Abstract
Background
To evaluate the prevalence and management of heart failure (HF) in very old patients in geriatric settings.
Methods
Members of the French Society of Geriatrics and Gerontology throughout France were invited to participate in a point prevalence survey and to include all patients ≥80 years old, hospitalized in geriatric settings, with HF (stable or decompensated) on June 18, 2012. General characteristics, presence of comorbidities, blood tests and medications were recorded.
Results
Among 7,197 patients in geriatric institution, prevalence of HF was 20.5% (n = 1,478): (27% in acute care, 24.2% in rehabilitation care and 18% in nursing home). Mean age was 88.2 (SD = 5.2) and Charlson co morbidity score was high (8.49 (SD = 2.21)). Left ventricular ejection fraction (LVEF) was available in 770 (52%) patients: 536 (69.6%) had a preserved LVEF (≥ 50%), 120 (15.6%) a reduced LVEF (< 40%), and 114 (14.8%) a midrange LVEF (40–49%). Prescription of recommended HF drugs was low: 42.6% (629) used Angiotensin Converting Enzyme Inhibitors (ACEI) or Angiotensin Receptor Blockers (ARBs), 48.0% (709) β-blockers, and 21.9% (324) ACEI or ARB with β-blockers, even in reduced LVEF. In multivariate analysis ACEI or ARBs were more often used in patients with myocardial infarction (1.36 (1.04–1.78)), stroke (1.42 (1.06–1.91)), and diabetes (1.54 (1.14–2.06)). β blockers were more likely used in patients with myocardial infarction (2.06 (1.54–2.76)) and atrial fibrillation (1.70 (1.28–2.28)).
Conclusion
In this large very old population, prevalence of HF was high. Recommended HF drugs were underused even in reduced LVEF. These results indicate that management of HF in geriatric settings can still be improved.
Similar content being viewed by others
Background
Low birth rates and higher life expectancy are transforming the demographics of Europe. The European Commission has estimated that by 2025, 20% of Europeans will be aged 65 years and older. The proportion of those aged 80 years and older in the Europe is expected to more than double between 2014 and 2050 from 5.1 to 10.9% [1]. Heart failure (HF) is a highly prevalent condition in the older population. After 80 years of age its prevalence varies between 15 and 20% [2,3,4]. Very old HF patients with multiple comorbidities are an important challenge for healthcare systems and are usually treated in geriatric institutions [5]. Despite a high prevalence and poor outcome, data on geriatric HF patients aged > 80 years from randomized controlled trials are limited and guidelines for this population are extrapolated from data on younger patients. The growing size of this population, however, requires more information on its optimal management. Although, HF treatment is thought to be beneficial to patients with reduced LVEF regardless of age, the existing literature indicates that the prescription of recommended treatments for HF is low in geriatric HF patients [6]. The aim of the current study was to carry out a point prevalence survey to provide up-to-date data on the prevalence and management of HF in individuals aged 80 years and older in geriatric settings in France and to explore factors influencing drug treatment.
Methods
Study design
This cross-sectional survey was initiated and conducted by the French Society of Geriatrics and Gerontology. A standardized questionnaire was emailed to 1600 geriatrician members of the French Society of Geriatrics and Gerontology throughout France, to evaluate the characteristics of patients with HF aged 80 years and older who were hospitalized in geriatric acute-care units (hospital department for acute patients with multiple chronic comorbidities), rehabilitation care units (hospital department for rehabilitation of patients with various neurological, orthopedic and other medical conditions following stabilization of their acute medical problems) or living in nursing homes (community-based institutions for disabled subjects with stable medical condition otherwise).
Participants
Inclusion criteria were: age 80 years or older, diagnosis of decompensated or stable HF and hospitalized in a geriatric care unit for at least 24 h or living in a nursing home and present in the facility at 9 am on June 18, 2012.
Decompensated HF was defined as presence of acute symptoms or signs of HF according to ESC guidelines [7, 8]. Stable heart failure was defined as history of hospitalization for heart failure and unchanged HF symptoms and signs for at least 1 month [7].
Data collection
For each patient, the following data were collected: demographic characteristics (age, gender, weight, systolic and diastolic blood pressure, heart rate) presence of cardiovascular comorbidities (hypertension, atrial fibrillation, history of myocardial infarction, history of stroke, diabetes, peripheral arterial disease, orthostatic hypotension) as well as non-cardiovascular comorbidities (anemia, renal insufficiency, malnutrition, dementia, falls (defined as ≥2 falls per year), chronic obstructive pulmonary disease (COPD), cancer), results of most recent blood tests (serum creatinine, electrolytes, albumin, hemoglobin, brain natriuretic peptide (BNP) and N-terminal brain natriuretic peptide (NTproBNP)), current medication (loop diuretic, thiazide diuretic, angiotensin converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), β-blocker, aldosterone receptor antagonist, ivabradine, digoxin, nitrates), and whether the patients was advised to follow a low salt diet.
Antiplatelet and anticoagulant (therapeutic dose or prophylactic dose), and the total number of drug classes (for cardiovascular or non-cardiovascular drugs) were also recorder. Anemia was defined according to the World Health Organization criteria as a hemoglobin level lower than 13 g/dL in men and lower than 12 g/dL in women [9]. Estimated glomerular filtration rate (eGFR) was calculated using the Cockcroft and Gault formula [10]. Severe renal insufficiency was defined as eGFR < 30 ml/min. Malnutrition was defined as serum albumin level < 35 g/L.
The date of the last echocardiogram was noted as well as the left ventricular ejection fraction (LVEF) value. Finally, the burden of comorbidities was calculated using the age adjusted Charlson Comorbidity Index [11, 12].
Procedure
Data were collected by the physician who was in charge of the patient on June 18, 2012. Physicians had 1 month until July 18, 2012 to send the data.
Ethical consideration
The study was conducted in accordance with the ethical standards set forth in the Declaration of Helsinki (1983). The entire study protocol was approved by the ethics committee of Nantes (Groupe Nantais d’Ethique dans le Domaine de la Santé, France), and the study complied with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement guidelines [13]. All patients’ data were anonymized in the local institution before they were uploaded to the central database.
