Abstract
Background
Liver diseases post-COVID-19 vaccination is extremely rare but can occur. A growing body of evidence has indicated that portal vein thrombosis, autoimmune hepatitis, raised liver enzymes and liver injuries, etc., may be potential consequence of COVID-19 vaccines.
Objectives
To describe the results of a systematic review for new-onset and relapsed liver disease following COVID-19 vaccination.
Methods
For this systematic review, we searched Proquest, Medline, Embase, PubMed, CINAHL, Wiley online library, Scopus and Nature through the Preferred Reporting Items for Systematic Reviews and Meta Analyses PRISMA guideline for studies on the incidence of new onset or relapsed liver diseases post-COVID-19 vaccination, published from December 1, 2020 to July 31, 2022, with English language restriction.
Results
Two hundred seventy-five cases from one hundred and eighteen articles were included in the qualitative synthesis of this systematic review. Autoimmune hepatitis (138 cases) was the most frequent pathology observed post-COVID-19 vaccination, followed by portal vein thrombosis (52 cases), raised liver enzymes (26 cases) and liver injury (21 cases). Other cases include splanchnic vein thrombosis, acute cellular rejection of the liver, jaundice, hepatomegaly, acute hepatic failure and hepatic porphyria. Mortality was reported in any of the included cases for acute hepatic failure (n = 4, 50%), portal vein thrombosis (n = 25, 48.1%), splanchnic vein thrombosis (n = 6, 42.8%), jaundice (n = 1, 12.5%), raised liver enzymes (n = 2, 7.7%), and autoimmune hepatitis (n = 3, 2.2%). Most patients were easily treated without any serious complications, recovered and did not require long-term hepatic therapy.
Conclusion
Reported evidence of liver diseases post-COIVD-19 vaccination should not discourage vaccination against this worldwide pandemic. The number of reported cases is relatively very small in relation to the hundreds of millions of vaccinations that have occurred and the protective benefits offered by COVID-19 vaccination far outweigh the risks.
Similar content being viewed by others
Background
Vaccinations against coronavirus disease 2019 (COVID-19) is a crucial step in ending the current worldwide pandemic. Vaccines such as Pfizer-BioNTech, Oxford Uni-AstraZeneca, Moderna, Johnson & Johnson, Sinovac-CoronaVac, Covishield, and Sinopharm have been developed rapidly, determined as safe, approved under emergency use authorization since early 2020 and had been used widely. As of 1 May 2022, there have been more than 5 billion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine doses administered globally [1]. Therefore, new safety, adverse effects, or toxicity concerns related to the COVID-19 vaccination have emerged. Adverse reactions to COVID-19 vaccines are commonly reported, but most are not hepatically mediated. Localized pain, fatigue, headache and muscle ache are the most prevalent adverse effects following COVID-19 vaccination [2]. Liver toxicity is rare with all vaccines used to prevent COVID-19, but can occur. A growing body of evidence has indicated that portal vein thrombosis [3,4,5], autoimmune hepatitis [6,7,8], raised liver enzymes [9,10,11] and liver injuries [12, 13], etc., may be potential consequence of COVID-19 vaccines. COVID-19 vaccines are usually administered in 2- or 3-dose series over a short time only [14, 15], and the symptoms and signs of the COVID-19 infection overshadow the mild and transient liver adverse effects that arises with some of the vaccines used to prevent COVID-19. Furthermore, instances of acute hepatitis [16], raised liver enzymes [17, 18] and liver injury [19] have been reported in patients with moderate and severe COVID-19 in which vaccines did not appear to play a role. Whether the association between SARS-CoV-2 vaccines and those liver diseases is coincidental or causal remains to be elucidated.
In light of newer case reports and case-series studies that were published to describe the incidence of hepatotoxicity in patients who received the COVID-19 vaccines, we provide a systematic review of the current literature to delineate the range of liver diseases that were elicited following COVID-19 vaccination. We expect our review to provide clinicians with a thorough understanding of these rare adverse events.
Methods
Design
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines PRISMA in conducting this systematic review [20]. The following electronic databases were searched: PROQUEST, MEDLINE, EMBASE, PUBMED, CINAHL, WILEY ONLINE LIBRARY, SCOPUS and NATURE with Full Text. We used the following keywords: (“COVID-19” OR “SARS-CoV-2” OR “Severe acute Respiratory Syndrome Coronavirus 2” OR “Coronavirus Disease 2019” OR “2019 novel coronavirus”) AND vaccine OR vaccination AND (“liver histopathology” OR “liver disease” OR “hepatic disease” OR “liver toxicity” OR “hepatotoxicity”). The search was limited to papers published in English between 1 December 2020 and 31 July 2022. Based on the title and abstract of each selected article, we selected those discussing and reporting occurrence of new-onset or relapsed liver disease following SARS-CoV-2 vaccination.
Inclusion–exclusion criteria
The inclusion criteria are as follows: (1) published case reports, case series and cohort studies that focused on new-onset or relapsed liver diseases following SARS-CoV-2 vaccination that included adults as population of interest; (2) studies of experimental or observational design reporting the incidence of new-onset or relapsed liver diseases in patients post-SARS-CoV-2 vaccination; and (3) the language was restricted to English. The exclusion criteria are as follows: (1) studies that did not report data on new-onset or relapsed liver diseases due to SARS-CoV-2 vaccination; (2) studies that did not report details on identified new-onset or relapsed liver disease cases following COVID-19 vaccination; (3) studies that reported new-onset or relapsed liver disease in patients with no history of COVID-19 vaccination; and (4) duplicate publications.
Data extraction
Six authors (Saad Alhumaid, Abbas Al Mutair, Ali Rabaan, Fatemah M. ALShakhs, Shin Jie Yong, and Hussain Ahmed Alsouaib) critically reviewed all of the studies retrieved and selected those judged to be the most relevant. Data were carefully extracted from the relevant research studies independently. Articles were categorized as case report or case-series studies. The following data were extracted from selected studies: authors; publication year; study location; study design and setting; age; proportion of male patients; patient ethnicity; time to hospital presentation with liver pathology from day of vaccination, medical comorbidities; vaccine brand and dose (if 1st dose, 2nd dose or 3rd dose); if liver pathology is new-onset or relapsed; patient clinical presentation; abnormal laboratory indicators; biopsy examination and radiological imaging findings; treatment given; assessment of study risk of bias; and treatment outcome (survived or died); which are noted in Table 1.
Quality assessment
The quality assessment of the studies was undertaken mainly based on the modified Newcastle–Ottawa Scale (NOS) to assess the quality of the selected studies [21]. Items related to the comparability and adjustment were removed from the NOS and items which focus on selection and representativeness of cases, and ascertainment of outcome and exposure are kept [22]. Modified NOS consists of five items each requires yes and no response to indicate whether bias was likely, and these items were applied to single-arm studies [22]. Quality of the study was considered good if all five criteria were met, moderate when four were met, and poor when three or less were met. Quality assessment was performed by six authors (Mohammed Hussain Al Khamees, Yaqoub Yousef Alatiyyah, Ali Ahmed Alsultan, Hassan N. Alshakhs, Haidar Abdullah Al Samaeel, and Rugayah Ahmed AlShayeb) independently, with any disagreement to be resolved by consensus.
Data analysis
We examined primarily the proportion of confirmed cases who suffered liver toxicity due to COVID-19 vaccination. This proportion was further classified based on the type of liver pathology induced by the COVID-19 vaccine (i.e., if portal vein thrombosis, autoimmune hepatitis or raised liver enzymes etc.). Descriptive statistics were used to describe the data. For continuous variables, mean and standard deviation were used to summarize the data; and for categorical variables, frequencies and percentages were reported. Microsoft Excel 2019 (Microsoft Corp., Redmond, USA) was used for all statistical analyses.
Results
Study characteristics and quality
A total of 1587 publications were identified (Fig. 1). After exclusion of duplicates and articles that did not fulfil the study inclusion criteria, one hundred and eighteen articles were included in the qualitative synthesis of this systematic review. The reports of two hundred and seventy-five cases identified from these articles are presented by groups based on confirmed diagnoses, laboratory, biopsy and imaging findings [3,4,5,6,7,8,9,10,11,12,13, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128]. The detailed characteristics of the included studies are shown in Table 1. There were 107 case report [3,4,5,6,7,8,9,10,11,12, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41, 43,44,45,46,47, 49,50,51, 55, 57,58,59, 61,62,63, 65,66,67,68, 70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125, 127, 128], and 11 case series [13, 42, 48, 52,53,54, 56, 60, 64, 69, 126] studies. These studies were conducted in United States (n = 20), Italy (n = 15), Germany (n = 10), United Kingdom (n = 9), Japan (n = 6), India (n = 5), Spain (n = 4), Saudi Arabia (n = 4), France (n = 4), Austria (n = 3), Switzerland (n = 4), Iran (n = 4), Republic of Korea (n = 3), Turkey (n = 2), Ireland (n = 2), Portugal (n = 2), Greece (n = 2), The Netherlands (n = 2), Denmark (n = 2), Singapore (n = 2), Brazil (n = 1), Oman (n = 1), Colombia (n = 1), China (n = 1), Israel (n = 1), Taiwan (n = 1), Kuwait (n = 1), Norway (n = 1), Mexico (n = 1), Malaysia (n = 1), Thailand (n = 1), Democratic Republic of the Congo (n = 1), and Australia (n = 1). Only two studies were made within multi-countries (n = 2) [60, 126]. The majority of the studies were single centre [3,4,5,6,7,8,9,10,11,12, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41, 43,44,45,46,47,48,49,50,51, 55,56,57,58,59, 61,62,63, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125, 127, 128] and only 8 studies were multi-centre [13, 42, 52,53,54, 60, 64, 126]. All case reports and case-series studies were assessed for bias using the modified NOS. Thirty-two studies were deemed to have high methodological quality, 83 moderate methodological quality, and 3 low methodological quality; Table 1.
