals to perform the therapy check flexibly and independently of time and location which was well accepted. This can be considered an advantage especially for the NH setting with geographically dispersed players [60] and different availabilities.
Another strength is the multi-professional approach involving not only prescribers but all healthcare professionals concerned [47, 61] and their direct interaction [9] based on equal comprehensive and updated patient-related data. There were no case conferences as all concerns and questions raised were resolved during the therapy check (in urgent cases by phone or during routine NH visits).
The primary outcome was investigated using a thoroughly validated implicit instrument [9, 62] involving medication-related and clinical parameters. The MAI, however, does not assess under-prescribing, ADEs and adherence [63].
The study’s main limitation is the non-controlled design which was chosen due to recruitment challenges based on the risk of contamination related to the fact that the study was conducted within one city where GPs treat patients in several different NHs [7]. Another limitation is the application of inference-statistical procedures although participants were not randomly recruited. Hence, results cannot be generalized.
The I-oP was developed and tested within this project. It was not a-priori integrated into the different EHRs used by participants; therefore, records in the platform had to be additionally performed for securing routine documentation. For GPs and pharmacies, this did not restrict the intervention; in the NHs, the therapy check was feasible only by utilizing study assistants and thereby involving nurses in a less direct fashion than planned. This may have caused delays and potential incompletion of the process as specialists’ new prescriptions were not always immediately included in the therapy check or may have been missed when occurring during the ongoing therapy check cycle. These challenges will be addressed by integrating the platform into primary documentation systems.
Even though the study combined medication-related and patient-related outcomes, other relevant endpoints according to the core outcome set for trials of medication review in multi-morbid older patients with polypharmacy were not measured; e.g. drug underuse, drug-related hospital admissions, health-related quality of life [64].
The rather high medication appropriateness could partly be related to selection bias (highly motivated GPs were probably more likely to participate) or study effects (awareness of GPs about surveillance may have influenced their prescribing behavior). The clinical pharmacists conducting MAI ratings were blinded regarding the single patient IDs, but not regarding the different times of measurements. A bias towards a generally more favorable evaluation at the end of the study can, therefore, not be excluded.
Another limitation results from the fact that two different clinical pharmacists conducted the MAI ratings. A calculation of Inter-Rater-Reliability was neither feasible within one testing time (because one person conducted the ratings at each time of measurement) nor between the different times of measurement (due to the effect of the intervention between the different testing times that would affect the results). Thus, the possibility of biased results due to inter-rater differences cannot fully be excluded. Nevertheless, both raters were supervised by the same adept clinical pharmacist who checked subsamples of all ratings before approving data for statistical analysis.
Implications for the future
To facilitate the use and allow optimal alignment with pre-existing workflows [65], the platform is planned to be connected with applied EHRs. Based on the experiences of the supervision process, we recommend thorough training to enable healthcare professionals to perform the therapy check autonomously in the daily routine.
Improvements in medication appropriateness were mainly shown for NHRs with high baseline MAI sum-scores; hence, for future application, the platform could be linked with a CDS counting numbers of drugs and/or based on a list of potentially inappropriate medications (e.g. STOPP/START criteria) [66] which alerts healthcare professionals to initiate a therapy check in cases with high numbers of drugs and/or presence of inappropriate medications.
Providing an app for mobile use could be an attractive solution [67]. In addition, the platform may be adapted for different settings, such as management of chronic diseases in ambulatory care.