Data Analysis
First, demographic characteristics and clinical variables of the patients were analyzed in the whole sample and according to the type of geriatric care unit (acute care, rehabilitation care and nursing home) using descriptive statistics: means and standard deviations for continuous variables, and numbers and percentages for categorical variables. Comparisons were made with analysis of variance (ANOVA) for continuous data and with χ2 test for categorical data.
Because of the non-normal distribution of the brain natriuretic peptide and N-terminal brain natriuretic peptide variables, they were log-transformed for the calculation of the statistics but for the sake of clarity the non-logged figures are presented in the tables. Demographic characteristics and clinical variables of the patients were then compared between patients with decompensated and stable heart failure with Student’s t-tests for the continuous variables and χ2 tests for the categorical variables.
Second, the differences in treatment between 3 groups of LVEF (preserved LVEF (≥ 50%), midrange LVEF (40–49%) and reduced LVEF (< 40%) [7] were graphically presented in a barplot and compared with χ2 tests.
Third, comparisons were made between patients with and without ACEI or ARBs treatment and between patients with and without β-blocker treatment. Two logistic regression models were built, one with ACEI or ARB and one with β-blockers as dependent variable and factors univariately associated (p < 0.10) with the dependent variables. In these 2 models, LVEF was not included in the dependent variables because it was only available in 52% of the sample. The results were graphically presented on two forest plots. Because ACEI, ARB and β-blockers are medication recommended for HF with reduced EF, as sensitivity analysis, we built 2 other models, one with ACEI or ARB and one with β-blockers as dependent variables restricted to patients with reduced LVEF.
All analyses were two-sided and a p-value < 0.05 was considered statistically significant. Data analysis was performed using R software version 3.2.3, (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
Results
Participants and patient characteristics
A total of 183 practitioners from 134 geriatric institutions participated in the survey: 58 from acute care units, 50 from rehabilitation care units and 75 from nursing homes. These practitioners were in charge of 7,197 patients: 1,397 acute care, 1,331 rehabilitation care and 4,469 nursing homes. Out of these 7,197 patients, 1,478 presented with HF on the day of the survey and were included in the study giving an overall HF prevalence of 20.5% (95% confidence interval 19.8–21.3%), 27.1% (25.2–29.1%) (N = 379) in acute care units, 24.2% (22.3–26.2%) (N = 322) in rehabilitation care units and 17.6% (16.7–18.5%) (N = 774) in nursing homes.
Demographic characteristics and past medical history of the sample are shown Table 1. The mean (SD) age was 88.2 (SD = 5.2) years and 68.9% (N = 1014) were women. The mean age adjusted Charlson comorbidity index score was 8.49 (2.21). The most common comorbidities were hypertension (77.6%), malnutrition (64.1%), anemia (59.9%), dementia (52.3%), atrial fibrillation (43.2%), depression (34.1%), history of myocardial infarction (28.5%), COPD (24.6%), diabetes (22.1%), peripheral arterial disease (20.0%), history of stroke (19.8%), orthostatic hypotension (18.5%) and cancer (16.3%). Patients were taking on an average of 8.43 (3.29) different drugs. Few patients (3.8% (57)) had a very low systolic blood pressure (< 100 mmHg in a sitting position). Heart rate was greater than 70 beats per minute (bpm) in 56.4% (816) of patients. In patients with permanent atrial fibrillation on the electrocardiogram, 40.7% (247) had a heart rate lower than 70 bpm whereas in patients in sinus rhythm on the electrocardiogram, 46.5% (369) had a heart rate lower than 70 (p = 0.04).
Only 780 (52.8%) patients had an available echocardiogram in their medical records. Ninety (11.6%) had an echocardiogram in the past week, 135 (17.4%) in the past 7–30 days, 181 (23.4%) in the past 1–6 months, 89 (11.5%) in the past 6–12 months, 107 (13.8%) in the past 12–24 months, and 172 (22.2%) over 2 years. Among the 780 patients with an available echocardiogram 770 had an LVEF calculated. The mean LVEF was 52.9% (14.4). 69.6% percent (536) had a preserved LVEF (LVEF ≥50%), 15.6% (120) had a reduced LVEF (LVEF < 40%), and 14.8% (114) had a midrange ejection fraction LVEF (40–49%).
Decompensated HF was reported in 21.4% (n = 316) and stable HF in 78.4% (n = 1158) for 4 subjects the information was missing.
For decompensated HF, the main precipitating factors leading to decompensation were infections (44.1%), atrial fibrillation (29.9%), anemia (19.7%) and acute coronary syndrome (10.0%).
Overall level of natriuretic peptide was elevated in this population. NTproBNP and BNP were higher in the decompensated than in the stable HF patients (10407 pg/mL (12393) vs. 4208 pg/mL (7621), p < .0001 and 1527 pg/mL (2141) vs. 729 pg/mL (15311), p < .0001).
Heart failure drug treatments
The type of HF drug treatments taken by patients is shown in Table 2. Patients with decompensated HF were more likely to receive loop diuretics and nitrates whereas patients with stable HF had higher prescriptions of ACEI or ARBs either alone or in combination with a β-blocker. A low salt diet was advised in 9.76% (144) most often in patients with decompensated HF (16.5% (52) vs. 7.95% (92), p < 0.0001). Overall, prescription of recommended HF drug treatments was low whether patients had decompensated or stable HF: 629 (42.6%) received ACEI or ARBs and 709 (48.0%) received β-blockers. The prescription of an ACEI or ARB in combination with a β-blocker was also very low (21.9% (324)), as was the prescription of aldosterone antagonists (5.95% (88)), ivabradine (0.88% (13)), nitrates (13.5% (199)) and digoxin (8.32% (123)).
Distribution of drug treatments according to preserved midrange and reduced LVEF
In the 770 patients with an available LVEF, there was low use of recommended HF drug treatments in patients with reduced LVEF. 64 (53.3%) used ACEI or ARB, 82 (68.3%) used β-blocker and 50 (41.7%) used a combination of β-blocker with an ACEI or ARB.
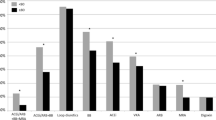
However, the use of ACEI or ARB or β-blocker was higher in patients with reduced LVEF than in patients with preserved or midrange LVEF (See Fig. 1). There was also more prescription of loop or thiazide diuretics and ivabradine among those with reduced or midrange LVEF than among those with preserved LVEF. No difference was observed for aldosterone antagonists, digoxin and nitrates according to LVEF categories.