Autoimmune hepatitis
Autoimmune hepatitis (AIH) was the first most-common liver disease reported following COVID-19 vaccination [eighty-three new onset cases [6,7,8, 37, 41, 68, 84, 85, 87, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 115, 117,118,119,120, 123, 124, 126, 127] and four previously known cases [43, 80, 86, 104]; and in fifty-one cases event if new-onset or relapsed was not reported [42]] (see Table 1). Most common clinical presentations in these AIH cases were fatigue (n = 75) [99, 102,103,104, 112, 118, 119, 124, 126, 127], jaundice (n = 68), [6,7,8, 37, 42, 68, 84, 85, 97, 99, 102, 104,105,106,107,108, 110, 112, 115, 117, 118, 123, 126, 127], nausea (n = 60) [68, 108, 112, 123, 126, 127], abdominal pain (n = 25) [7, 37, 68, 105, 126], pruritus (n = 10) [6, 37, 99, 101, 105, 110, 117, 127], itching (n = 10) [126], dark urine (n = 10) [6, 7, 68, 84, 103, 104, 106, 108, 110, 123], hepatomegaly (n = 6) [6, 7, 85, 102, 103, 123], fever (n = 5) [84, 104, 117, 123], malaise (n = 4) [84, 85, 97, 112], anorexia (n = 4) [8, 102, 104, 112], and yellow eyes (n = 4) [8, 103, 112, 118]. Four of the AIH cases were asymptomatic [43, 80, 86, 87]. The median interquartile range (IQR) age of this group was 59 [41 to 72], with an increased female predominance in AIH patients diagnosed after COVID-19 vaccination in most of the studies [n = 90, 65.2%] [6,7,8, 43, 68, 80, 84, 86, 87, 97, 99, 103, 105,106,107,108, 110, 112, 115, 118,119,120, 123, 124, 126], and majority of the patients belonged to White (Caucasian) (n = 34, 24.6%) [6, 7, 41,42,43, 68, 80, 85,86,87, 97, 99, 102, 103, 105,106,107,108, 112, 120, 127] and Asian (n = 13, 9.4%) [8, 84, 110, 115, 117,118,119, 123, 124] ethnicity. The median (IQR) time between the COVID-19 vaccination and time of presentation was 14 (7–20) days. Seventy-seven, twenty-nine, and twenty-nine of these one hundred-thirty eight cases were reported following Pfizer-BioNTech (eight after the first dose, eight after the second dose and three after the third dose) [6, 41, 43, 68, 84, 87, 99, 105, 106, 112, 115, 119, 120, 123, 124, 127], Moderna (nine after the first dose and three after the second dose) [7, 8, 80, 85, 97, 99, 102, 103, 107, 108, 117, 126], and Oxford Uni-AstraZeneca (three after the first dose, two after the second dose and one after the third dose) [37, 86, 99, 101, 115, 126] vaccination; respectively. Ten AIH patients had a history of thyroid gland disorders [Hashimoto’s thyroiditis (n = 6) [42, 103, 106, 112] and hypothyroidism (n = 4) [68, 86, 104, 127]] and seven patients had no medical history (n = 7, 5.1%) [85, 97, 110, 115, 117, 119, 123], however, some of the patients had a past medical history of hypertension (n = 17, 12.3%) [6, 101, 112, 118, 126], diabetes mellitus (n = 15, 10.9%) [102, 104, 126], hyperlipidaemia (n = 6, 4.3%) [8, 84, 105, 106, 115, 118], and rheumatoid arthritis (n = 2, 1.4%) [42]. Some of those AIH cases presented with a previous known history of hepatic pathologies [undetermined pre-existing liver disease (n = 12, 8.7%) [126], nonalcoholic fatty liver disease (n = 7, 5.1%) [126], primary biliary cholangitis (n = 5, 3.6%) [41, 80, 84, 115, 126], hepatitis C infection (n = 2, 1.4%) [124, 126], liver transplant recipient (n = 2, 1.4%) [43, 126], hepatitis B infection (n = 1, 0.7%) [120], autoimmune hepatitis (n = 1, 0.7%) [43], jaundice (n = 1, 0.7%) [104], liver cirrhosis (n = 1, 0.7%) [41], or hypertransaminasemia (n = 1, 0.7%) [86]]. Radiological imaging was unremarkable for a high number of the AIH cases (n = 22, 15.9%) [6, 8, 43, 68, 80, 85,86,87, 97, 99, 102, 104, 105, 108, 110, 115, 117, 119, 120, 124] or not reported (n = 100, 72.5%) [41, 42, 126], nevertheless, liver biopsy revealed histopathological findings consistent with AIH in all cases except for one patient [42]. Patients who suffered AIH post-COVID-19 vaccination were more likely to have positive antinuclear antibodies (n = 92) [6,7,8, 37, 41, 42, 80, 84,85,86,87, 97, 99, 101,102,103,104, 106, 108, 110, 112, 115, 117,118,119, 123, 124, 126], elevated immunoglobulin G (n = 89) [7, 8, 37, 41, 68, 80, 84,85,86,87, 97, 101,102,103,104,105,106,107,108, 110, 112, 115, 117,118,119,120, 123, 124, 126], raised liver enzymes (n = 55) [6,7,8, 37, 41,42,43, 68, 80, 84,85,86,87, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 115, 117,118,119,120, 123, 124, 126, 127], raised bilirubin (n = 41) [6,7,8, 37, 41, 42, 68, 80, 84, 85, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 115, 117, 118, 123, 124], positive anti-smooth muscle antibodies (n = 24) [8, 37, 42, 97, 103, 107, 108, 112, 118, 126], or high international normalized ratio (n = 6) [80, 84, 99, 104]. As expected, most prescribed pharmacotherapy agents in these AIH cases were steroids (n = 82) [6,7,8, 37, 41, 43, 68, 80, 84,85,86,87, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 115, 118, 120, 123, 124, 126, 127] and azathioprine (n = 20) [37, 41, 43, 68, 80, 86, 97, 103, 110, 112, 118, 126], however, pharmacotherapy was not reported in a high number of these AIH patients (n = 12, 8.7%) [42]. Clinical outcomes of the AIH patients with mortality were documented in 3 (2.2%) [99, 104, 115], while 123 (89.1%) of the AIH cases recovered [6,7,8, 37, 41, 43, 68, 80, 84,85,86,87, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 115, 117,118,119,120, 123, 124, 126, 127] and final treatment outcome was not reported in many AIH patients (n = 12, 29.3%) [42].
Portal vein thrombosis
Portal vein thrombosis (PVT) was the second most common liver pathology reported following COVID-19 vaccination (fifty-two new-onset cases), with extra-cranial thrombosis (n = 21) [3, 5, 52,53,54, 56, 59, 61, 63,64,65,66,67, 76, 91, 95, 98, 111], headache (n = 20) [3,4,5, 31, 51,52,53, 55, 58, 64,65,66,67, 91, 111, 116], intracranial hemorrhage (n = 17) [3, 31, 53, 54, 95, 111], abdominal pain (n = 16) [3,4,5, 52, 53, 57,58,59, 61, 65, 76, 91, 96, 98, 116], cerebral venous sinus thrombosis (n = 13) [30, 31, 54, 55, 66, 95, 109], vomiting (n = 8) [3, 31, 53, 64, 67, 96, 98], fever (n = 8) [52, 53, 58, 64, 67, 96, 116], nausea (n = 6) [31, 53, 66, 96, 116] and seizures (n = 5) [3, 31, 61, 67, 111] as the common clinical presentations in these cases (see Table 1). The median interquartile range (IQR) age of this group was 47.5 (32.5 to 55) years, with an increased female predominance in PVT patients diagnosed after COVID-19 vaccination in most of the studies [n = 28, 53.8%] [4, 30, 31, 51, 53, 58, 61, 62, 64, 65, 67, 76, 91, 96, 98, 109, 111, 116], and majority of the patients belonged to White (Caucasian) (n = 44, 84.6%) [3,4,5, 30, 49, 51,52,53,54, 57, 61,62,63,64,65,66, 76, 91, 95, 96, 98, 109, 111, 116] and Indian (n = 6, 11.8%) [31, 55, 56, 59] ethnicity. The median (IQR) time between the COVID-19 vaccination and time of presentation was 10 (7–13) days. Forty-five of these fifty-one cases (forty-four after the first dose and one after the second dose) were reported following Oxford Uni-AstraZeneca vaccination [3,4,5, 31, 49, 51, 52, 54,55,56, 58, 59, 61, 62, 64, 65, 67, 76, 91, 95, 96, 98, 109, 111]. The remaining six PVT cases were reported after Johnson & Johnson COVID-19 vaccination [30, 53, 57, 63, 66]. Fourteen PVT patients were donors after brain death (n = 14, 27.4%) [95, 109] and seven patients had no medical history (n = 7, 13.7%) [4, 5, 52, 64, 66], however, some of the patients had a past drug history of regular intake of oral contraceptive pills (n = 6, 11.5%) [31, 51, 53, 54, 64, 111, 116]. Few PVT patients had pre-existing diabetes mellitus (n = 3) [56, 59, 67], migraine (n = 3) [51, 65, 111], thyroid gland disorders [hypothyroidism and goiter] (n = 4), and obesity (n = 3) [61, 63, 116]. Nevertheless, medical history was not reported for five PVT cases [3, 30, 62, 91, 98] and there were four PVT cases with previously established diagnoses of liver diseases [alcoholic cirrhosis (n = 2), nonalcoholic fatty liver disease (n = 1), and hepatitis C (n = 1)] [56, 57]. Radiological imaging shown PVT in almost all the patients who were included in this review and thought to have had developed PVTs post-COVID-19 vaccination [3,4,5, 30, 31, 49, 51,52,53,54,55,56,57,58,59, 61,62,63,64,65,66,67, 76, 91, 95, 96, 98, 109, 111], however, only a total of three cases presenting with PVT following COVID-19 vaccination were diagnosed based on liver histopathology [30, 54, 98, 116]. Patients who suffered PVT post-COVID-19 vaccination were more likely to have thrombocytopenia (n = 36) [3,4,5, 30, 31, 49, 51,52,53,54,55, 57, 58, 61,62,63,64,65,66,67, 76, 91, 95, 96, 98, 109, 111], high D-dimer (n = 34) [3,4,5, 30, 31, 49, 51,52,53,54,55, 57, 58, 61,62,63,64,65,66,67, 91, 95, 98, 109], positive antibodies directed against platelet factor 4 (n = 23) [3,4,5, 30, 49, 51,52,53,54,55, 57, 58, 61, 63, 65, 66, 76, 91, 111], high international normalized ratio (n = 10) [31, 53, 57, 63, 64, 67, 91, 98], high activated partial thromboplastin time (n = 8) [53, 54, 62, 67, 98, 109], low haemoglobin (n = 7) [61,62,63, 67, 76, 96, 98], and raised liver enzymes (n = 7) [51, 57, 58, 65, 67, 91, 96]. As expected, most prescribed pharmacotherapy agents in these PVT cases were the anticoagulants (n = 26, 51%), including unspecified type of heparins (n = 10), unspecified type of anticoagulants (n = 9), fondaparinux (n = 9), argatroban (n = 7), apixaban (n = 5), dalteparin (n = 3), rivaroxaban (n = 3), warfarin (n = 1), danaparoid (n = 1), or tinzaparin (n = 1) [3,4,5, 30, 31, 49, 51,52,53, 55,56,57,58,59, 61, 63,64,65,66,67, 76, 91, 96, 111, 116]. Many patients were also prescribed intravenous immunoglobulin (n = 19, 37.2%) [3,4,5, 30, 31, 49, 52, 53, 55, 57, 58, 61, 63, 65, 66, 76, 91, 111] and steroids (n = 11, 21.6%) [5, 49, 52, 57, 58, 61, 63, 64], however, pharmacotherapy was not reported in a high number of these PVT patients (n = 18, 35.3%) [54, 95, 98, 109]. Clinical outcomes of the PVT patients with mortality were documented in 25 (48.1%) [30, 31, 53, 54, 56, 61, 62, 64, 67, 95, 98, 109], while 23 (44.2%) of the PVT cases recovered [3,4,5, 49, 51,52,53,54,55,56,57,58,59, 61, 63, 65, 66, 76, 96, 111, 116] and few PVT patients were in a coma (n = 3, 5.9%) [64].