Conclusion
This study was the first to evaluate the impact of a HIT-based intervention addressing communication in the German NH setting. Medication appropriateness increased substantially especially for NHRs with high numbers of drugs and/or inappropriate medication at baseline. Furthermore, some results indicate that there may also be a remote impact on patient-related outcomes in terms of mitigating the natural decline. An appropriate controlled study in course of future application of the communication platform is required to confirm these effects. Future research will be needed to investigate the association between improvement of medication appropriateness and patient-relevant outcomes.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADE:
-
Adverse drug event
- BESD:
-
Beobachtung von Schmerz bei Demenz, German version of PAINAD
- CDS:
-
Clinical decision support
- CMAI-D:
-
Cohen Mansfield Agitation Inventory
- CPOE:
-
Computerized physician order entry
- DDIs:
-
Drug-drug interactions
- DOS:
-
Delirium Observation Screening Scale
- DRKS:
-
German Clinical Trials Register
- DSS:
-
Dementia Screening Scale
- GP:
-
General practitioner
- EHR(s):
-
Electronic health records
- HIT:
-
Health information technology
- InTherAKT:
-
“Initiative zur Therapiesicherheit in der Altenhilfe durch Kooperation und Teamwork”- Initiative for medication safety in long-term care via cooperation and teamwork
- I-oP:
-
InTherAKT- online Platform
- IQR:
-
Inter-Quartile-Range
- M:
-
Mean
- MD:
-
Median
- MAI:
-
Medication Appropriateness Index
- MAI-Mean:
-
Weighted MAI mean-score
- MAI-Sum:
-
Weighted MAI sum-score
- MMSE:
-
Mini Mental State Examination
- NH(s):
-
Nursing home(s)
- NHR(s):
-
Nursing home resident(s)
- PAINAD:
-
Pain Assessment in Advanced Dementia Scale
- PIP:
-
Potentially inappropriate prescription
- S-DDIs:
-
Severe drug-drug interactions
- RCT:
-
Randomized controlled trial
- SD:
-
Standard deviation
- STOPP/START criteria:
-
Screening Tool of Older People’s Prescriptions/Screening Tool to Alert to Right Treatment
- TUG:
-
Timed Get Up and Go test
- VRS:
-
Verbal Rating Scale
- WHO:
-
World Health Organization
References
Fialová D, Topinková E, Gambassi G, Finne-Soveri H, Jónsson PV, Carpenter I, et al. Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293(11):1348–58.
Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005;118(3):251–8.
Frazier SC. Health outcomes and polypharmacy in elderly individuals: an integrated literature review. J Gerontol Nurs. 2005;31(9):4–11.
Flaherty JH, Perry HM, Lynchard GS, Morley JE. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55(10):M554–9.
Muhlack DC, Hoppe LK, Weberpals J, Brenner H, Schöttker B. The Association of Potentially Inappropriate Medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc. 2017;18(3):211–20.
Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ. 2003;169(6):549–56.
Mahlknecht A, Nestler N, Bauer U, Schüßler N, Schuler J, Scharer S, et al. Effect of training and structured medication review on medication appropriateness in nursing home residents and on cooperation between health care professionals: the InTherAKT study protocol. BMC Geriatr. 2017;17(1):24.
Balzer K, Butz S, Bentzel J, Boukhemair DS, Luehmann D. Beschreibung und Bewertung der fachärztlichen Versorgung von Pflegeheimbewohnern in Deutschland http://portal.dimdi.de/de/hta/hta_berichte/hta298_bericht_de.pdf. Accessed 02 Dec 2014.
Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, Hanlon JT. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84.
Olsson IN, Curman B, Engfeldt P. Patient focused drug surveillance of elderly patients in nursing homes. Pharmacoepidemiol Drug Saf. 2010;19(2):150–7.
Barr N, Vania D, Randall G, Mulvale G. Impact of information and communication technology on interprofessional collaboration for chronic disease management: a systematic review. J Health Serv Res Policy. 2017. https://doi.org/10.1177/1355819617714292.
Lapane KL, Hughes CM, Christian JB, Daiello LA, Cameron KA, Feinberg J. Evaluation of the fleetwood model of long-term care pharmacy. J Am Med Dir Assoc. 2011;12(5):355–63.
Anrys P, Strauven G, Boland B, Dalleur O, Declercq A, Degryse JM, et al. Collaborative approach to optimise MEdication use for older people in nursing homes (COME-ON): study protocol of a cluster controlled trial. Implement Sci. 2016;11:35.
McKibbon KA, Lokker C, Handler SM, Dolovich LR, Holbrook AM, O'Reilly D, et al. The effectiveness of integrated health information technologies across the phases of medication management: a systematic review of randomized controlled trials. J Am Med Inform Assoc. 2012;19(1):22–30.