Factors associated with prescription of ACEI or ARB in very old patients with HF
Additional file 1: Table S1 shows the characteristics of patients according to ACEI or ARB use.
In a multivariate analysis, after adjustment for predictors of ACEI or ARB prescription identified by univariate analysis, ACEI or ARBs were more likely prescribed in patients with cardiovascular diseases including history of myocardial infarction (OR: 1.36 (95% CI: 1.04–1.78)), history of stroke (OR: 1.42 (95% CI: 1.06–1.91)), and diabetes (OR: 1.54 (95% CI: 1.14–2.06)) (Fig. 2). Determinants of the non-prescription of ACEI or ARB included malnutrition (serum albumin levels < 35 g/L) (OR: 0.74 (95% CI: 0.57–0.95)) and eGFR between 30 and 50 ml/min (OR: 0.64 (95% CI: 0.45–0.91)) and eGFR < 30 ml/min (OR: 0.46 (95% CI: 0.32–0.67)).
Factors associated with ACE inhibitor or ARB and with β-blocker prescription. * p < 0.05; ** p < 0.01; *** p < 0.001. ACEI, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; eGFR, estimated glomerular filtration rate calculated with Cockcroft formula; MI, myocardial infarction
When the model was run among patients with reduced LVEF, predictors of ACEI or ARB prescription were then diabetes (OR: 2.23 (95% CI: 1.18–4.31), p = 0.02), history of myocardial infarction (OR: 3.26 (95% CI: 1.83–5.96), p < .0001) and eGFR between 30 and 50 ml/min (OR: 0.22 (95% CI: 0.09–0.54), p = 0.001) and eGFR < 30 ml/min (OR: 0.35 (95% CI: 0.14–0.83), p = 0.02).
Factors associated with prescription of β-blockers in very old patients with HF
Additional file 1: Table S2 shows the characteristics of patients according to β-blockers use.
Multivariate analysis indicated that β blockers were more likely prescribed in patients with myocardial infarction (OR: 2.06 (95% CI: 1.54–2.76)) and atrial fibrillation (OR: 1.70 (95% CI: 1.28–2.28)) (Fig. 2). Determinants of the non-prescription of a β-blocker were older age (OR: 0.96 (95% CI: 0.93–0.99) and infection (OR: 0.69 (95% CI: 0.53–0.91)).
When the model was run among patients with reduced LVEF, β blockers prescription was then no more associated with any of the predictors. However myocardial infarction (OR: 1.77 (95% CI: 0.98–3.24), p = 0.06), atrial fibrillation (OR: 1.68 (95% CI: 0.94–3.06), p = 0.08) and infection (OR: 0.61 (95% CI: 0.35–1.08), p = 0.09) were marginally associated with β blocker prescriptions.
Discussion
This large cross-sectional survey study provides information on the current management of very old patients with HF in geriatric settings in France. The overall prevalence of HF was 20.5% (ranging from 27% in acute care departments to 18% in nursing home).
Prescriptions of guideline-recommended HF drug treatments were low whether patients had decompensated or stable HF. Less than half of the subjects received ACEI or ARBs or β-blockers. The combination of an ACEI or ARB with a β-blocker was prescribed in less than 22% of the subjects.
The prevalence of HF in this study is in the same range as reported in a systematic review which indicates that about 20% (15–45%) of nursing homes residents are affected by HF [4].
Published studies on the management of HF in geriatric patients with multiple comorbidities are sparse, even though this population is growing rapidly. In the current study, geriatric HF patients had a high number of comorbidities, in particular non-cardiovascular such as malnutrition, dementia and falls that are generally not evaluated in clinical trials. This result is in line with the SAGE study indicating that in nursing home 32% of HF patients have at least six other concurrent diseases [14] and with the Swedish Heart Failure Registry database, that reported a higher incidence of cardiovascular and non-cardiovascular comorbidities in patients 85 years and older [15].
In the current study, only 53% of patients had an available echocardiogram in their medical records, and among those, 70% had a preserved LVEF. These data highlight the difficulties in obtaining an echocardiogram for patients in geriatric care setting because of the difficulties in transporting patients to a cardiology unit, and lower physician demand for diagnostic confirmation.
Our results confirm previous observations of a higher prevalence of HF with preserved LVEF among the very old [2, 16,17,18,19]. In octogenarians hospitalized for HF in the Euro Heart Failure Survey (EHFS), only 38.4% of patients had a known LVEF and in 60% of these LVEF were preserved [16].
In our study, regardless of whether HF was stable or decompensated, the prescription of recommended treatments for HF such as ACEI (or ARB) and β-blocker was low. Particularly the combination of an ACEI (or ARB) and a β-blocker was very low (less than a quarter of subjects). Among subjects with reduced LVEF, the rate of prescriptions of ACEI (or ARB) or β-blocker was still sub-optimal (53% for ACEI or ARB and 68% % for β-blocker) even though they were more often prescribed than in midrange and preserved LVEF. The greater prevalence of HF with preserved LVEF was therefore not the only reason for the low use of ACEI (or ARB) and β-blockers in older patients. The underuse of recommended HF treatments in the very old age has already been reported in other studies [6, 15, 16, 20,21,22]. In a study collecting data on 19 long-term care facilities only 41 and 38% prescription of ACEI and β-blockers respectively were reported in eligible HF patients [6]. In a large Canadian study in older home-care patients only 28% were receiving the recommended combination therapy of an ACEI and β-blocker [20]. Similar findings were reported in the Swedish Heart Failure Registry database [15] and in an Italian cardiology database [22]. A comparison of HF therapy between the Euro Heart Failure survey I and the EHFS II found a significant increase in prescription rates of recommended HF drugs in HF octogenarians at discharge in EHFS II [2], but these data mainly concerned patients hospitalized in cardiology units. Our data indicate that prescription guidelines remain less implemented in geriatric settings. A number of potential reasons for the low prescription of recommended therapies in very old HF patients can be hypothesized. Some factors may be related to the patients (comorbidities, poor tolerance, non-adherence) and some to the prescribers (fear of side effects, lack of awareness of guidelines, diagnostic uncertainty, focus on symptomatic improvement rather than outcome, reluctance to modify existing therapies in very old patients) [16, 23]. Moreover, the lack of definite evidence from specific randomized trial in very old population may also explain the low use of ACEI or beta-blocker. However, all the randomized trials conducted in HF patients with reduced LVEF have shown a benefit of those drugs regardless of age. Also, there is evidence from small trials and observational datasets that HF medications do improve outcomes in older HF patients, including quality of life related outcomes [2, 16].