Raised liver enzymes
Raised liver enzymes (RLEs) was the third most-common disease (twenty-six cases) reported following COVID-19 vaccination from our review (twenty-four new onset cases [9,10,11, 23, 25,26,27,28, 32, 33, 36, 38,39,40, 46, 70, 77, 79, 83, 94, 114, 121] and two relapsed cases [89, 93]) (see Table 1). Most common clinical presentations in those cases who presented with RLEs post-COVID-19 vaccination were fever (n = 11) [9, 10, 25, 28, 38, 70, 77, 79, 83, 114, 121], rash (n = 8) [25, 32, 38, 39, 79, 83, 89, 94], oedema (n = 8) [25, 32, 40, 79, 83, 89], weakness (n = 6) [26, 28, 46, 77, 79, 83, 89], fatigue (n = 5) [9, 25,26,27, 83], shortness of breath (n = 5) [26, 77, 78, 83, 89], vomiting (n = 5) [9, 11, 39, 77, 89], abdominal pain (n = 5) [39, 46, 83, 89, 121], headache (n = 5) [23, 33, 38, 83, 121], and myalgia (n = 4) [9, 25, 38, 77]. The median interquartile range (IQR) age of this group was 49 (32.7 to 68.2), with a similar gender rate in patients who presented with RLEs found after COVID-19 vaccination in all of the studies [female (n = 13) [10, 11, 27, 28, 32, 33, 36, 39, 40, 78, 83, 93, 94] and male (n = 13) [9, 23, 25, 26, 38, 46, 70, 77, 89, 114, 121]], and majority of the patients belonged to White (Caucasian) (n = 13, 50%) [9, 11, 23, 25,26,27, 32, 38, 46, 77, 83, 93, 94, 121] and Arab (n = 4, 15.4%) [39, 114] ethnicity. The median (IQR) time between the COVID-19 vaccination and time of presentation was 7 (4.5–11.5) days. Eleven, nine, and four of these twenty-five cases were reported following Pfizer-BioNTech (five after the first dose and six after the second dose) [9, 10, 25, 27, 28, 38,39,40, 79, 83, 93], Oxford Uni-AstraZeneca (eight after the first dose and one after the second dose) [23, 32, 33, 36, 46, 77, 114], and Moderna (four after the first dose) [26, 70, 89, 94] vaccination; respectively. Only two cases presented with RLEs were reported after Johnson & Johnson COVID-19 vaccination [11, 121]. Six of the patients who presented with RLEs had hypertension [11, 27, 33, 38, 83, 89] and nine patients had no medical history (n = 9, 34.1%) [9, 10, 26, 28, 32, 39, 40, 46, 121], however, few of those cases presented with a previous known history of hepatic diseases [chronic hepatitis B (n = 1) [83], alcohol-associated liver disease (n = 1) [70], chronic liver disease (n = 1) [36], portal hypertension (n = 1) [36], hepatitis C infection (n = 1) [89], and compensated alcoholic liver cirrhosis (n = 1) [25]]. Radiological imaging was unremarkable for a high number of the cases who presented with RLEs (n = 10, 40%) [11, 23, 25, 28, 38, 77, 79, 83, 93, 94] or not performed (n = 3, 12%) [10, 27, 36], nevertheless, few cases shown fatty liver and gallbladder polyps (n = 1) [70], liver cirrhosis (n = 1) [89], and abruptly collapsed hepatic veins (n = 1) [40]. Liver biopsy revealed histopathological findings consistent with leukocytoclastic vasculitis (n = 1) [36], drug reaction with eosinophilia (n = 1) [32], giant cell arteritis (n = 1) [23], plasmacytoid dendritic cells (n = 1) [10], and dermatomyositis (n = 1) [28]; however, histopathological examination was not performed in most of the cases (n = 18, %) [9, 11, 25,26,27, 33, 38, 39, 70, 77, 79, 83, 89, 93, 114, 121]. Patients who suffered RLEs post-COVID-19 vaccination were more likely to have high C-reactive protein (n = 14) [10, 11, 23, 25, 32, 33, 36, 38, 70, 89, 114, 121], thrombocytopenia (n = 13) [10, 11, 27, 33, 39, 83, 89, 93, 94, 114, 121], high lactate dehydrogenase (n = 11) [10, 11, 26, 27, 33, 36, 39, 46, 83, 89, 93], raised bilirubin (n = 10) [10, 11, 26, 27, 38, 39, 79, 83, 89, 114], low haemoglobin (n = 7) [11, 27, 33, 38, 39, 114], high creatinine (n = 6) [10, 11, 28, 33, 46, 77, 89], high reticulocyte count (n = 5) [26, 27, 39, 83, 93], high D-dimer (n = 5) [10, 33, 40, 46, 114], raised white blood cells (n = 4) [11, 27, 39, 77], high leukocytes (n = 4) [26, 33, 36, 79], and high ferritin (n = 4) [10, 38, 79, 89]. Most prescribed pharmacotherapy agents in patients with RLEs post-COVID-19 vaccination were steroids (n = 19) [9,10,11, 23, 25,26,27,28, 32, 33, 36, 38, 39, 46, 70, 78, 83, 93, 94], intravenous immunoglobulin (n = 8) [9, 10, 27, 28, 33, 40, 93, 94], and antibiotics (n = 7) [9, 46, 114, 121]. Clinical outcomes of the RLEs patients with mortality were documented in 2 (7.7%) [46, 114], while 22 (84.6%) of the RLEs cases recovered [9, 10, 23, 25,26,27,28, 32, 33, 36, 38,39,40, 70, 77, 79, 83, 93, 94, 114, 121] and final treatment outcome was not reported in two RLEs patients (n = 2, 7.7%) [11, 89].
Acute liver injury
Acute liver injuries (ALIs) was the fourth most-common disease (twenty-one cases) reported following COVID-19 vaccination from our review [sixteen new onset cases [12, 13, 44, 99, 113, 122] and five relapsed cases [13]] (see Table 1). Most common clinical presentations in patients who presented with ALIs post-COVID-19 vaccination were abdominal tenderness (n = 3) [12, 113], jaundice (n = 2) [44, 113], yellow eyes (n = 2) [12, 44], weakness (n = 2) [12, 44], and vomiting (n = 2) [12, 113]. The median interquartile range (IQR) age of this group was 61 (41.5–68), with a female predominance in ALIs patients diagnosed after COVID-19 vaccination in most of the studies [n = 14, 66.7%] [12, 13, 99, 113, 122], and ethnicity was not reported for majority of the patients (n = 16, 80%) [13]. The median (IQR) time between the COVID-19 vaccination and time of presentation was 24 (7.5–31) days. Sixteen and four of these twenty cases were reported following Pfizer-BioNTech [12, 13, 99, 113, 122] and Moderna [13] vaccination; respectively. Only one case presented with liver injury was reported after Sinopharm COVID-19 vaccination [44]. Most of those cases presented with a previous known history of hepatic diseases [chronic liver disease (n = 6) [13], AIH (n = 4) [13], cirrhosis (n = 3) [13], hepatitis C virus (n = 1) [13], drug-induced liver injury (n = 1) [13], alcohol-associated liver disease (n = 1) [99], and liver transplant recipient (n = 1) [99]]. Radiological imaging was unremarkable for few cases who presented with ALIs (n = 4, 19%) [13, 99, 122], however, liver biopsy revealed histopathological findings consistent with AIH in one case [13] but biopsy examination was not made for many patients (n = 10, 47.6%) [13, 44, 99, 113, 122]. Patients who suffered ALIs post-COVID-19 vaccination were more likely to have raised liver enzymes (n = 20) [12, 13, 44, 99, 122], raised bilirubin (n = 15) [12, 13, 44, 99], high international normalized ratio (n = 8) [13, 113], positive antinuclear antibodies (n = 5) [13], and positive anti-smooth muscle antibodies (n = 4) [13]. Most prescribed pharmacotherapy agents in patients who suffered ALIs post-COVID-19 vaccination were steroids (n = 8) [13] and N-acetylcysteine (n = 3) [13, 113]. All patients who experienced ALIs after COVID-19 vaccination recovered (n = 21, 100%) [12, 13, 44, 99, 113, 122].
Splanchnic vein thrombosis
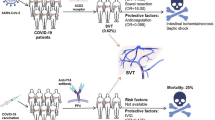
Splanchnic vein thrombosis (SVT) was the fifth most-common disease (fourteen cases) reported following COVID-19 vaccination from our review (fourteen new onset cases [47, 48, 50, 60]) (see Table 1). Most common clinical presentations in patients who presented with SVT post-COVID-19 vaccination were abdominal tenderness (n = 2) [47, 50], fatigue (n = 2) [48, 60], nausea (n = 2) [47, 60], and headache (n = 2) [50, 60]. The median interquartile range (IQR) age of this group was 55 (48.2 to 61), with a female predominance in SVT patients diagnosed after COVID-19 vaccination in most of the studies (n = 12, 60%) [47, 48, 50, 60], and all patients belonged to the White (Caucasian) ethnicity (n = 20, 100%) [47, 48, 50, 60]. The median (IQR) time between the COVID-19 vaccination and time of presentation was 8.5 (6.7–13.2) days. Thirteen of these fourteen SVT cases were reported following Oxford Uni-AstraZeneca vaccination [47, 48, 60] and only one case presented with SVT was reported after Johnson & Johnson COVID-19 vaccination [50]. Unexpectedly, most of the SVT cases had no medical history (n = 11, 73.3%) [47, 48, 50, 60]. Radiological imaging for SVT patients shown cerebral venous thrombosis (n = 9) [60], disseminated intravascular coagulation (n = 5) [60] and pulmonary embolisms (n = 3) [60]. Patients who experienced SVT post-COVID-19 vaccination were more likely to have thrombocytopenia (n = 14) [47, 48, 50, 60], positive for antibodies directed against platelet factor 4 antibodies (n = 13) [48, 50, 60], high D-dimer (n = 10) [47, 48, 50, 60], high activated partial thromboplastin time (n = 6) [50, 60], high international normalized ratio (n = 5) [60], and low fibrinogen (n = 5) [50, 60]. Most prescribed pharmacotherapy agents in patients who suffered SVTs post-COVID-19 vaccination were the heparins (n = 7, 50%) [48, 50, 60], anticoagulants (n = 4, 28.6%) [47, 48, 50, 60], and intravenous immunoglobulin (n = 3, 21.4%) [47, 48, 50]. Clinical outcomes of the SVT patients with mortality were documented in 6 (42.8%) [60], while 8 (57.1%) of the SVT cases recovered [47, 48, 50, 60].
