Abstract
Background
Gastric cancer ranks high in terms of morbidity and mortality worldwide. Multimodal therapy is therefore essential for locally advanced gastric cancer. Recent studies have demonstrated that both perioperative chemotherapy and neoadjuvant chemoradiotherapy can improve the prognosis of patients. However, the completion rate of chemotherapy after surgery remains low, which may affect survival. Thus, identifying the best way to combine radiotherapy, chemotherapy and surgery is important. The aim of this study was to explore the toxicity and efficacy of the total neoadjuvant therapy modality for locally advanced gastric cancer.
Methods
This study will be a prospective, multicenter, single-arm, phase II clinical trial. Patients diagnosed with locally advanced (stage cT3-4 and cN positive, AJCC 8th) gastric cancer and gastroesophageal junction adenocarcinoma will be enrolled. Patients will initially receive radiotherapy (95% planned target volume: 45 Gy/25 f) and concurrent chemotherapy (S-1: 40–60 mg twice a day) followed by six cycles of consolidated chemotherapy (SOX, consisting of S-1 and oxaliplatin) and surgery. The primary objective will assess pathological complete response; the secondary objectives will include toxicities assessing surgical complications, the tumor downstaging rate and the R0 resection rate.
Discussion
Investigation of total neoadjuvant therapy in gastric cancer is limited. The goal of this trial is to explore the efficacy and toxicity of total neoadjuvant therapy for locally advanced gastric cancer and gastroesophageal junction adenocarcinoma.
Trial registration: Clinicaltrials.gov (NCT04062058, August 20, 2019).
Similar content being viewed by others
Background
In recent years, the morbidity of gastric cancer (GC) has declined worldwide, while the percentage of esophagogastric junction (EGJ) adenocarcinoma has increased rapidly [1]. In China, GC still ranks second in terms of morbidity and mortality [2], with most patients diagnosed at a locally advanced stage. Although radical surgery is the main treatment approach for these patients, the risk of recurrence remains high after surgery [3], and the prognosis is extremely poor. Hence, the combination of radiotherapy, chemotherapy and surgery is essential. Attempts to combine these three important treatment methods remain a research hotspot; in the early years, radiotherapy and chemotherapy were mostly administered after surgery. The INT0116 study enrolled 556 patients diagnosed with GC, all of whom underwent radical surgery. The results showed that compared with surgery alone, adjuvant chemoradiotherapy significantly improved overall survival [4]. Gradually, as D2 resection has become more widespread, the value of adjuvant chemoradiotherapy has been challenged, especially after the results of the ARTIST 2 trial were published [5]. Recently, some phase II or III studies have verified the value of neoadjuvant radiotherapy for locally advanced GC and EGJ cancer in terms of local control and the R0 reaction rate [6, 7]. With advancements in neoadjuvant therapy, the MAGIC, FFCD and FLOT4 trials have confirmed that perioperative chemotherapy will improve patient prognosis [8,9,10]. Currently, both perioperative chemotherapy and preoperative concurrent chemoradiotherapy are recommended for patients with locally advanced GC according to the National Comprehensive Cancer Network (NCCN) guidelines [11]. However, the completion rate of therapy after surgery is lower than that before surgery, which may influence survival outcomes [12]. Therefore, total neoadjuvant therapy is a promising treatment modality. The POET study is the first phase III clinical trial comparing induced chemotherapy followed by neoadjuvant chemoradiotherapy (NCRT) and surgery with neoadjuvant chemotherapy (NCT). The study ultimately enrolled 119 patients due to slow enrollment. The pathological complete response (pCR) rates for NCRT and NCT were 14.3% and 1.9%, respectively (P = 0.03), and the 5-year overall survival rates were 39.5% and 24.4%, respectively (P = 0.055) [13]. NCRT showed a tendency to improve survival; however, the dose of radiotherapy was 30 Gy, and the radiotherapy machine and chemotherapy regimen were outdated. Based on the current advancements in intensity-modulated radiotherapy equipment and chemotherapy regimens, our study will assess a newly designed total neoadjuvant therapy comprising NCRT followed by consolidated chemotherapy and surgery for locally advanced GC and EGJ adenocarcinoma. The objective of this trial is to confirm the feasibility and safety of this treatment mode. We aim to provide additional clinical evidence to support the administration of new neoadjuvant treatments.