Finally, the difficulty to get cardiologic advice in a nursing home may also explain the lower prescription of recommended HF drugs in this population, suggesting a potential interest of telemedicine in this population. Multimodal and guideline-based HF management may improve HF patients in geriatrics settings [24]. Specific education on HF for geriatricians and the development of specific tools like portable ultrasound scanner should be investigated in geriatric care units or nursing homes [24].
Similar to previous studies [16], there was a high use of diuretics reflecting the importance of reducing symptoms and maintaining quality of life as the main goals of treatment in very old HF patients. The low use of digoxin was in line with guidelines that caution against their use in the elderly and in patients with reduced renal function [7]. We observed a low use of nitrates in decompensated HF probably because of an increased risk of orthostatic hypotension in the elderly.
Our data show that in very old HF patients managed in geriatric care units or nursing homes, the factors determining the prescription of first-line HF treatments (ACEI or ARB and β-blocker) were history of myocardial infarction and stroke, diabetes for ACEI or ARBs and history of myocardial infarction and atrial fibrillation for β-blockers. Factors influencing the nonprescription of HF treatment were non-cardiovascular comorbidities (malnutrition and renal insufficiency for ACEI (or ARBs) and infections for β-blockers).
Among patients with reduced LVEF, history of stroke and malnutrition were not any more significantly associated with prescription of ACEI or ARBs. For prescription of β-blockers, history of myocardial infarction and atrial fibrillation and infections were not any more significant but the estimates were in the same range as in the whole sample. This is in all likelihood related to a lack of power due to the small sample size.
Over half of the patients in the current study had on average a heart rate faster than 70 bpm, even though a number of large-scale studies have shown that elevated heart rate is associated with morbidity and mortality in HF patients with reduced or preserved LVEF independently of age [25,26,27,28,29].
The OPTIMIZE-HF Registry observed that among 10,696 patients with HF and LVEF < 40, 28.6% did not receive β-blockers and only 6.7% of patients received the target dose of β-blockers [30]. These results stress the difficulties of obtaining such goals in real life mainly in the older people.
Interestingly, BNP or NTproBNP levels remained very high even in stable HF, suggesting a suboptimal treatment. Meanwhile, natriuretic peptide level increases with age and comorbidities (renal dysfunction, atrial fibrillation, left ventricular hypertrophy…) making it difficult to interpret in geriatric population [31].
Our study has several limitations. It is an observational study performed on 1 day and only comprising hospitalized or institutionalized older individuals who might not be representative of the whole elderly population. A reporting bias cannot be ruled out as the accuracy and completeness of the data were entirely reliant upon physicians’ declarations, although the questionnaire was designed to limit variability in readers’ interpretations by asking only factual data. In addition, while selection bias could not be eliminated, investigators were asked to take into account all the patients in their care on the day of the survey. Information on duration and dose of treatments was not collected. No follow-up was available to analyze the adequacy between underuse of recommended HF and mortality. Activities of daily living and frailty status were not recorded in this study because it was difficult to assess in hospitalized patient with HF and unfortunately their status before the hospitalization had not been assessed. Thus, this potential cause for the underuse of medications was not assessed. Meanwhile cognitive status that is a classical cause of underuse of medications was taken into account. Lastly data were collected in 2012 and might not exactly reflect the current status.
The study also had some strengths. This study provides important data on the management of HF failure in patients over 80 years of age in geriatric care unit or nursing home. Few data exist for this population, particularly in real-life settings. Our study had a large sample size and included very old (mean age 88.2 (5.2)) non-selected ‘real-life’ HF patients with numerous comorbidities like dementia, malnutrition, anemia, renal insufficiency, depression, orthostatic hypotension (Charlson score = 8.49). Lastly few studies have analyzed the management of HF according to 3 classes of LVEF (reduced, midrange and preserved LVEF) in geriatric patients.
Conclusion
Our results show a high prevalence of HF in patients aged 80 years and older cared for in a geriatric care unit or nursing home (20.5%), characterized by a high prevalence of preserved LVEF and a high burden of cardiovascular as well as non-cardiovascular comorbidities. There was a low prescription of recommended treatment in the overall HF population with less than half of patients receiving an ACEI or ARB and less than a quarter receiving an ACEI or ARB in combination with a β-blocker. This under-prescription was also observed in patients with reduced LVEF. Overall, these results indicate that management of HF in patients cared for in geriatric settings can still be improved.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ANOVA:
-
Analysis of variance
- ARB:
-
Angiotensin II receptor blocker
- BNP:
-
Brain natriuretic peptide
- COPD:
-
Chronic obstructive pulmonary disease
- EHFS:
-
Euro heart failure survey
- HF:
-
Heart failure
- LVEF:
-
Left ventricular ejection fraction
- NTproBNP:
-
N-terminal brain natriuretic peptide
- SD:
-
Standard deviation
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
References
Eurostat. People in the EU – population projections. 2015. https://ec.europa.eu/eurostat/statistics-explained/index.php/People_in_the_EU_-_population_projections#Population_projections.
Komajda M, Hanon O, Hochadel M, Lopez-Sendon JL, Follath F, Ponikowski P, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: euro heart failure survey II. Eur Heart J. 2009;30:478–86.
Paren P, Schaufelberger M, Bjorck L, Lappas G, Fu M, Rosengren A. Trends in prevalence from 1990 to 2007 of patients hospitalized with heart failure in Sweden. Eur J Heart Fail. 2014;16:737–42.
Daamen MAMJ, Hamers JPH, Gorgels APM, Brunner-La Rocca H, Tan FES, van Dieijen-Visser MP, et al. Heart failure in nursing home residents; a cross-sectional study to determine the prevalence and clinical characteristics. BMC Geriatr. 2015;15:167.
Laveau F, Hammoudi N, Berthelot E, Belmin J, Assayag P, Cohen A, et al. Patient journey in decompensated heart failure: an analysis in departments of cardiology and geriatrics in the greater Paris University hospitals. Arch Cardiovasc Dis. 2017;110:42–50.