Acute cellular rejection of the liver
Acute cellular rejection of the liver (ACRL) was the sixth most-common disease (eight cases) reported following COVID-19 vaccination from our review (six new onset and two relapsed cases [29, 34, 69, 82]) (see Table 1). The median interquartile range (IQR) age of this group was 59.5 (52.5–64.7), with a male predominance in ACRL patients diagnosed after COVID-19 vaccination in most of the studies [n = 5, 62.5%] [34, 69], and all patients belonged to the White (Caucasian) ethnicity (n = 8, 100%) [29, 34, 69, 82]. The median (IQR) time between the COVID-19 vaccination and time of presentation was 11 (7.5–17.2) days. Four of these eight ACRL cases were reported following Pfizer-BioNTech vaccination [29, 34, 69] and four of these eight ACRL cases were reported after Moderna COVID-19 vaccination [69, 82]. All of the ACRL cases had previous medical history related to the liver [non-alcoholic steatohepatitis-related cirrhosis (n = 3) [69], alcohol-related cirrhosis (n = 2) [69], history of acute cellular rejection (n = 2) [69], autoimmune cirrhosis (n = 1) [29], cryptogenic cirrhosis (n = 1) [34], cirrhosis (n = 1) [82], end-stage liver disease (n = 1) [29], hepatitis C virus (n = 1) [82], and hepatocellular carcinoma (n = 1) [82]]. Liver biopsy for the ACRL cases shown typical features consistent with acute liver rejection [mixed portal inflammation of predominantly mixed activated lymphocytes, bile duct injury, and endotheliitis] (n = 7, 87.5%) [29, 34, 69, 82]. Patients who experienced ACLR post-COVID-19 vaccination were more likely to have raised liver enzymes (n = 6) [29, 34, 69, 82], raised bilirubin (n = 5) [34, 69], and thrombocytopenia (n = 2) [29, 34]. Most prescribed pharmacotherapy agents in patients who suffered ACRL post-COVID-19 vaccination were the steroids (n = 12), IVIG (n = 2) [29, 34], immunosuppressants (n = 4) [tacrolimus(n = 2), everolimus (n = 1) and cyclosporine (n = 1)] [69], and mycophenolate mofetil (n = 2) [69, 82]. All patients who experienced ACRL after COVID-19 vaccination recovered (n = 8, 100%) [29, 34, 69, 82].
Jaundice
Jaundice was the seventh most-common disease (eight cases) reported following COVID-19 vaccination from our review (six new onset cases [71,72,73,74,75, 81] and two relapsed cases [90, 125]) (see Table 1). The median interquartile range (IQR) age of this group was 55 [39 to 60], with a similar gender rate in patients who presented with jaundice found after COVID-19 vaccination in all of the studies [female (n = 4) [73, 75, 81, 90] and male (n = 4) [71, 72, 74, 125]], and most patients belonged to the White (Caucasian) ethnicity (n = 4, 50%) [73, 81, 90, 125] and Arab (n = 2, 28.6%) [71, 75] ethnicity. The median (IQR) time between the COVID-19 vaccination and time of presentation was 4 (2.2–9.2) days. Six and two of these eight jaundice cases were reported following Pfizer-BioNTech COVID-19 vaccination [72, 73, 75, 81, 90, 125] and Oxford Uni-AstraZeneca COVID-19 vaccination [71, 74]; respectively. Few of the jaundice cases had no medical history (n = 3, 37.5%) [72, 74, 75]. Patients who experienced jaundice post-COVID-19 vaccination were more likely to have raised bilirubin (n = 7) [72,73,74,75, 81, 90, 125], raised liver enzymes (n = 5) [72, 74, 81, 90, 125], thrombocytopenia (n = 4) [71, 72, 74], high reticulocyte count (n = 4) [71,72,73, 75], low Hb (n = 4) [71,72,73, 75], and high LDH (n = 3) [71, 74, 75]. Most prescribed pharmacotherapy agents in patients who suffered jaundice post-COVID-19 vaccination were the steroids (n = 4) [71,72,73, 81] and rituximab (n = 3) [71, 72, 75]. All patients who experienced jaundice after COVID-19 vaccination recovered (n = 7, 87.5%) [71,72,73,74,75, 81, 125] except one case who had a history of portal hypertension, hepatitis B and C, and hepatic cirrhosis and patient eventually expired [90].
Acute hepatic failure
Acute hepatic failure (AHF) was reported in four cases following COVID-19 vaccination from our review [four new onset cases [35, 45, 78, 128]], with abdominal pain (n = 3) [45, 78, 128], nausea (n = 2) [35, 78], myalgia (n = 2) [45, 78], and fatigue (n = 2) [35, 45] as the common clinical presentations in these cases (see Table 1). The median patient age ranged from 24 to 53 years across studies. Two of the AHF cases were males and one patient was female [ethnicity: White (Caucasian) = 2 [35, 45] and Persian = 2 [78, 128]]. AHF occurred in patients within 1–10 days due to the use of Pfizer-BioNTech COVID-19 vaccination [35, 45] or Oxford Uni-AstraZeneca COVID-19 vaccination [78, 128]. Three of the AHF cases had no medical history (n = 3, 75%) [35, 45, 78]. Patients who experienced AHF post-COVID-19 vaccination were more likely to have raised liver enzymes (n = 4) [35, 45, 78, 128], raised bilirubin (n = 3) [45, 78, 128], and high INR (n = 3) [45, 78, 128]. The most prescribed pharmacotherapy agent in patients who suffered AHF post-COVID-19 vaccination was the steroids (n = 4, 100%) [35, 45, 78, 128], and one AHF patient received a new liver transplant [45]. Among these AHF patients, two patients survived [35, 45] and two patients deceased [78, 128].
Hepatomegaly
Hepatomegaly was reported in three cases following COVID-19 vaccination from our review (three new onset cases [24, 88, 100]) (see Table 1). The median patient age ranged from 22 to 69 years across studies. All cases were females (n = 3, 100%) [ethnicity: White (Caucasian) = 2 [88, 100] and Indian = 1 [24]]. Patients developed hepatomegaly within 1–10 days after receiving Oxford Uni-AstraZeneca (n = 1) [100], Pfizer-BioNTech (n = 1) [88], and Covishield (n = 1) [24] COVID-19 vaccination. Two patients who developed hepatomegaly post COVID-19 vaccination had no medical history [88, 100], however, one patient had a history of infective jaundice [24]. Patients who experienced hepatomegaly post-COVID-19 vaccination were more likely to have thrombocytopenia (n = 2) [24, 100], high C-reactive protein (n = 2) [88, 100], high erythrocyte sedimentation rate (n = 2) [24, 88], and high lactate dehydrogenase (n = 2) [24, 100]. The most prescribed pharmacotherapy agent in patients who suffered hepatomegaly post-COVID-19 vaccination was the steroids (n = 3, 100%) [24, 88, 100]. All patients who experienced hepatomegaly after COVID-19 vaccination recovered (n = 3, 100%) [24, 88, 100].
Hepatic porphyria
Hepatic porphyria was reported in a 34 year-old white female following the Oxford Uni-AstraZeneca vaccine, with development of abdominal pain, red urine, and hyponatremia, needing intensive care admission [one new onset case [92]] (see Table 1). Patient experienced syndrome of inappropriate antidiuretic hormone then acute hepatic porphyria was diagnosed, and the patient recovered completely after treatment with hemin [92].
Discussion
A considerable range of liver diseases were observed following COVID-19 vaccination. As the dominant pathology reported in our review, AIH is defined as a chronic, inflammatory disease of the liver that is characterized by circulating autoantibodies and elevated serum globulin levels [129]. AIH occurs globally in all ethnicities and affects children and adults of all ages, with a female predominance [130]. A loss of tolerance against the patient’s own liver antigens is regarded as the main underlying pathogenetic mechanism, which is probably triggered by environmental agents such as pathogens and xenobiotics, in genetically susceptible individuals [130]. Although the mechanisms associated with COVID-19 vaccination and AIH are still unknown, molecular mimicry has emerged as the most likely process associated with this phenomenon [131]. Indeed, antibodies against the spike protein S1 of SARS-CoV-2 had a high affinity against some human tissue proteins [132]. As Pfizer-BioNTech, Oxford Uni-AstraZeneca, and Moderna vaccines code the same viral protein [133], they can trigger autoimmune diseases in predisposed patients. Diagnosis of AIH is based upon characteristic serologic and histologic findings and exclusion of other forms of chronic liver disease [134]. AIH can often be strongly suspected based upon clinical and laboratory features, and thus a liver biopsy is not always necessary in patients with typical findings on noninvasive testing [135]. Findings in liver biopsy correlate with reports of AIH following SARS-CoV-2 vaccination. Necroinflammatory hepatitis was observed in all cases of AIH following vaccination with Pfizer-BioNTech [6, 41, 43, 68, 84, 87, 99, 105, 106, 112], Moderna [7, 8, 80, 85, 97, 99, 102, 103, 107, 108], Oxford Uni-AstraZeneca [37, 86, 99, 101], Covishield [104] and Sinovac-CoronaVac [110] vaccine. AIH is a relatively rare; and AIH patients should receive anti-SARS-CoV-2 vaccination when the disease activity is controlled by immunosuppressive therapy [136]. Patients with new acute onset of AIH following anti-SARS-Cov-2 vaccine should be managed as suggested by current guidelines of American Association for the Study of Liver Diseases [137], British Society of Gastroenterology [138] and European Association for the Study of the Liver [139] that recommend the initial use of therapy with either glucocorticoid monotherapy or a combination of a glucocorticoid and azathioprine. The aim of treatment is induction of stable remission. Biochemical remission is defined as lowering of transaminase and immunoglobulin G levels to normal [130] and without treatment, the survival rate in patients with symptomatic AIH at five years is approximately 50 percent [140]. However, with treatment, the 10 year survival rate is approximately 90 percent [141]. Subsequent management will depend on how the patient responds to the initial treatment (remission, incomplete response, failed treatment, drug intolerance) and whether the patient relapses if treatment is withdrawn [137,138,139].