Methods
Study design and objectives
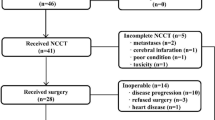
This study will be a single-arm, open-label and multicenter phase II trial. Patients diagnosed with locally advanced (stage cT3-4 and cN positive, AJCC 8th) GC or EGJ adenocarcinoma will be recruited. Before recruitment, each patient will receive gastroscopy, endoscopic ultrasound, and chest-abdomen-pelvis enhanced CT for tumor detection and stages. A multidisciplinary team will screen patients meeting the enrollment conditions. The clinical T stage will be identified by imaging features and laparoscopy information. Metastatic regional nodes will be considered based on their size and biological characteristics. After careful screening, patients will be initially treated with NCRT. The technique of Intensity-Modulated Radiation Therapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT) will be adopted in this trial, with a dose of 45 Gy/25 f to the planned target volume (PTV), and the concurrent chemotherapy will be S-1. Then, the patients will begin six courses of neoadjuvant consolidated chemotherapy (NCCT) using the S-1 and oxaliplatin (SOX) regimen. Finally, patients will undergo surgery after total neoadjuvant therapy. An overview of the study design is shown in Fig. 1.
The primary objective is the assessment of a pathological complete response (pCR), which is defined as no tumor cells in the primary tumor and metastatic regional lymph node. Secondary objectives include assessing toxicities during NCRT and NCCT, surgical complications, the tumor downstaging rate and the R0 resection rate. Recruitment began in November 2019 and is expected to be finished in September 2023. Seven centers in China are participating in this study. The protocol was approved by the institutional ethics committees of these centers and was registered at ClinicalTrials.gov with registration number NCT04062058.
Eligibility criteria
In this multicenter phase II clinical trial, patients aged 18–70 years with Karnofsky performance status (KPS) ≥ 70; histologically proven gastric cancer or Siewert II/III EGJ adenocarcinoma [14]; and clinical stage T3–4 and N positive without distant metastases were enrolled. No other anticancer treatments were allowed before enrollment. Laboratory investigation requirements were as follows: white blood cell (WBC) count ≥ 3.0 × 109/L, platelet count (PLT) ≥ 100 × 109/L, hemoglobin count (Hb) ≥ 10 g/dl, neutrophil count ≥ 1.5 × 109/L; total bilirubin ≤ 1.5 times the upper limit of normal (ULN); and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 1.5 × ULN; serum creatinine (SCr) ≤ 1.0 × ULN; alkaline phosphatase (AKP) ≤ 2.5 × ULN. Patients agreed to sign an informed consent form before enrollment.
The exclusion criteria included patients aged more than 70 years or less than 18 years, prior anticancer treatment, pregnant or lactating females, metastatic disease, history of myocardial infarction or cerebral infarction within the past 6 months, active infections of tuberculosis or hepatitis, and refusal to provide informed consent.
Treatment
Total NCRT will involve NCRT followed by six cycles of consolidated chemotherapy and surgery.
Neoadjuvant chemoradiotherapy
Before the simulation, patients will be instructed to fast for at least 4 h and drink 300 ml semifluid diet 10 min before CT (4DCT) simulation and daily radiotherapy to maintain a consistent stomach volume. The patients will lie on the table in the supine position with their arms crossed above their heads and will be stabilized by a thermoplastic mask under free-breathing conditions. CT scanning will be performed from the clavicle to the fifth lumbar spine, with a scanning thickness of 0.5 cm. IMRT or VMAT will be recommended during radiotherapy. The 45 Gy dose of radiotherapy will be delivered in 25 fractions. Concurrent chemotherapy is S-1 administered at a dose of 40 to 60 mg twice daily according to body surface area (BSA) (Table 1).