Mann JL, Evans TS. A review of the management of heart failure in long-term care residents. Consult Pharm. 2006;21:222–8.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200.
McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847.
WHO. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51.
Rouaud A, Hanon O, Boureau A, Chapelet G, de Decker L. Comorbidities against quality control of VKA therapy in non-valvular atrial fibrillation: a French national cross-sectional study. PLoS One. 2015;10:e0119043.
Gambassi G, Forman DE, Lapane KL, Mor V, Sgadari A, Lipsitz LA, et al. Management of heart failure among very old persons living in long-term care: has the voice of trials spread? The SAGE study group. Am Heart J. 2000;139:85–93.
Holmstrom A, Sigurjonsdottir R, Edner M, Jonsson A, Dahlstrom U, Fu ML. Increased comorbidities in heart failure patients >/= 85 years but declined from >90 years: data from the Swedish heart failure registry. Int J Cardiol. 2013;167:2747–52.
Komajda M, Hanon O, Hochadel M, Follath F, Swedberg K, Gitt A, et al. Management of octogenarians hospitalized for heart failure in euro heart failure survey I. Eur Heart J. 2007;28:1310–8.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS research group. Cardiovascular health study. Am J Cardiol. 2001;87:413–9.
McMurray JJV, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–56.
Foebel AD, Heckman GA, Hirdes JP, Tyas SL, Tjam EY, McKelvie RS, et al. Clinical, demographic and functional characteristics associated with pharmacotherapy for heart failure in older home care clients: a retrospective, population-level, cross-sectional study. Drugs Aging. 2011;28:561–73.
Mahjoub H, Rusinaru D, Souliere V, Durier C, Peltier M, Tribouilloy C. Long-term survival in patients older than 80 years hospitalised for heart failure. A 5-year prospective study. Eur J Heart Fail. 2008;10:78–84.
Pulignano G, Del Sindaco D, Tavazzi L, Lucci D, Gorini M, Leggio F, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF registry). Am Heart J. 2002;143:45–55.
Steinman MA, Patil S, Kamat P, Peterson C, Knight SJ. A taxonomy of reasons for not prescribing guideline-recommended medications for patients with heart failure. Am J Geriatr Pharmacother. 2010;8:583–94.
Shang X, Lu R, Liu M, Xiao S, Dong N. Heart rate and outcomes in patients with heart failure with preserved ejection fraction: a dose-response meta-analysis. Medicine (Baltimore). 2017;96:e8431.
Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75.
McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94.
Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94.
Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in heart failure with preserved ejection fraction study (I-PRESERVE). Circ Heart Fail. 2011;4:27–35.
DeVore AD, Mi X, Mentz RJ, Fonarow GC, Van Dyke MK, Maya JF, et al. Discharge heart rate and beta-blocker dose in patients hospitalized with heart failure: findings from the OPTIMIZE-HF registry. Am Heart J. 2016;173:172–8.
Heckman GA, Shamji AK, Ladha R, Stapleton J, Boscart V, Boxer RS, et al. Heart failure Management in Nursing Homes: a scoping literature review. Can J Cardiol. 2018;34:871–80.
Plichart M, Orvoen G, Jourdain P, Quinquis L, Coste J, Escande M, et al. Brain natriuretic peptide usefulness in very elderly dyspnoeic patients: the BED study. Eur J Heart Fail. 2017;19:540–8.
Acknowledgments
We are grateful to the participants for their cooperation, to all the geriatric care units and to the Société Française de Gériatrie et Gérontologie (SFGG).
The SFGG study group: Etienne Guibert [3–5], Fanny Ghazali [6], Alain Pesce [7], Bérangère Beauplet [8], Jean-Dominique Roger [9], Isabelle Carrière [10], Boubacar Timbely [11], Houria Idiri [12], Jean-Pierre Constensoux [13], Anne-Marie Durocher [14], Delphine Dubail [1, 2, 15, 16], Marc Fargier [10], Marie-Christine Godart [19], Marie-Agnès Manciaux [19], Angèle Néouze [20], Sabine Duranton Trevet [21], Régine Payen [22], Denata Mutelica [23], Oarda Bahri [24], Laurent Parrad [24], Aichouna Aroui-Sidelhadj [25], Adeline Lalu-Fraisse [26], Jean-Louis Supiot [27], Elise Thiel [28], Luc Walter [29], Guillaume Barthelat [30], Chantal Delemasure [31], Jean-Jacques Domerego [30], Catherine Maillet [30], Marcel Barjaud [32], Rachid Benaissa [1, 2], Catherine Couturier [18], Marie-Line Gaubert Dahan [33], Alain Jean [34], Maurice Viala [35], Benoît Bartheleme [36], Edith Blanchet [17], Noël Blettner [37], Marie-Christine Bourdiol [38], Corinne Bruhat [39, 40], Joanne Jenn [41], Eric Kiledjian [42], Sophie Lochet [43], Céline Martinand [44], Nicolas Ruillière [45], Hanitra Andrianasolo [46], Jean-Paul Cotinat [47], Aurélie Courrier [48], Laure De Decker [49], Denis Jamin [50], Bruno Martin [51], Odile Martinet [52], Samir Merbah [53], Elena Paillaud [53], Nathalie Ruel [54], Anne-Marie Barisic [30], Emmanuelle Comps [55], Marie Danet [41], Laurent de Bataille [56], Sandrine Estivin [55], Armelle Gentric [55], Dominique Huvent-Grelle [14], Léna Joffredo [1, 2], Sophie Moulias [57], Agnès Rouaud [58], Dominique Adelving-Denuit [59], Emmanuel