PVT is defined as the sudden onset of portal venous occlusion due to thrombus [142]. PVT can develop in the main body of the portal vein or its intrahepatic branches and may even extend to the splenic or superior mesenteric veins and occlusion may be complete or partial [142]. The pathogenesis of PVT associated with the use of COVID-19 vaccines against SARS-CoV-2 is suggested as the result of the viral proteins and free deoxyribonucleic acid in the vaccine binding to platelet factor 4 to generate a neoantigen that subsequently leads to the development of antibodies against platelet factor 4 which activate platelets and promote clotting [143]. It should be noted the risk of PVT after vaccination against SARS-CoV-2 do not appear to be higher than the background risks in the general population, a finding consistent with the rare and sporadic nature of this syndrome [54]. Anticoagulation therapy for patients with acute PVT due to COVID-19 vaccination is recommended [144]. Therapeutic anticoagulation is one of the primary treatments for PVT and is used unless there is a contraindication such as expanding intracerebral hemorrhage [144]. The choice of anticoagulant depends on the patient's clinical status and anticipated need to stop anticoagulation (based on risk of bleeding or need for an invasive procedure) [144]. Rapid anticoagulation can be achieved by starting PVT patient on low molecular weight heparin, with a switch to non-heparin anticoagulant agents, such as argatroban, danaparoid, fondaparinux, or direct oral anticoagulants (such as apixaban, edoxaban, or rivaroxaban) once the patient's condition has stabilized and no invasive procedures are planned [144]. Administration of intravenous immune globulin (IVIG) should not be delayed for PVT post-COVID-19 vaccination especially for individuals with thrombocytopenia [143]. Evidence supporting the use of IVIG comes from its use in other forms of autoimmune heparin induced thrombocytopenia which is the closest comparison to PVT, and IVIG would be expected to have direct antibody-mediated toxic effects [54]. Plasma exchange with plasma rather than albumin could also be effective in temporarily reducing levels of pathologic antibodies and providing some correction of the coagulopathy in terms of the hypofibrinogenemia [144]. Avoidance of platelet transfusions is critical, because such treatment would provide a substrate for further antibody-mediated platelet activation and coagulopathy [54].
RLEs post-COVID-19 vaccination led to nearly 74.5% of cases of liver injuries and about 3.8% cases of AHF. From all the spontaneous reports that we included in our review from patients who received Pfizer-BioNTech, Oxford Uni-AstraZeneca, Moderna, Johnson & Johnson, Sinovac-CoronaVac, Covishield, and Sinopharm vaccines worldwide between 1 December 2020 and 31 July 2022, there are reports of one hundred and six patients having abnormal liver function analysis [6,7,8,9,10,11,12,13, 23, 25,26,27,28,29, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46, 51, 57, 58, 60, 65, 67,68,69,70, 72, 74, 77,78,79,80,81,82,83,84,85,86,87, 89,90,91, 93, 94, 96, 97, 99, 101,102,103,104,105,106,107,108, 110, 112, 114] and out of these who had the RLEs there are seventy nine patients having COVID-19 vaccine-induced liver injuries [6,7,8, 12, 13, 29, 34, 35, 37, 41,42,43,44,45, 51, 57, 58, 60, 65, 67,68,69, 72, 74, 78, 80,81,82, 84,85,86,87, 90, 91, 96, 97, 99, 101,102,103,104,105,106,107,108, 110, 112] and ultimately four cases ending up with AHF [35, 45, 78, 99]. This systematic review shown the pooled incidence of cases with acute liver injuries diagnosed after COVID-19 vaccination was much higher in women [12, 13, 99, 113], which is consistent with a previously reported finding that shown female gender is more susceptible for drug-induced liver injury [145]. This may be related to the fact that these drugs often produce drug-induced liver injury with autoimmune features, and women are more susceptible to drug-induced AIH [146]. Liver injury, which is chronic in nature, increases in severity over time [147]. Cirrhosis, fatty liver, fibrosis and cancer are examples of chronic liver injuries. However, ALIs occur rapidly and may include COVID-19 vaccine-induced liver failure [147]. Serum levels of liver enzymes and bilirubin are commonly used for the noninvasive diagnosis of liver injury. But these diagnostic parameters are not specific in nature and cannot be used to identify a specific type of liver injury [148]. For instance, liver enzymes may increase in people due to no liver injury (e.g., alcohol, obesity or muscle damage) [149]. Furthermore, serum aminotransferase levels may rise too late for therapeutic intervention (e.g., acute toxicity of paracetamol) [150]. Therefore, serum RLEs and bilirubin may not delineate between different types of liver injury and do not always correlate well with the severity of the liver disease and prediction of clinical outcome; they are general rather than specific indicators. While it is important to recognize and treat RLEs after COVID-19 vaccination, it is equally important not to always label these infrequent cases with RLEs as serious, particularly when there are no objective findings. Most of the identified cases with RLEs post-COVID vaccination recovered and should not discourage vaccination against SARS-CoV-2.
Patients with chronic liver diseases (CLDs), particularly cirrhosis, hepatocellular malignancies, candidates for liver transplantation, and immunosuppressed individuals after liver transplantation appear to be at increased risk of COVID-19 infections, which in turn translates into increased mortality [151]. Therefore, vaccination against various diseases including COVID-19, administered as early as possible in patients with CLDs, is an important protective measure [152]. However, due to impaired immune responses in these patients, the immediate and long-term protective response through immunization may be incomplete [152]. Patients with advanced CLD have deficiencies in innate and humoral immunity [153, 154] and liver transplant recipients require immunosuppressant medications and have blunted antibody responses following SARS-CoV-2 vaccinations [155]. CLDs patients and liver transplant recipients were shown to develop substantially lower immunological response and undetectable or suboptimal poor antibody responses [155, 156] even after three doses of COVID-19 vaccine [157,158,159]. Currently, effective measures to improve immunogenicity to the COVID-19 vaccine in this population remain unknown and are urgently needed [155]. Although there may be big concerns that COVID-19 vaccines could lead to immunologically mediated rejection of the liver [29, 34, 69, 82], luckily, acceptance rate for COVID-19 vaccination among liver transplant recipients is extremely high [160, 161]. It is worth mentioning that several controlled trials and case series studies showed no increased risk of rejection with standard vaccination against SARS-CoV-2 compared with non-vaccinated controls [155, 162,163,164,165,166]. It is important to note that all cases of ACRL post-COVID-19 vaccination included in this review were easily treated without any serious complications and these findings should not be used to discourage vaccination for COVID-19 in patients with CLDs or liver transplant recipients [29, 34, 69, 82]. Vaccination against SARS-CoV-2 for patients with CLDs and hepatobiliary cancer, as well as for liver transplant recipients is recommended and should be prioritised in household members of patients with those liver pathologies, and in healthcare professionals caring for these patients [152].
SVT including portal, mesenteric, splenic vein thrombosis and the Budd-Chiari syndrome, is a manifestation of unusual site venous thromboembolism [167]. SVT presents with a lower incidence than deep vein thrombosis of the lower limbs and pulmonary embolism, with PVT and Budd-Chiari syndrome being respectively the most and the least common presentations of SVT [167]. SVT represents an extremely rare entity but which can be quite severe and worrisome for healthcare providers, and perhaps, not that “infrequent” [168]. Because almost all SVT and PVT cases reported post-COVID-19 vaccination occurred as a result of Oxford Uni-AstraZeneca vaccine use [3,4,5, 31, 47,48,49, 51, 52, 54,55,56, 58,59,60,61,62, 64, 65, 67, 76, 91, 95, 96, 98, 109, 111], while six PVT cases [30, 53, 57, 63, 66] and one SVT case [50] were reported after Johnson & Johnson COVID-19 vaccination, clinicians should be more suspicious to the scarce existence of PVT or SVT in patients with symptoms like severe abdominal pain, nausea or vomiting, fatigue, melena, and persistent high fevers within the setting of previous exposure to the Oxford Uni-AstraZeneca COVID-19 vaccine.
From the one hundred seventy-three cases that were evaluated in our review, Oxford Uni-AstraZeneca (79 cases) [3,4,5, 23, 31,32,33, 36, 37, 46,47,48,49, 51, 52, 54,55,56, 58,59,60,61,62, 64, 65, 67, 71, 74, 76,77,78, 86, 91, 92, 95, 96, 98,99,100,101, 109, 111, 114], Pfizer-BioNTech (57 cases) [6, 9, 10, 12, 13, 25, 27,28,29, 34, 35, 38,39,40,41, 43, 45, 68, 69, 72, 73, 75, 79, 81, 83, 84, 87, 88, 90, 93, 99, 105, 106, 112, 113], and Moderna (24 cases) [7, 8, 13, 26, 69, 70, 80, 82, 85, 89, 94, 97, 99, 102, 103, 107, 108] appear to be the most frequent COVID-19 vaccines associated with post-vaccination liver disease development (see Fig. 2). The higher number of cases can be attributed to the immune response generated to those COVID-19 vaccines [131, 132, 143] or probably due to the fact that the vast majority of cases were reported from a select number of countries across North America, Europe, and Asia, where Oxford Uni-AstraZeneca, Pfizer-BioNTech and Moderna vaccines have been more accessible and commonly available in established vaccination programs [169, 170].
Limitations
First, while most of the evidence discussed were based on few case series and many case reports, many of these are small and performed in single centers and not necessarily generalizable to the current COVID-19 vaccination settings. Second, all studies included in this review were retrospective in design which could have introduced potential reporting bias due to reliance on clinical case records. Third, the study population included adult patients and hence its results cannot be generalized to pediatric patients. Last, study was not registered in Prospero, an international prospective register of systematic reviews, as this might have added extra work and the merit was mostly limited to the avoidance of duplication.
Conclusion
A range of liver diseases post-COIVD-19 vaccination may occur at extremely rare rate and is likely to be immune-mediated. Reported evidence of liver diseases post-COIVD-19 vaccination should not discourage vaccination. The number of reported cases is relatively very small in relation to the hundreds of millions of vaccinations that have occurred and the protective benefits offered by COVID-19 vaccination far outweigh the risks.
Availability of data and materials
Data are available upon request, please contact author for data requests.
Abbreviations
- ACRL:
-
Acute cellular rejection of the liver
- AHF:
-
Acute hepatic failure
- AIH:
-
Autoimmune hepatitis
- ALIs:
-
Acute liver injuries
- COVID-19:
-
Coronavirus disease 2019
- NOS:
-
Newcastle–Ottawa scale
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- PVT:
-
Portal vein thrombosis
- RLEs:
-
Raised liver enzymes
- SVT:
-
Splanchnic vein thrombosis
References
World Health Organization. COVID-19 vaccination dashboard 2022 [01 May 2022]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: Should we be concerned? Toxicol Rep. 2021;8:871–9.