Based on the EORTC-ROG expert opinion and third edition of the Japan Gastric Cancer Association guideline [15, 16], the radiation field will include the gross tumor, metastatic regional nodes and elective regional lymph node stations. The gross tumor volume (GTV) will be defined as a primary tumor determined by available technology. The diagnosis of metastatic regional lymph nodes will require comprehensive judgment based on their size and morphology. For example, for metastatic regional lymph nodes, the short axis diameter of the lymph nodes should be larger than 8 mm; a rounded shape, necrosis in the center of the lymph node, and clustered lymph nodes should be observed; marked enhancement should be identified during enhanced CT; and lymph nodes should be enlarged significantly during the examination. A metastatic regional lymph node will be defined as a metastatic regional node (GTVnd). The clinical target volume (CTV) contains GTV, GTVnd and elective regional lymph node stations according to the location of the tumor (Table 2).
Referring to the ICRU-62 report [17], the planned target volume will be derived from CTV plus 1 cm in the cranio-caudal direction and 0.5 to 0.7 cm in the anterior–posterior and left–right directions. The dose limitations of the organs at risk (OARs) will be as follows: Dmax stomach ≤ 50 Gy, V30 liver < 26%, V20 kidney < 26%, Dmean kidney < 18 Gy, Dmax spinal cord PRV ≤ 40 Gy, V10 bone marrow < 26%, Dmax intestine ≤ 42 Gy, and V40 intestine < 10% (for EGJ cancer, heart and lung should be included, V30 heart < 26%, and V20 lung < 20%).
Cone beam computed tomography (CBCT) scans will be performed daily during the first week and then once a week at least based on the clinical situation. Routine blood tests will be monitored weekly, and biochemical tests will be performed every two weeks during NCRT. Patients will take a 3-week break, and then a physical examination will be conducted before NCCT. The contents of the examination are shown in Table 3.
Neoadjuvant consolidated chemotherapy
The NCCT regimen will involve six cycles of SOX, with each cycle lasting for 21 days. Oxaliplatin will be delivered intravenously at a dose of 130 mg/m2 on day 1, and S-1 will be given orally at a dosage of 40 to 60 mg twice daily according to BSA (Table 1) on days 1 to 14. Patients will take a seven-day break after 14 days of chemotherapy, and complete physical examination will be performed every 3 cycles.
Surgery
Standard radical surgery will be performed 6 weeks after the last cycle of chemotherapy. For the type of resection, laparoscopic will be recommended. For the Siewert II EGJ, laparoscopy can also be combined with thoracoscopy. For the proximal GC and EGJ, esophagectomy and total or subtotal gastrectomy will be recommended. For the middle or distal GC, subtotal gastrectomy will be recommended. For the gastrointestinal tract reconstruction, Billroth I or Billroth II reconstruction will be used for subtotal gastrectomy and Roux-en-Y reconstruction will be used for total gastrectomy. D2 resection will be recommended. The definition of D2 lymph node dissection can be referred to NCCN guidelines [11]. At least 16 or greater lymph nodes are required to examine during the surgery. If the disease progresses during neoadjuvant therapy, a multiple disciplinary team (MDT) will be required to discuss the subsequent treatment.
Tumor response and toxicity criteria
The contents of the tumor efficacy assessments during therapy and follow-up are listed in Table 3. Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines will be adopted as the tumor response evaluation criteria [18]. Toxicities occurring during therapy will be assessed based on Common Terminology Criteria of Adverse Events (CTCAE) version 3.0. It will be necessary to reduce the dose or to stop treatment when serious adverse effects occur. During neoadjuvant or concurrent chemotherapy, when grade III gastrointestinal, neurotoxic or grade IV leukopenia occurs without recovery within 5 days after treatment, the dose will be reduced to 80% of the original dose. Chemotherapy will be stopped if any grade IV adverse effects occur except leukopenia. Radiotherapy will be stopped if a patient does not recover in 7 days when grade III gastrointestinal occurs after treatment. Any grade IV adverse effect except leukopenia will result in termination of radiotherapy. When a patient recovers to grade 0-I, radiotherapy may continue without receiving concurrent chemotherapy. It will be unnecessary to adjust the radiation dose. Surgical complications will be defined as anastomotic leakage, anastomotic bleeding or abdominal infection and others within 30 days after surgery. Tumor pathological response will be assessed by using the tumor response grading (TRG) system proposed by Mandard et al. [19]. All adverse events will be recorded in a case report form (CRF). Adverse events will be reported to the ethical institution committee within 24 h and dealt with immediately.