Field TS, Rochon P, Lee M, Gavendo L, Baril JL, Gurwitz JH. Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J Am Med Inform Assoc. 2009;16(4):480–5.
Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–52.
Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc. 2008;56(12):2225–33.
McGregor JC, Weekes E, Forrest GN, Standiford HC, Perencevich EN, Furuno JP, et al. Impact of a computerized clinical decision support system on reducing inappropriate antimicrobial use: a randomized controlled trial. J Am Med Inform Assoc. 2006;13(4):378–84.
Ash J, Sittig D, Poon E, Guappone K, Campbell E, Dykstra R. The extent and importance of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc. 2007;14(4):9.
Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59(7):1238–45.
Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, Hughes C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014;10:CD008165.
Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, Bowie P. Clinical medication review by a pharmacist of elderly people living in care homes--randomised controlled trial. Age Ageing. 2006;35(6):586–91.
Frankenthal D, Lerman Y, Kalendaryev E. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–65.
García-Gollarte F, Baleriola-Júlvez J, Ferrero-López I, Cuenllas-Díaz Á, Cruz-Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc. 2014;15(12):885–91.
Pitkälä KH, Juola AL, Kautiainen H, Soini H, Finne-Soveri UH, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(12):892–8.
Connolly MJ, Boyd M, Broad JB, Kerse N, Lumley T, Whitehead N, et al. The aged residential care healthcare utilization study (ARCHUS): a multidisciplinary, cluster randomized controlled trial designed to reduce acute avoidable hospitalizations from long-term care facilities. J Am Med Dir Assoc. 2015;16(1):49–55.
Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing. 2004;33(6):612–7.
Hanlon JT, Schmader KE. The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging. 2013;30(11):893–900.
Sauter A, Sauter W, Bender H. Blended learning: effiziente integration von E-learning und Praesenztraining. Neuwied: Luchterhand; 2002.
Aly F, Hellmann G, Möller H. Aktionsplan zur Verbesserung der Arzneimitteltherapiesicherheit in Deutschland- Spezifikation für einen patientenbezogenen Medikationsplan. https://www.akdae.de/AMTS/Medikationsplan/docs/Medikationsplan_aktualisiert.pdf. Accessed 06 Nov 2017.
PCNE Guidelines for Retrospective Medication Review in Pharmacy V0. http://www.pcne.org/upload/files/91_Messerli_2015.pdf. Accessed 16 Jan 2017.
Thuermann P, Jaehde U. Abschlussbericht zum Projekt Arzneimitteltherapiesicherheit in Alten- und Pflegeheimen: Querschnittsanalyse und Machbarkeit eines multidisziplinären Ansatzes. Bundesgesundheitsministerium (BMG). https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/Abschlussbericht_Arzneimitteltherapiesicherheit_in_Alten-_und_Pflegeheimen_Querschnittsanalyse_und_Machbarkeit_eines_multidisziplinaeren_Ansatzes.pdf. Accessed 06 Nov 2017.
Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51.
Samsa GP, Hanlon JT, Schmader KE, Weinberger M, Clipp EC, Uttech KM, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47(8):891–6.
Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–35.
Koehler L, Weyerer S, Schäufele M. Proxy screening tools improve the recognition of dementia in old-age homes: results of a validation study. Age Ageing. 2007;36(5):549–54.
Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50.
Roesler M, Frey U, Retz-Junginger P, Supprian T, Retz W. Diagnostik der Demenzen: Standardisierte Untersuchungsinstrumente im Überblick. Fortschr Neurol Psychiat. 2003;71:187–98.
Stoffel O. Cohen-Mansfield agitation inventory (CMAI). Pflegewissenschaft. 2010;12(1):55–9.
Podsiadlo D, Richardson S. The timed "up & go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8.
UpToDate® Lexicomp® Drug Interactions. http://www.uptodate.com/home/drugs-drug-interaction. Accessed 12 Dec 2017.