Graf T, Thiele T, Klingebiel R, Greinacher A, Schäbitz W-R, Greeve I. Immediate high-dose intravenous immunoglobulins followed by direct thrombin-inhibitor treatment is crucial for survival in sars-Covid-19-adenoviral vector vaccine-induced immune thrombotic thrombocytopenia VITT with cerebral sinus venous and portal vein thrombosis. J Neurol. 2021;268(12):4483–5.
Öcal O, Stecher S-S, Wildgruber M. Portal vein thrombosis associated with ChAdOx1 nCov-19 vaccination. Lancet Gastroenterol Hepatol. 2021;6(8):676.
Strobel D, Haberkamp S, Zundler S. Portal vein thrombosis due to vaccine-induced immune thrombotic thrombocytopenia (VITT) after Covid vaccination with ChAdOx1 nCoV-19. Ultraschall Med Eur J Ultrasound. 2021;42(05):551–2.
Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75(1):222–4.
Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis after COVID-19 vaccine–more than a coincidence. J Autoimmun. 2021;125: 102741.
Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75(5):1250–2.
Chai Q, Nygaard U, Schmidt RC, Zaremba T, Møller AM, Thorvig CM. Multisystem inflammatory syndrome in a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine. Acta Paediatr. 2022;111(1):125–7.
Watanabe H, Ashida M, Satomi N, Koike Y, Kuwatsuka S, Murota H. Histopathological analysis of skin reactions after coronavirus disease 2019 vaccination: increment in number of infiltrated plasmacytoid dendritic cell. J Dermatol. 2022;49(7):732–5.
Yocum A, Simon EL. Thrombotic thrombocytopenic purpura after Ad26. COV2-S vaccination. Am J Emerg Med. 2021;49:441.e3-441.e4.
Mann R, Sekhon S, Sekhon S. Drug-induced liver injury after COVID-19 vaccine. Cureus. 2021;13(7):e16491.
Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: a multicenter case series. J Hepatol. 2022;76(1):211–4.
Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, Lewis N, Natarajan K, Stenehjem E, Grannis SJ. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION network, 10 states, August 2021–January 2022. Morb Mortal Wkly Rep. 2022;71(7):255.
Centers for Disease Control and Prevention. COVID-19 vaccines for people who are moderately or severely immunocompromised 2022 [07 May 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
Wander P, Epstein M, Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115(6):941–2.
Al Mutair A, Alhumaid S, Alhuqbani WN, Zaidi ARZ, Alkoraisi S, Al-Subaie MF, AlHindi AM, Abogosh AK, Alrasheed AK, Alsharafi AA. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: an observational cohort study. Eur J Med Res. 2020;25(1):1–8.
Alhumaid S, Al Mutair A, Al Alawi Z, Al Salman K, Al Dossary N, Omar A, Alismail M, Al Ghazal AM, Jubarah MB, Al SH. Clinical features and prognostic factors of intensive and non-intensive 1014 COVID-19 patients: an experience cohort from Alahsa, Saudi Arabia. Eur J Med Res. 2021;26(1):1–13.
Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. U Eur Gastroenterol J. 2020;8(5):509–19.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott Ott Hosp Res Inst. 2011;2(1):1–12.
Bazerbachi F, Sawas T, Vargas EJ, Prokop LJ, Chari ST, Gleeson FC, Levy MJ, Martin J, Petersen BT, Pearson RK. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87(1):30-42.e12.
Sauret A, Stievenart J, Smets P, Olagne L, Guelon B, Aumaître O, André M, Trefond L. Case of giant cell arteritis after SARS-CoV-2 vaccination: a particular phenotype? J Rheumatol. 2022;49(1):120.
Patil S, Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol. 2021;20(10):3103.
Mücke VT, Knop V, Mücke MM, Ochsendorf F, Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect Dis. 2021;21(1):1–6.
Gaignard M-E, Lieberherr S, Schoenenberger A, Benz R. Autoimmune hematologic disorders in two patients after mRNA COVID-19 vaccine. HemaSphere. 2021;5(8):e618.
Gadi SR, Brunker PA, Al-Samkari H, Sykes DB, Saff RR, Lo J, Bendapudi P, Leaf DE, Leaf RK. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion. 2021;61(11):3267–71.
Wu M, Karim M, Ashinoff R. COVID-19 vaccine-associated dermatomyositis. JAAD Case Rep. 2022;23:58–60.
Valsecchi M, Lauterio A, Crocchiolo R, De Carlis R, Pugliano M, Centonze L, Ferla F, Zaniboni M, Veronese S, Podda GM. New-onset antibodies to platelet factor 4 following liver transplantation from a donor with vaccine-induced thrombotic thrombocytopenia. Liver Transpl. 2022;28(2):314–6.
Uzun G, Bohnert BN, Althaus K, Nann D, Nadalin S, Heyne N, Fend F, Haap M, Bakchoul T. Organ donation from a brain dead donor with vaccine-induced immune thrombotic thrombocytopenia after Ad26. COV2. S: the risk of organ microthrombi. Transplantation. 2022;106(3):e178–80.
Tiwari AM, Zirpe KG, Gurav SK, Bhirud LB, Suryawanshi RS, Kulkarni SS. Case of suspected SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia (VITT): the dilemma for organ donation. Indian J Crit Care Med. 2022;26(4):515.
O’Connor T, O’Callaghan-Maher M, Ryan P, Gibson G. Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID-19 vaccine. JAAD Case Rep. 2022;20:14–6.
Khajavirad N, Salehi M, Haji Ghadery A, Khalili H, Arab Ahmadi M, Dehghan Manshadi SA, Zare DA. Serious events following COVID-19 vaccination with ChAdOx1 nCoV-19 vaccine (Vaxzevria): a short case series from Iran. Clin Case Rep. 2022;10(2): e05390.
Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transpl. 2022;28(1):134–7.
Hieber M-L, Sprute R, Eichenauer DA, Hallek M, Jachimowicz RD. Hemophagocytic lymphohistiocytosis after SARS-CoV-2 vaccination. Infection. 2022:1–6.
Fritzen M, Funchal GDG, Luiz MO, Durigon GS. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97(1):118–21.
Camacho-Domínguez L, Rodríguez Y, Polo F, Gutierrez JCR, Zapata E, Rojas M, Anaya J-M. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022;5:100140.
Brown M, Garbajs NZ, Garces JPD, Tekin A, Bansal V, Das S, Muigai M, Boisseau R, Khan SA, Gajic O. 218: a case of adult multisystem inflammation syndrome following COVID-19 vaccine. Crit Care Med. 2022;50(1):93.
Alrashdi M, Alanazi S, Almoaqly K, Alshaya A, Alanazi S. Systemic lupus erythematosus with acute pancreatitis and vasculitic rash following COVID-19 vaccine: a case report and literature review. Clin Rheumatol. 2022:1–6.
Sung PS, Oh JS, Choi JI. Acute Budd-Chiari syndrome with thrombotic thrombocytopenia after BNT162b2 mRNA vaccination. Liver Int. 2022;42(6):1447.
Romero-Salazar FL, Lista MV, Gómez-Domínguez E, Ibarrola-Andrés C, Gómez RM, Vázquez IF. SARS-CoV-2 vaccine, a new autoimmune hepatitis trigger? Rev Esp Enferm Dig Organo Of Soc Esp Patol Dig. 2022;114(9):567–8.
Rigamonti C, Coco B, Brunetto M, Labanca S, Giannini E, Magro B, Fagiuoli S, Baroni GS, Sgamato C, Miele L. Clinical features of patients with new onset of autoimmune hepatitis following SARS-CoV-2 vaccination. Dig Liver Dis. 2022;54:S48.
Mahalingham A, Duckworth A, Griffiths WJ. First report of post-transplant autoimmune hepatitis recurrence following SARS-CoV-2 mRNA vaccination. Transpl Immunol. 2022;72:101600.
Ghorbani H, Rouhi T, Vosough Z, Shokri-Shirvani J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: a case study of a 62-year-old patient. Int J Surg Case Rep. 2022;93: 106926.
Efe C, Harputluoğlu M, Soylu NK, Yilmaz S. Letter to the editor: Liver transplantation following severe acute respiratory syndrome-coronavirus-2 vaccination-induced liver failure. Hepatology. 2022;75(6):1669–1671.
Cirillo E, Esposito C, Giardino G, Azan G, Fecarotta S, Pittaluga S, Ruggiero L, Barretta F, Frisso G, Notarangelo LD. Case report: severe rhabdomyolysis and multiorgan failure after ChAdOx1 nCoV-19 vaccination. Front Immunol. 2022;13:1146.
van Dijk MM, Veldman HD, Aarts F, Barten DG, van den Bergh JP, Dielis AW. A case of unusual mild clinical presentation of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with splanchnic vein thrombosis. Ann Hepatol. 2022;27(1): 100590.
Tiede A, Sachs UJ, Czwalinna A, Werwitzke S, Bikker R, Krauss JK, Donnerstag F, Weißenborn K, Höglinger G, Maasoumy B. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood J Am Soc Hematol. 2021;138(4):350–3.
Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt A-H, Skattør TH, Tjønnfjord GE. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–30.
Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26. COV2. S vaccination. N Engl J Med. 2021;384(20):1964–5.
Tølbøll Sørensen AL, Rolland M, Hartmann J, Harboe ZB, Roed C, Jensen TØ, Kolte L, El Fassi D, Hillingsø J, Radziwon-Balicka A. A case of thrombocytopenia and multiple thromboses after vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2. Blood Adv. 2021;5(12):2569–74.
Thaler J, Jilma P, Samadi N, Roitner F, Mikušková E, Kudrnovsky-Moser S, Rettl J, Preiss R, Quehenberger P, Pabinger I. Long-term follow-up after successful treatment of vaccine-induced prothrombotic immune thrombocytopenia. Thromb Res. 2021;207:126–30.
See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, Streiff MB, Rao AK, Wheeler AP, Beavers SF. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26. COV2. S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56.
Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–11.
Ramdeny S, Lang A, Al-Izzi S, Hung A, Anwar I, Kumar P. Management of a patient with a rare congenital limb malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and thrombocytopenia (VITT). Br J Haematol. 2021;195(3):299.
Premkumar M, Bhujade H, Karki T, Chaluvashetty SB, Kaur H, Duseja AK, Singh V. New portal vein thrombosis in cirrhosis-is the thrombophilia exacerbated due to vaccine or COVID-19? J Clin Exp Hepatol. 2021;12(3):1025–8.
Major A, Carll T, Chan CW, Christenson C, Aldarweesh F, Wool GD, Cohen KS. Refractory vaccine-induced immune thrombotic thrombocytopenia (VITT) managed with delayed therapeutic plasma exchange (TPE). J Clin Apheresis. 2022;37(1):117–21.