Alix [60], Marielle Berlioz-Thibal [61], Jean-Paul Bernard [62], Denis Branciforti [63], William Chatelier [30], Isabelle Defouilloy [64], Amandine Devun [65], Mourad Kacher [66], Marc Mennecart [67], Pascal Meyvaert [68], Patricia Rolland [61], Catherine Sagot [69], Philippe Veau [70], Marie-Thérèse Becerro Hallard [71], Souad Benhamed [72], Michel Béra [73], Frédéric Espagne [74, 75], Virginie Fossey Diaz [33], Wiltord Jarzebowski [66], Camille Loiseau-Breton [20], Anne Sonnic [18, 56], Marie-Mériadec Tanguy [8], Thalie Traissac [76], Valéry Antoine [35], Anne Chahwakilian [1, 2], Denis Federico [77], Xavier Galimard [78], Hariniaina Jailany [1, 2], Mireille Jurus-Frichet [52], Frederique Lenormand [79], Lise Lorentz [80], Jean-François Louvet [81], Annick Maltaverne [82], Valérie Revel Da Rocha [79], Sandrine Thibeaud [83], Sabiha Ahmine [84], Assia Bendris-Benaissa [85], Sophie Blanchemain [8], Clémence Boully [1, 2], Cyrille Cantet [86], Cécile Charpentier [87], Pascal Chevalet [18], Dominique Clairet [88,89], Autila Crépin [90], Michel Davy [91], Jean-François Desson [92], Olivier Drunat [33], Yannick Gasnier [93], Yvette Giaccardi [94], Farid Hacini [95], Laëtitia Hengel-Di Nisi [96], Claire Houette [97], Claude Jeandel [17], Aurélie Lafargue [41], Eloïse Lefur-Musquer [61], Marina Monnier [17], Andriamasimanana Rakotoarisoa De Rozier [85], Claire Rouquet [98], Christel Salvietti [30], Alexia Serayet [99], Stephanie Vancompernolle [90], Pascale Vincent [100,101], Joelle Auffret Burguin [102], Edouard Beretti [103], Carine Bouayi [17], Yasmina Boudali [1, 2], Hélène Chossonnery [104], Sébastien Colas [105], Marie-Hélène Fix [18], Isabelle Gantois [106], Anne-Laure Godard [107], Felipe Greslou [17], Stéphane Herbaud [53], Gabriel Malerba [108], Olivier Mellier [52], Eglantine Nemitz [87], Eric Pautas [66], Jean-Michel Pratico [109], Nicolas Redureau [110], Anne Richard [111], Dominique Rivière [112], Jean-Antoine Rosati [113], Aude Simon [114], Julien Zirnhelt [111], Cédric Annweiler [115], Marie-Ange Blanchon [65], Frédéric Bloch [1,2,64], Edouard Chaussade [1, 2], Dominique Darmedru [45], Florence Delamarre-Damier [116], Sophie Deprecq [117], Michèle Escande [118], Catherine Hernandez [119], Mélanie Hervouet [52], Irina Ivancov [107], Florian Labourée [1, 2], Carmelo Lafuente-Lafuente [66], Florence Leonel [107], Rachid Mahmoudi [105], Philippe Morlon [120], Etienne Ojardias [65], Lalaina Rakotoarisoa [85], Kokouvi Soadjede [117], Laurence Vaillard [121], Gabriel Abitbol [1, 2], Sylvie Chaillou [7], Stéphanie Thomas [17], Marc Jegou [122], Anna Kearney-Schwartz [106], Véronique Larraillet [123], Gilles Loggia [8], Marie Lombard [124], Christelle Mischis [125], Brigitte Pichot Duclos [126], Saholy Razafindrainibe [85], Achille Tchalla [127], Catherine Terrat [65], Christine Yves Deville [128], Yassine Benamacht [128], Gilles Berrut [18], David Brugnon [129], Emmanuelle Ferry [120], Valery Gautier [1,2130], Olivier Gilly [89], Emeline Proye [131], Georges Rakocevic [132], Typhaine Riaudel [18], Laure Schmitt [133], Patrick Friocourt [134], Jean-Sébastien Vidal [1, 2], Olivier Hanon [1, 2].
Affiliations
[1] Assistance Publique des Hopitaux de Paris, Hopital Broca, 75013 PARIS, France
[2] Université Paris-Descartes, Sorbonne Paris-Cité, Equipe d’Accueil 4468, PARIS, France
[3] Ma Maison, Les Petites sœurs des pauvres, 33000 BORDEAUX, France
[4] Ma Maison, Les Petites sœurs des pauvres, 47000 AGEN, France
[5] Ma Maison, Les Petites sœurs des pauvres, 17100 SAINTES, France
[6] GH Nord-Vienne, Pole 4, Gériatrie, Soins de suite, HAD, 86100 CHATELLERAULT, France
[7] CH Princesse-Grace, Centre Rainier III, 98000 MONACO, Monaco
[8] CHU de Caen, Départementfilière gériatrique, 14000 CAEN, France
[9] CH d’Annecy-Genevois, USLD La Tonnelle, 74600 SEYNOD, France
[10] CH de Saint-Galmier, 42330 SAINT-GALMIER, France.
[11] CH de Meaux, Service soins de suite, 77100 MEAUX, France.
[12] CHI Le Molinel, 59290 WASQUEHAL, France.
[13] EHPAD Mon Repos, 44140 AIGREFEUILLE-SUR-MAINE, France.
[14] CHRU de Lille, Hôpital des Bateliers, 59037 LILLE, France.
[15] ORPEA Clamart Maison Blanche, 92140 CLAMART, France.
[16] Orpea Résidence La Chanterelle, 93310 LE PRE-SAINT-GERVAIS, France.
[17] CHU de Montpellier, Centre Antonin Balmès, 34000 MONTPELLIER, France.
[18] CHU de Nantes, Hôpital Bellier, 44300 NANTES, France.
[19] CHU de Nancy, Centre de long séjour Saint-Stanislas, 54000 NANCY, France.
[20] Hôpital Bichat, Assistance Publique des Hopitaux de Paris, 75018 PARIS, France.
[21] CH Durécu-Lavoisier, 76160 DARNÉTAL, France.
[22] EHPAD Sainte Camille, 62000 ARRAS, France.
[23] Hôpitaux universitaires de Strasbourg, Pôle de gériatrie, Service de long séjour, 67091 STRASBOURG, France.
[24] CHU de Rouen, Médecine interne gériatrique, 76000 ROUEN, France.
[25] CH de Sainte Foy La Grande, 33220 SAINTE-FOY-LA-GRANDE, France.
[26] CHU Dijon Bourgogne, Centre gériatrique Champmaillot, 21000 DIJON, France.
[27] EHPAD Le Verger, 44470 MAUVES-SUR-LOIRE, France.
[28] CHU de Bordeaux, EHPAD Lormont, 33310 LORMONT, France.