Luebke N, Meinck M, von Renteln-Kruse W. Der Barthel-index in der Geriatrie. Eine Kontextanalyse zum Hamburger Einstufungsmanual. Z Gerontol Geriat. 2004;37:11.
Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. EPCRC: studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manag. 2011;41(6):1073–93.
Schuler MS, Becker S, Kaspar R, Nikolaus T, Kruse A, Basler HD. Psychometric properties of the German "pain assessment in advanced dementia scale" (PAINAD-G) in nursing home residents. J Am Med Dir Assoc. 2007;8(6):388–95.
Rose O, Mennemann H, John C, Lautenschläger M, Mertens-Keller D, Richling K, et al. Priority setting and influential factors on acceptance of pharmaceutical recommendations in collaborative medication reviews in an ambulatory care setting - analysis of a cluster randomized controlled trial (WestGem-study). PLoS One. 2016;11(6):e0156304.
Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, Hughes CM. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235.
Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016;2:CD009095.
Aevarsson O, Skoog I. A longitudinal population study of the mini-mental state examination in the very old: relation to dementia and education. Dement Geriatr Cogn Disord. 2000;11(3):166–75.
Helvik AS, Høgseth LD, Bergh S, Šaltytė-Benth J, Kirkevold Ø, Selbæk G. A 36-month follow-up of decline in activities of daily living in individuals receiving domiciliary care. BMC Geriatr. 2015;15:47.
Vinks TH, Egberts TC, de Lange TM, de Koning FH. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs Aging. 2009;26(2):123–33.
Payne RA, Abel GA, Avery AJ, Mercer SW, Roland MO. Is polypharmacy always hazardous? A retrospective cohort analysis using linked electronic health records from primary and secondary care. Br J Clin Pharmacol. 2014;77(6):1073–82.
Aronson JK. Polypharmacy, appropriate and inappropriate. Br J Gen Pract. 2006;56(528):484–5.
Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scand J Prim Health Care. 2012;30(1):3–9.
Malone DC, Liberman JN, Sun D. Effect of an educational outreach program on prescribing potential drug-drug interactions. J Manag Care Pharm. 2013;19(7):549–57.
Gagne JJ, Maio V, Rabinowitz C. Prevalence and predictors of potential drug-drug interactions in Regione Emilia-Romagna. Italy J Clin Pharm Ther. 2008;33(2):141–51.
Raschi E, Piccinni C, Signoretta V, Lionello L, Bonezzi S, Delfino M, et al. Clinically important drug-drug interactions in poly-treated elderly outpatients: a campaign to improve appropriateness in general practice. Br J Clin Pharmacol. 2015;80(6):1411–20.
Ferrah N, Lovell JJ, Ibrahim JE. Systematic review of the prevalence of medication errors resulting in hospitalization and death of nursing home residents. J Am Geriatr Soc. 2017;65(2):433–42.
Eslami S, de Keizer NF, Abu-Hanna A. The impact of computerized physician medication order entry in hospitalized patients--a systematic review. Int J Med Inform. 2008;77(6):365–76.
Weissenborn M, Haefeli WE, Peters-Klimm F, Seidling HM. Interprofessional communication between community pharmacists and general practitioners: a qualitative study. Int J Clin Pharm. 2017;39(3):495–506.
Kramer A, Richard A, Epstein A, Winn D, May K. Understanding the costs and benefits of health information technology in nursing homes and home health agencies: case study findings. In. U.S. Department of Health and Human Services: ASPE- Office of The Assistant Secretary for Planning and Evaluation http://aspe.hhs.gov/daltcp/reports/2009/HITcsf.pdf. Accessed 07 Nov 2017.
Fried TR, Niehoff KM, Street RL, Charpentier PA, Rajeevan N, Miller PL, et al. Effect of the tool to reduce inappropriate medications on medication communication and Deprescribing. J Am Geriatr Soc. 2017;65(10):2265–71.
Prados-Torres A, Del Cura-González I, Prados-Torres D, López-Rodríguez JA, Leiva-Fernández F, Calderón-Larrañaga A, et al. Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (multi-PAP project). Implement Sci. 2017;12(1):54.