Lin C-Y, Wang C-H, Hsiao P-J. Unusual fever, headache, and abdominal pain in a healthy woman. Gastroenterology. 2021;161(5):1387–9.
Kulkarni AV, Reddy J, Singh JR, Sreekanth V, Reddy A, Sharma M, Nutalapati C, Rao PN, Reddy DN. Exercise caution with ChAdOx1 COVID-19 vaccination in chronic Budd-Chiari syndrome with a thrombophilic genetic predisposition. J Clin Exp Hepatol. 2021;12(2):716–7.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101.
De Michele M, Iacobucci M, Chistolini A, Nicolini E, Pulcinelli F, Cerbelli B, Merenda E, Schiavo O, Sbardella E, Berto I. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat Commun. 2021;12(1):1–7.
D’agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, Sirabella G, Marano I, Granata V, Grassi R. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285.
Curcio R, Gandolfo V, Alcidi R, Giacomino L, Campanella T, Casarola G, Rossi R, Chiatti L, D’abbondanza M, Commissari R. Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of janssen AD26. COV2. S vaccination. Int J Infect Dis. 2022;116:154–6.
Ciccone A, Zanotti B. The importance of recognizing cerebral venous thrombosis following anti-COVID-19 vaccination. Eur J Intern Med. 2021;89:115–7.
Asmat H, Fayeye F, Alshakaty H, Patel J. A rare case of COVID-19 vaccine-induced thrombotic thrombocytopaenia (VITT) involving the veno-splanchnic and pulmonary arterial circulation, from a UK district general hospital. BMJ Case Rep CP. 2021;14(9): e244223.
Asif S, Kesireddy M, Koepsell SA, Gonzalez-Castellon MA, Gundabolu K, Baljevic M. Cerebral venous sinus thrombosis due to thrombosis with thrombocytopenia syndrome following Ad26. COV2. S: a first real-world case report of a male subject. Neurohosp. 2021;12(2):346–51.
Aladdin Y, Algahtani H, Shirah B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 vaccine. J Stroke Cerebrovasc Dis. 2021;30(9): 105938.
Fimiano F, D’Amato D, Gambella A, Marzano A, Saracco G, Morgando A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following Covid-19 vaccination? Liver Int. 2022;42(5):1204–5.
Sarwar R, Adeyi OA, Lake J, Lim N. Acute cellular rejection in liver transplant recipients following vaccination against COVID‐19: a case series. Liver Transpl. 2022;28(8):1388–1392.
Kishimoto M, Ishikawa T, Odawara M. Subacute thyroiditis with liver dysfunction following coronavirus disease 2019 (COVID-19) vaccination: report of two cases and a literature review. Endocr J. 2022;69(4):947–57.
Al-Ahmad M, Al-Rasheed M, Shalaby NAB. Acquired thrombotic thrombocytopenic purpura with possible association with AstraZeneca-Oxford COVID-19 vaccine. EJHaem. 2021;2(3):534–6.
Yoshida K, Sakaki A, Matsuyama Y, Mushino T, Matsumoto M, Sonoki T, Tamura S. Acquired thrombotic thrombocytopenic purpura following BNT162b2 mRNA coronavirus disease vaccination in a Japanese patient. Intern Med. 2022;61(3):407–12.
Pérez-Lamas L, Moreno-Jiménez G, Tenorio-Núñez MC, Velázquez-Kennedy K, Jiménez-Chillón C, Astibia-Mahillo B, Núñez-Torrón C, García-Gutiérrez V, Jiménez-Martín A, Vallés-Carboneras A, López-Jiménez JF. Hemolytic crisis due to Covid-19 vaccination in a woman with cold agglutinin disease. Am J Hematol. 2021;96(8):E288–E291.
Peralta-Amaro AL, Tejada-Ruiz MI, Rivera-Alvarado KL, Cobos-Quevedo ODJ, Romero-Hernández P, Macías-Arroyo W, Avendaño-Ponce A, Hurtado-Díaz J, Vera-Lastra O, Lucas-Hernández A. Atypical Kawasaki disease after COVID-19 vaccination: a new form of adverse event following immunization. Vaccines. 2022;10(1):126.
Al Aoun S, Motabi I. Cold agglutinin disease after COVID-19 vaccine. Br J Haematol. 2021;195(5):650.
Umbrello M, Brena N, Vercelli R, Foa RA, Femia M, Rossi U, Podda GM, Cortellaro F, Muttini S. Successful treatment of acute spleno-porto-mesenteric vein thrombosis after ChAdOx1 nCoV-19 vaccine. A case report. J Crit Care. 2021;65:72–5.
Tan A, Stepien KM, Narayana STK. Carnitine palmitoyltransferase II deficiency and post-COVID vaccination rhabdomyolysis. QJM Int J Med. 2021;114(8):596–7.
Sohrabi M, Sobhrakhshankhah E, Ziaei H, AtaeeKachuee M. Acute liver failure after vaccination against of COVID-19; a case report and review literature. Respir Med Case Rep. 2022;35: 101568.
Sharabi A, Shiber S, Molad Y. Adult-onset still’s disease following mRNA COVID-19 vaccination. Clin Immunol (Orlando, Fla). 2021;233: 108878.
Zhou T, Fronhoffs F, Dold L, Strassburg CP, Weismüller TJ. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis–should we be more vigilant? J Hepatol. 2022;76(1):218–20.
Wong CY, Rios EJ. Cutaneous hypersensitivity reaction with acute hepatitis following COVID-19 vaccine. JAAD Case Rep. 2021;16:44–6.
Vyhmeister R, Enestvedt CK, VanSandt M, Schlansky B. Steroid-resistant acute cellular rejection of the liver after severe acute respiratory syndrome coronavirus 2 mRNA vaccination. Liver Transpl. 2021;27(9):1339–42.
Waqar SHB, Khan AA, Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int J Hematol. 2021;114(5):626–9.
Suzuki Y, Kakisaka K, Takikawa Y. Autoimmune hepatitis after COVID-19 vaccination: need for population based-epidemiological study. Hepatology. 2021;75(3):759–60.
Tun GSZ, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2021;76(3):747–9.
Torrente S, Castiella A, Garmendia M, Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol Hepatol. 2021;45:115–6.
Palla P, Vergadis C, Sakellariou S, Androutsakos T. Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology. 2022;75(2):489–90.
Manzo C, Natale M, Castagna A. Polymyalgia rheumatica as uncommon adverse event following immunization with COVID-19 vaccine: a case report and review of literature. Aging Med. 2021;4(3):234–8.
Malayala SV, Mohan G, Vasireddy D, Atluri P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus. 2021;13(3):e14099.
Lensen R, Netea MG, Rosendaal FR. Hepatitis C virus reactivation following COVID-19 vaccination–a case report. Int Med Case Rep J. 2021;14:573.
Kadam N, Ramavathu KV, Kamath N, Min KK. Imaging findings in a patient with suspected vaccine induced immune thrombotic thrombocytopenia. BJR Case Rep. 2022;8(1):20210138.
Jud P, Hackl G, Reisinger AC, Horvath A, Eller P, Stadlbauer V. Red urine and a red herring–diagnosing rare diseases in the light of the COVID-19 pandemic. Z Gastroenterol. 2021;60(9):1326–31.
Jawed M, Khalid A, Rubin M, Shafiq R, Cemalovic N, editors. Acute immune thrombocytopenia (ITP) following COVID-19 vaccination in a patient with previously stable ITP. Open forum infectious diseases. Oxford University Press US; 2021.
Hines A, Shen JG, Olazagasti C, Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep CP. 2021;14(7): e242678.
UK Donor VITT Transplant Study Group, Greenhall GHB, Ushiro-Lumb I, Pavord S, Currie I, Perera MTPR, Hartog H, Hill QA, Mohamed I, Khurram MA, Motallebzadeh R, Jones G, Marshall A, Pollok JM, Torpey N, Pettigrew GJ, Mehra S, Sharma H, Calder F, Kessaris N, Nath J, Roy D, Oniscu GC, Clancy M, Santhanakrishnan K, Mascaro J, Lim S, Berman M, Madden S, Mumford L, Mirza D, Watson C, McGowan O, Thorburn D, Ravanan R, Hunt BJ, Callaghan CJ, Roberts DJ, Forsythe J. Organ transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia. Am J Transplant. 2021 Dec;21(12):4095–4097.
Graça LL, Amaral MJ, Serôdio M, Costa B. Extensive thrombosis after COVID-19 vaccine: Cause or coincidence? BMJ Case Rep CP. 2021;14(8): e244878.
Goulas A, Kafiri G, Kranidioti H, Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following Covid-19 mRNA vaccination. More than a coincidence? Liver Int. 2022;42(1):254–5.
Fanni D, Saba L, Demontis R, Gerosa C, Chighine A, Nioi M, Suri JS, Ravarino A, Cau F, Barcellona D, Botta MC, Porcu M, Scano A, Coghe F, Orrù G, Van Eyken P, Gibo Y, La Nasa G, D'aloja E, Marongiu F, Faa G. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: a report of an autoptic case and review of the literature. Eur Rev Med Pharmacol Sci. 2021;25(15):5063–5069.
Erard D, Villeret F, Lavrut P-M, Dumortier J. Autoimmune hepatitis developing after COVID 19 vaccine: presumed guilty? Clin Res Hepatol Gastroenterol. 2021;46(3):101841.
Cory P, Lawrence H, Abdulrahim H, Mahmood-Rao H, Hussein A, Gane J. Lessons of the month 3: haemophagocytic lymphohistiocytosis following COVID-19 vaccination (ChAdOx1 nCoV-19). Clin Med. 2021;21(6):e677–9.
Clayton-Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75(5):1249–50.
Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, Cerny A, Dayer E, Vergani D, Beretta-Piccoli BT. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J Autoimmun. 2021;123: 102706.
Vuille-Lessard É, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123: 102710.
Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123: 102688.
Lodato F, Larocca A, D’Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021;75(5):1254–6.
Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021;75(3):728–9.
McShane C, Kiat C, Rigby J, Crosbie Ó. The mRNA COVID-19 vaccine–A rare trigger of autoimmune hepatitis? J Hepatol. 2021;75(5):1252–4.
Londoño M-C, Gratacós-Ginès J, Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination–still casualty? J Hepatol. 2021;75(5):1248–9.
Centonze L, Lauterio A, De Carlis R, Ferla F, De Carlis L. Successful liver transplantation from a deceased donor with vaccine-induced thrombotic thrombocytopenia causing cerebral venous sinus and hepatic veins thrombosis after ChAdOx1 nCov-19 vaccination. Transplantation. 2021;105(10): e144.
Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2021;75(3):757–9.