Sample size calculation
The primary objective is to determine the pCR rate in this trial. According to the results of the prospective phase II trial previously conducted at our center [20], the pCR rate of patients who received NCT was 14% (P0). We assume that after NCRT and six cycles of NCCT, the pCR rate will reach 28% in this study. Taking into consideration the loss of patients and the optimal two-stage design of phase II clinical trials, the test level α is 0.05 with a power of 80%. The required sample size of the study will be 82 patients. After 33 patients are enrolled, a planning interim analysis will be conducted. If the number of patients who received pCR is less than five, the study will be closed.
Statistical analysis
For descriptive statistics, categorical variables are described by frequency, rate, or 95% confidence interval. Continuous variables are described by the mean, median, quartile or standard deviation. For comparisons between groups, categorical variables will be compared by using the chi-square test or Fisher’s exact test. For continuous variables, the paired t test or Wilcoxon test will be used to compare changes between baseline and endpoint. The Kaplan–Meier method will be used to predict overall survival probabilities at specific time points. All statistical tests will be two-sided. The level of significance will be set to P < 0.05.
Follow-up
After completing the treatment according to the protocol, patients will receive the examinations listed in Table 3. Patients will be examined every 3 months in the first 2 years, every 6 months during years 3 to 5, and then once a year after 5 years. During follow-up, investigators will collect information on surgical complications, late toxicity, other treatments, and recurrence and survival.
Quality assurance
The quality assurance (QA) team associated with this study will include experienced experts in the field of radiotherapy, dosimetry, medical physics, medicine, surgery, imaging, pathology, and data censors. Diagnostic images before and after neoadjuvant treatment will be reviewed centrally and independently by two radiologists. The first three cases of target volume delineation from each center will be reviewed centrally and then checked randomly. Data censors in this study will stay in communication with branch-centers and check the quality of the data collection randomly.
Data collection and management
Every center needs at least one physician to be responsible for enrolling patients in this trial and arranging patient therapy. Two physicians will collect data and complete the CRF at their center. When modifying data, the researcher will need to sign their name and record the date of modification on the CRF. The CRF will be regarded as raw material, and all data in the CRF will be archived electronically on a specific computer. All electronic documents will be confidential, and only the data manager will have the password. Only the project leader will have the right to use the database, and other researchers will not be allowed to use the database unless permitted.
Discussion
GC ranks high in terms of morbidity worldwide, and multidisciplinary treatments are urgently needed, particularly for locally advanced GC. Recent published or ongoing clinical trials associated with NCRT or NCT are listed in Table 4. In our study, the objective will be to explore the short-term efficacy and safety of total neoadjuvant therapy for locally advanced GC and EGJ adenocarcinoma. The results may offer more therapeutic options for physicians to offer these patients.
The CROSS study was the first to report a significant survival benefit for the NCRT group compared with the surgery alone group among patients with EGJ adenocarcinoma [7]. With the development of NCT, the choice between NCRT and NCT has become controversial. The POET study was the first to compare NCRT with NCT and found that NCRT significantly downstaged tumor stage and improved the pCR rate [13]. One meta-analysis confirmed that NCRT can improve local control compared with NCT, but improvements in overall survival were not observed [28].