[29] EHPAD de Bergheim, 68750 BERGHEIM, France.
[30] Clinique Les Sources, 06100 NICE, France.
[31] EHPAD La Sabotière, 59260 HELLEMMES-LILLE, France.
[32] EHPAD Sainte Elisabeth, 63210 ROCHEFORT-MONTAGNE, France.
[33] Hôpital Bretonneau, Assistance Publique des Hopitaux de Paris, 75018 PARIS, France.
[34] Hôpital La Rochefoucauld, Assistance Publique des Hopitaux de Paris, 75014 PARIS, France.
[35] CHU de Nîmes, Médecine gériatrique, 30000 NIMES, France.
[36] CH de Rouffach, EHPAD Maison Saint-Jacques, 68250 ROUFFACH, France.
[37] CHR de Metz-Thionville, Hôpital de Mercy, 57000 METZ, France.
[38] CH Saint Jean d’Angély, Service de médecine géraitrique aiguë, 17415 SAINT-JEAN-D’ANGÉLY, France.
[39] Hôpital Baugeois Vallée, 49150 BAUGE, France.
[40] Hôpital Saint-Nicolas, 49000 ANGERS, France.
[41] CHU de Bordeaux, Hôpital Xavier Arnozan, 33604 PESSAC, France.
[42] CH de Vienne Lucien Hussel, Service de gériatrie, 38200 VIENNE, France.
[43] EHPAD Jardin des Ainés, 34190 GANGES, France.
[44] EHPAD Breton, GH Eaubonne Montmorency, 95600 EAUBONNE, France.
[45] CH Ain Val de Saône, Site Pont de Veyle 01290 PONT-DE-VEYLE, France.
[46] CHU de Martinique, Centre Emma Ventura, 97200 FORT de France, France.
[47] EHPAD Les Plaines, 49800 TRÉLAZÉ, France.
[48] Hôpital Cœur du Bourbonnais, 03500 SAINT-POURÇAIN-SUR-SIOULE, France.
[49] CHU de Nantes, Hôpital Nord-Laennec, Médecine aiguë gériatrique, 44300 NANTES, France.
[50] EHPAD Les Papillons d’Or, 63120 COURPIERRE, France.
[51] Etablissement de santé Baugeois Vallée, SSR, 49250 BEAUFORT-EN-VALLÉE, France.
[52] Etablissement de santé Jean Lachenaud, 83600 FREJUS, France.
[53] Hôpital Henri-Mondor, Assistance Publique des Hopitaux de Paris, 94000 CRÉTEIL, France.
[54] CH d’Annecy-Genevois, EHPAD Saint François de Sales, 74000 ANNECY, France.
[55] CHRU Brest, Hopital de la Cavale Blanche, 29200 BREST, France.
[56] CHU de Nantes, EHPAD La Seilleraye, 44470 CARQUEFOU, France.
[57] Hôpital Amboise-Paré, Assistance Publique des Hopitaux de Paris, UCSG, 92100 BOULOGNE, France.
[58] CHU de Nantes, Maison Beauséjour, 44300 NANTES, France.
[59] EHPAD Les Fassoles, 21240 TALANT, France.
[60] CH du Mans, Pôle de gériatrie, 72000 LE MANS, France.
[61] CHU de Nantes, Hôpital Saint-Jacques, Maison Pirmil, 44300 NANTES, France.
[62] Fondation Pomme, 64400 OLORON-SAINTE-MARIE, France.
[63] EHPAD RESIDENCE DE L’ ONAC, 06140 VENCE, France.
[64] CHU d’Amiens-Picardie, Centre Saint Victor, 80000 AMIENS, France.
[65] CHU de Saint Etienne, Hôpital La Charité, 42000 SAINT-ÉTIENNE, France.
[66] Hôpital Charles-Foix, Assistance Publique des Hopitaux de Paris, 94200 IVRY-SUR-SEINE, France.
[67] CHU de Tours, Médecine interne gériatrique, 37000 TOURS, France.
[68] EHPAD Le Manoir, 67150 GERSTHEIM, France.
[69] Hôpital Pitié-Salêtrière, Assistance Publique des Hopitaux de Paris, Service de médecine géraitrique, 75013 PARIS, France.
[70] EHPAD CH George Sand, 18160 CHEZAL-BENOÎT, France.
[71] Hôpital Layné, 40000 MONT-DE-MARSAN, France.
[72] Hôpital de La Collégiale, Assistance Publique des Hopitaux de Paris, 75005 PARIS, France.
[73] CH de Sillé, Service SSR, 72140 SILLÉ-LE-GUILLAUME, France.
[74] EHPAD La Colombe, 63112 BLANZAT, France.
[75] EHPAD La Sainte Famille, 63000 CLERMONT-FERRAND, France.
[76] CHU de Bordeaux, Hôpital Saint-André, 33000 BORDEAUX, France.
[77] Hospices Civils de Lyon, Hôpital Charpennes, 69100 VILLEURBANNE, France.
[78] CHI de Poissy/Saint-Germain-en-Laye, Site hospitalier de Saint-Germain-en-laye, 78100 SAINT-GERMAIN-EN-LAYE, France.
[79] CH de Pau, Unité urgence gériatrique, 64000 PAU, France.
[80] CH départemental de Bischwiller, Pôle médecine et réadaptation, 67240 BISCHWILLER, France.
[81] EHPAD Sainte Anne, 61450 LA FERRIÈRE-AUX-ÉTANGS, France.
[82] EHPAD Henri Vincent, 69100 VILLEURBANNE, France.
[83] GH Sud Ile-de-France Hôpital de Melun-Sénart, SSR St Liesne, 77000 MELUN, France.
[84] Hospices Civils de Lyon, Hôpital Pierre-Garraud, 69005 LYON, France.
[85] Hôpital Georges-Clémenceau, Assistance Publique des Hopitaux de Paris, 91750 CHAMPCUEIL, France.
[86] Hôpital Charles-Richet, Assistance Publique des Hopitaux de Paris, 95400 VILLIERS-LE-BEL, France.
[87] CH des quatres villes, SSR gériatrique, 92310 SÈVRES, France.
[88] CHI de Toulon La Seyne sur Mer, Hôpital Clemenceau La Garde, 83000 TOULON, France.
[89] Hôpital Clémenceau, 83130 LA GARDE, France.