Gómez Aguirre N, Caudevilla Martínez A, Bellostas Muñoz L, Crespo Avellana M, Velilla Marco J, Díez-Manglano J. Polypathology, polypharmacy, medication regimen complexity and drug therapy appropriateness. Rev Clin Esp. 2017;217(5):289–95.
Beuscart JB, Knol W, Cullinan S, Schneider C, Dalleur O, Boland B, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21.
Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15(5):585–600.
Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (screening tool of older Person's prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
Handler S, Boyce R, Ligons F, Perera S, Nace D, Hochheiser H. Use and perceived benefits of Mobile devices by physicians in preventing adverse drug events in the nursing home. J Am Med Dir Assoc. 2013;14(12):12.
Acknowledgements
We thank the nursing home residents and their legal representatives as well as the nurses and participating nursing homes, GPs, medical assistants, pharmacists and pharmaceutical technicians who gave their consent to participate in this project. We express our gratitude to Ralf Becker and Oliver Schwalbe for their contribution in study conceptualization, recruitment and congressional activities, and to Sarah Waltermann and Nina Schürholz, who participated in the coordination of the study and managed the organization of recruitment, data collection and project reporting. We thank Nadine Schüssler, Stephanie Hemling and Sebastian Scharer who collaborated in methodical and organizational conceptualization and in the execution of the interprofessional training. We express our gratitude to Isabel Waltering for helpful discussions regarding the MAI score and for participation in the study conceptualization and in the training part of the intervention. We also thank the study assistants, especially David Bruns, for their valuable cooperation in the data collection and intervention phase.
Funding
The study was granted by commercial funding (Grünenthal GmbH, Germany), the city of Muenster (Germany) and the local government of Salzburg (Austria). Pharmaceutical funding aimed to explore a relevant topic of public health in terms of social sponsoring, excluding direct drug-related research. None of the sponsors had any access or influence on conceptual design, collection, evaluation or presentation of data.
Author information
Authors and Affiliations
Contributions
JO and MF were the initiators and scientific project leaders, JO raised the study funding. NN was the coordinator of the project and organized the application for ethical approval, recruitment, training of the study assistants, data collection and development of the I-oP. UB participated in the conception and design of the study, supervised the development of the data collecting tool, contributed methodical expertise and performed statistical analysis of the baseline data. LK was responsible for methodical expertise, statistical analysis and interpretation of data. JS, GH and MF developed and executed the interprofessional training, NL and NN conducted and analyzed the focus group interviews. NL was responsible for supervision of the study assistants and participants during the intervention phase. DZ developed the online platform. GH participated in the development of the primary outcome, LF conducted MAI ratings. AM designed the study protocol, tested the online platform in the developing phase, contributed to MAI subgroup analysis and wrote the manuscript, which was revised and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All study participants were informed in detail about the study and signed informed consent according to the Declaration of Helsinki. The study protocol was registered on 2015-09-02 via the German Clinical Trials Register (ID: DRKS00007900, available also on the Meta-registry of the WHO http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00007900); due to bureaucratic delays, registration was completed 4 months after obtaining the first written consent and 6 weeks after commencement of the first data collection.
Ethical approval was obtained from the responsible Ethics Committee (Ethics Committee of the Medical Association Westphalia-Lippe and of the Faculty of Medicine of the University of Muenster) on 2015-03-27 (approval number 2015–147-f-S).
Consent for publication
Not applicable.
Competing interests
NN: In the last 5 years I have received lecture honoraria from Grünenthal and Mundipharma.
GH: In the last 5 years I have received lecture honoraria from Grünenthal.
JO: In the last 5 years I have received lecture honoraria from Pfizer, Grünenthal and Mundipharma.
All other authors declare to have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mahlknecht, A., Krisch, L., Nestler, N. et al. Impact of training and structured medication review on medication appropriateness and patient-related outcomes in nursing homes: results from the interventional study InTherAKT. BMC Geriatr 19, 257 (2019). https://doi.org/10.1186/s12877-019-1263-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-019-1263-3