Bersinger S, Lagarde K, Marlu R, Pernod G, Payen J-F. using nonheparin anticoagulant to treat a near-fatal case with multiple venous thrombotic lesions during ChAdOx1 nCoV-19 vaccination-related vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021;49(9):e870–3.
Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J Autoimmun. 2021;125: 102745.
Alqarni MM, Faloudah AZ, Alsulaihebi AS, Halawani HK, Khan AS. A Case of Hepatotoxicity After Receiving a COVID-19 Vaccine. Cureus. 2021;13(12):e20455.
Alkindi S, Elsadek RA, Pathare AV. Safety Warning for ChAdOx1 nCov-19 Vaccine in Patients with Sickle Cell Disease. Mediterr J Hematol Infect Dis. 2021;13(1):e2021059.
Shahrani S, Sooi CY, Hilmi IN, Mahadeva S. Autoimmune hepatitis (AIH) following coronavirus (COVID-19) vaccine-No longer exclusive to mRNA vaccine? Liver Int. 2022;42(10):2344–2345.
Repp ML, Cohen S, Kibbey C. Acute portal vein thrombosis secondary to COVID-19 vaccination. Cureus. 2022;14(7):e26825.
Nyein CM, Liew ZHS, Leow W-Q, Yeong PSJ, Ho GH. Severe de novo liver injury after Moderna vaccination–not always autoimmune hepatitis. J Hepatol. 2022;77(2):556–8.
Mekritthikrai K, Jaru-Ampornpan P, Komolmit P, Thanapirom K. Autoimmune hepatitis triggered by COVID-19 vaccine: the first case from inactivated vaccine. ACG Case Rep J. 2022;9(7):e00811.
Lee SK, Kwon JH, Yoon N, Lee SH, Sung PS. Immune-mediated liver injury represented as overlap syndrome after SARS-CoV-2 vaccination. J Hepatol. 2022;77(4):1209–11.
Lasagna A, Lenti MV, Cassaniti I, Sacchi P. Development of hepatitis triggered by SARS-CoV-2 vaccination in patient with cancer during immunotherapy: a case report. Immunotherapy. 2022;14(12):915–25.
Kyungu FM, Katumba AM, Kamwira HL, Mayikuli AV, Mala A, Banza MI, Manirou HM, Tosali FB, Mulenga PC. Acute acalculous cholecystitis following COVID-19 vaccination: a case report. Pan Afr Med J. 2022;41:291.
Kawasaki Y, Matsubara K, Hori M, Isome K. Liver injury and cytopenia after BNT162b2 COVID-19 vaccination in an adolescent. Pediatr Int. 2022;64(1): e15178.
Kang SH, Kim MY, Cho MY, Baik SK. Autoimmune hepatitis following vaccination for SARS-Cov-2 in Korea: Coincidence or autoimmunity?. J Korean Med Sci. 2022;37(15):e116.
Hasegawa N, Matsuoka R, Ishikawa N, Endo M, Terasaki M, Seo E, Tsuchiya K. Autoimmune hepatitis with history of HCV treatment triggered by COVID-19 vaccination: case report and literature review. Clin J Gastroenterol. 2022;15(4):791–5.
Guri Y, Vosbeck J, Dickenmann M, Jetter A, Bernsmeier C. Exacerbation of familial intrahepatic cholestasis in conjunction with COVID-19 vaccination. J Hepatol. 2022;77(3):872–874.
Efe C, Kulkarni AV, Terziroli Beretta‐Piccoli B, Magro B, Friedrich Staettermayer A, Cengiz M, Clayton‐Chubb D, Lammert C, Bernsmeier C, Gül Ö. Liver injury after SARS‐CoV‐2 vaccination: Features of immune‐mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022. https://doi.org/10.1002/hep.32572.
Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Alizei ES, Zoldan K, Krimmel L, Bronsert P, Schwabenland M. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77(3):653–9.
Barary M, Sharifi-Razavi A, Rakhshani N, Sio TT, Ebrahimpour S, Baziboroun M. Fulminant hepatitis following COVID-19 vaccination: a case report. Clin Case Rep. 2022;10(7): e6066.
Harputluoglu M, Caliskan AR, Akbulut S. Autoimmune hepatitis and liver transplantation: Indications, and recurrent and de novo autoimmune hepatitis. World J Transpl. 2022;12(3):59.
Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, Lohse AW, Montano-Loza AJ. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4(1):1–21.
Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68(5):310–3.
Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol (Orlando, Fla). 2020;217: 108480.
Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25.
van Beers JJ, Koek GH, Damoiseaux JG. The role of autoantibodies in the diagnosis of autoimmune liver disease: lessons learned from clinical practice. J Appl Lab Med. 2022;7(1):259–67.
Björnsson E, Talwalkar J, Treeprasertsuk S, Neuhauser M, Lindor K. Patients with typical laboratory features of autoimmune hepatitis rarely need a liver biopsy for diagnosis. Clin Gastroenterol Hepatol. 2011;9(1):57–63.
Lleo A, Cazzagon N, Rigamonti C, Cabibbo G, Lai Q, Muratori L, Carbone M. Clinical update on risks and efficacy of anti-SARS-CoV-2 vaccines in patients with autoimmune hepatitis and summary of reports on post-vaccination liver injury. Dig Liver Dis. 2022;54(6):722–6.
Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72(2):671–722.
Gleeson D, Heneghan MA. British Society of gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60(12):1611–29.
Lohse AW, Chazouilleres O, Dalekos G, Drenth J, Heneghan M, Hofer H, Lammert F, Lenzi M. EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004.
Heneghan MA. Autoimmune hepatitis: Treatment 2022 [05 May 2022]. Available from: https://www.uptodate.com/contents/autoimmune-hepatitis-treatment.
Gossard AA, Lindor KD. Autoimmune hepatitis: a review. J Gastroenterol. 2012;47(5):498–503.
Garfield HSKOA-AK. Portal vein thrombosis: StatPearls; 2022 [13 September 2022]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534157.
Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood J Am Soc Hematol. 2021;138(22):2256–68.
Bogovic N, Doenecke A, Hart C, Lürken L, Heimerl S, Eissnert C, Schlitt HJ, Bitterer F. Covid19 vaccination-associated portal vein thrombosis–an interdisciplinary clinical challenge. Clin Res Hepatol Gastroenterol. 2022;46(8):101932.
Amacher DE. Female gender as a susceptibility factor for drug-induced liver injury. Hum Exp Toxicol. 2014;33(9):928–39.
de Lemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL, editors. Drug-induced liver injury with autoimmune features Seminars in liver disease. New York: Thieme Medical Publishers; 2014.
Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13(3):131–49.
Verslype C. Evaluation of abnormal liver-enzyme results in asymptomatic patients. Acta Clin Belg. 2004;59(5):285–9.
Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Sarcopenic obesity in liver cirrhosis: possible mechanism and clinical impact. Int J Mol Sci. 2021;22(4):1917.
Klein-Schwartz W, Doyon S. Intravenous acetylcysteine for the treatment of acetaminophen overdose. Expert Opin Pharmacother. 2011;12(1):119–30.
Fraser J, Mousley J, Testro A, Smibert OC, Koshy AN, editors. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: a systematic review and quantitative analysis Transplantation proceedings. Amsterdam: Elsevier; 2020.
Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74(4):944–51.
Liaskou E, Hirschfield GM. Cirrhosis-associated immune dysfunction: novel insights in impaired adaptive immunity. EBioMedicine. 2019;50:3.
Lebossé F, Gudd C, Tunc E, Singanayagam A, Nathwani R, Triantafyllou E, Pop O, Kumar N, Mukherjee S, Hou TZ. CD8+ T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis-associated immune dysfunction. EBioMedicine. 2019;49:258–68.
Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–8.
Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434–9.
Meunier L, Sanavio M, Dumortier J, Meszaros M, Faure S, Beodya JU, Echenne M, Boillot O, Debourdeau A, Pageaux GP. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int. 2022;77:S771.
Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, Izopet J, Kamar N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transpl. 2021;22(1):322.
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–2.
Costantino A, Invernizzi F, Centorrino E, Vecchi M, Lampertico P, Donato MF. COVID-19 vaccine acceptance among liver transplant recipients. Vaccines. 2021;9(11):1314.
Giannini EG, Marenco S. High acceptance rate of COVID-19 vaccination in liver transplant recipients. J Hepatol. 2021;75(2):483–4.
Boyarsky BJ, Ou MT, Greenberg RS, Teles AT, Werbel WA, Avery RK, Massie AB, Segev DL, Garonzik-Wang JM. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56–7.
Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, Garonzik-Wang JM. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–6.
Sookaromdee P, Wiwanitkit V. Seroconversion after coronavirus disease 2019 vaccination. Liver Transpl. 2022;28(3):514.
Calleri A, Saracco M, Pittaluga F, Cavallo R, Romagnoli R, Martini S. Seroconversion after coronavirus disease 2019 vaccination in patients awaiting liver transplantation: Fact or fancy? Liver Transpl. 2022;28(2):180–7.
Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, Tobian AA, Massie AB, Hamilton JP, Garonzik-Wang JM. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27(12):1852–6.
Valeriani E, Riva N, Di Nisio M, Ageno W. Splanchnic vein thrombosis: current perspectives. Vasc Health Risk Manag. 2019;15:449.
Porres-Aguilar M, Lazo-Langner A, Panduro A, Uribe M. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: an emerging cause of splanchnic vein thrombosis. Ann Hepatol. 2021;23: 100356.
Basak P, Abir T, Al Mamun A, Zainol NR, Khanam M, Haque MR, Milton AH, Agho KE. A global study on the correlates of gross domestic product (GDP) and COVID-19 vaccine distribution. Vaccines. 2022;10(2):266.
Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA. 2020;323(24):2462–3.
Acknowledgements
We would like to thank authors and their colleagues who contributed to the availability of evidence needed to compile this article. We would also like to thank the reviewers for very helpful and valuable comments and suggestions for improving the paper.
Funding
None.
Author information
Authors and Affiliations
Contributions
SA, AAM, AR, FMA, SJY, and AA-O contributed equally to the systematic review. SA, AAM, FMA, and AAR were the core team leading the systematic review. SA, AAM, AR, FMA, SJY, and HAA identified and selected the studies. MHAK, YYA, AAA, HNA, HAAS, and RAA did the quality assessment of the studies. SA, FN, AK, JM, FA, ASAM, HRA-T, AHA, MEA, MEA, and MAA collected the data. SA, AAM, OPC, EHA, DAA, HAA, AAA, AHA, FHA, KH, JAA-T, AAR, and AA-O drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alhumaid, S., Al Mutair, A., Rabaan, A.A. et al. New-onset and relapsed liver diseases following COVID-19 vaccination: a systematic review. BMC Gastroenterol 22, 433 (2022). https://doi.org/10.1186/s12876-022-02507-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02507-3