[90] Groupement des Hôpitaux de l’Institut Catholique de Lille, Hôpital Saint Philibert, SSR gériatrique, 59160 LOMME, France.
[91] EHPAD Maison Saint Gabriel, 50400 GRANVILLE, France.
[92] CH de Bayeux, 14400 BAYEUX, France.
[93] Hôpital Les Acacias, 65503 VIC-EN-BIGORRE, France.
[94] Résidence Fontdivina, 06240 BEAUSOLEIL, France.
[95] La Buissonnière 42350 LA TALAUDIÈRE, France.
[96] Centre médical MGEN Trois-Épis, 68410 TROIS-ÉPIS, France.
[97] CH de Bazas, 33430 BAZAS, France.
[98] SSR Le Belvédère Korian, 40150 LABENNE, France.
[99] Hôpital Rothschild, Assistance Publique des Hopitaux de Paris, 75012 PARIS, France.
[100] EHPAD La Provalière, 73140 SAINT-MICHEL-DE-MAURIENNE, France.
[101] Hôpital de Modane, 73500 MODANE, France.
[102] CH de Quimperlé, 29300 QUIMPERLÉ, France.
[103] Ehpad Residence Le Bocage, 13821 LA PENNE-SUR-HUVEAUNE, France.
[104] Centre de Soins et de Réadaptation Les Tilleroyes, 25000 BESANÇON, France.
[105] CHU de Reims, Hopital Maison-Blanche, 51000 REIMS, France.
[106] CHU de Nancy, Hôpitaux de Brabois, 54500 VANDŒUVRE-LÈS-NANCY, France.
[107] CHU de Montpellier, SSR Bellevue, 34000 MONTPELLIER, France.
[108] CHU de Nancy, Hôpital Saint-Julien, Centre Paul Spillmann, 54000 NANCY, France.
[109] EHPAD Les Aiguerelles, 34130 MAUGUIO, France.
[110] CH Côte de Lumière, 85109 LES SABLES-D’OLONNE, France.
[111] CH Denain, Court séjour géraitrique, 74370 PRINGY, France.
[112] EHPAD LA BRUYERE, 19160 NEUVIC, France.
[113] EHPAD Chantelle, 03110 CHANTELLE, France.
[114] Clinique du Parc de Belleville-CLINEA, 75020 PARIS, France.
[115] CHU d’Angers, Service de gérontologie clinique, 49000 ANGERS, France.
[116] Maison de retraite Montfort, 85290 SAINT-LAURENT-SUR-SÈVRE, France.
[117] CH d’Arras, 62000 ARRAS, France.
[118] Hôpital Privé Marseille - Vert Coteau, Service court séjour, 13012 MARSEILLE, France.
[119] Hôpital de Fourvière, 69005 LYON, France.
[120] CH de Monluçon, 03100 MONTLUÇON, France.
[121] EHPAD Champmaillot, 2100 DIJON, France.
[122] Hôpital Emile-Roux, Assistance Publique des Hopitaux de Paris, 94450 LIMEIL-BRÉVANNES, France.
[123] CHU de Martinique, Site Mango-Vulcin, Unité mobile de gériatrie, 97232 LE LAMENTIN, France.
[124] CHS La Chartreuse, USLD/EHPAD Les Vergers de la Chartreuse, 21000 DIJON, France.
[125] CH de Valence, 26000 VALENCE, France.
[126] CH de Cholet, 49300 CHOLET, France.
[127] CHU de Limoges, 87000 LIMOGES, France.
[128] CH de Ssint-Quentin, 02100 SAINT-QUENTIN, France.
[129] CH du Mont-Dore, 63240 LE MONT-DORE, France.
[130] CH de Laval, 53000 LAVAL, France.
[131] CH de Valenciennes, 59300 VALENCIENNES, France.
[132] EHPAD La Verte Colline, 27540 IVRY-LA-BATAILLE, France.
[133] Centre départemental de repos et de soin, 68000 COLMAR, France.
[134] CH de Blois, Médecine interne gériatrique, 41000 BLOIS, France.
Funding
The Société Française de Gériatrie et Gérontologie (SFGG, French Society of Geriatrics and Gerontology) financed the data management. The SFGG participated in the design of this study, analysis, interpretation of data or in writing the manuscript through its members.
Author information
Authors and Affiliations
Consortia
Contributions
CB has participated in the analysis of the data and drafted the manuscript, JSV has participated in the analysis and interpretation of the data and substantively revised the manuscript. EG, FNG, AP, BB, JDR, IC, BT, HI, JPC, AMD, DD, MF are participated in the acquisition of the data and substantively revised the manuscript. GB, CJ are made substantial contributions to the conception participated in the acquisition of the data. OH has made substantial contributions to the conception participated in the acquisition of the data and substantively revised the manuscript. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study). All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the ethical standards set forth in the Declaration of Helsinki (1983). The entire study protocol was approved by the ethics committee of Nantes (Groupe Nantais d’Ethique dans le Domaine de la Santé, France), and the study complies with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement guidelines.
No consent to participate was sought for the subjects in accordance to the French ethics rules because the study was observational (related to usual care), and no nominative data were collected (Code de la Santé Publique – Article L1121–1. https://www.legifrance.gouv.fr). All patients’ data were anonymized at the patients’ sites before they were uploaded to the study center.
Consent for publication
N/A
Competing interests
OH received consultant/advisory/lecture fees from Bayer, Boehringer-Ingelheim, BMS, Pfizer, Novartis, Servier, Astra-Zeneca, Vifor.
CJ received consultant/advisory/lecture fees from Bayer, Boehringer-Ingelheim, BMS, Pfizer, Novartis, Servier, Vifor.
GB received consultant/advisory/lecture fees from Bayer, BMS, Pfizer, Novartis, Vifor.
CB, JSV, EG, FNG, AP, BB, JDR, IC, BT, HI, JPC, AMD, DD, MF have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1
Table S1 Characteristics of patients according to ACEI or ARB use. Table S2 Characteristics of patients according to β-blocker use. (DOCX 47 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Boully, C., Vidal, JS., Guibert, E. et al. National survey on the management of heart failure in individuals over 80 years of age in French geriatric care units. BMC Geriatr 19, 204 (2019). https://doi.org/10.1186/s12877-019-1215-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-019-1215-y