Currently, the value of NCRT for EGJ is clearly verified and recommended by the NCCN guidelines [11]. However, the value of NCRT for middle and distal GC is still lack of reliable evidence. The RTOG9904 study included 42 patients with GC and firstly found the value of NCRT in GC. The pCR rate was 26%, and the 1-year OS was 72% in this study [29]. Recently, Liu et al. included 40 patients with GC who received NCRT. The pCR rate was 13.9% and the medial survival time was 30.3 months [22]. Ahmed et al. included 32 patients with GC who received NCRT. The pCR rate was 18.8% and the 2-year OS was 51.3% [30]. Although, some phase II clinical trial has found the value of NCRT in middle and distal GC, it still needs evidence from larger sample size prospective clinical trials.
Perioperative therapy has advanced rapidly, and 6–8 cycles of perioperative chemotherapy are currently recommended according to the NCCN guidelines [11]. In the MAGIC study, six cycles of an ECF/ECX (stepirubicin, cisplatin and fluorouracil or capecitabine) regimen were used before and after surgery; although overall survival improved, only 41.6% of the patients completed the planned six cycles [9]. In the FLOT4 study, only 46% of patients completed all perioperative treatments [8]. In the CRITICS trial, the completion rate of adjuvant chemotherapy and chemoradiotherapy was 60% [12]. As mentioned above, the complete percentage of adjuvant therapy was quite low. Hence, in this trial, all perioperative treatment was administered before surgery. Moreover, preoperative chemotherapy may reduce the surgery rate for potential distant metastasis patients. In recent years, an increasing number of investigators have focused on the use of a total neoadjuvant therapy modality in patients with gastrointestinal tumors, especially those with rectal cancer [31], yet similar studies on GC are quite limited. Kim et al. explored the induction of NCT followed by NCRT for GC patients; adjuvant chemotherapy was needed for patients whose tumors were not downstaged to stage 0 to I after surgery. A total of 42 patients were enrolled in their study; 33.3% were pathologically downstaged to stage 0-I after preoperative treatment, and the 3-year overall survival rate was 75.5% [23]. The results of the use of total neoadjuvant therapy approaches for GC and EGJ adenocarcinoma phase II clinical trials were published at the 2019 ASTRO (American Society for Therapeutic Radiology and Oncology) meeting. Patients received 8 cycles of induction chemotherapy (FOLFIRINOX: Oxaliplatin, Irinotecan, Fluorouracil and leucovorin) followed by NCRT and surgery. Of the 25 patients enrolled in this trial, 20 underwent surgery, and the pCR rate was 35%. The results of this trial demonstrated the promising effects of total neoadjuvant therapy [32]. To further improve the downstaging rate, we prolonged the interval time between radiotherapy and surgery. The strategy of receiving NCRT followed by NCCT and surgery is rarely reported for GC.
We previously conducted a preliminary study to evaluate the efficacy of NCRT for locally advanced GC in Chinese patients. Among the 11 patients enrolled, two received the paclitaxel and carboplatin combined with radiotherapy. One patient experienced a serious adverse effect and concurrent chemotherapy interruption in the early stage. The other choked while eating half a month after chemoradiotherapy. The other nine patients received one drug with no serious reactions [33]. We moved all postoperative chemotherapy to before surgery considering toxicities, and we consider S-1 to be an appropriate choice when combined with radiotherapy.
Regarding the choice of NCT regimen, Sah et al. [34] compared FLOT (Oxaliplatin, Leucovorin, Fluorouracil and Docetaxel) and SOX regimens for locally advanced GC, with little difference in toxicity and prognosis. The SOX regimen has been widely used in Eastern clinical trials [24, 35, 36] and has been recommended in guidelines [11, 37]. Because our study is an exploratory study, six cycles of NCT are recommended according to the patients’ degree of tolerance. We previously designed a prospective study for GC and EGJ cancer. Patients who were diagnosed at clinical stage T3-4 or N positive were randomly assigned to the perioperative chemotherapy group or NCRT combined with surgery and adjuvant chemotherapy group. Surgery was performed 4–10 weeks after the completion of radiotherapy and chemotherapy. During the 2018 ASTRO meeting, we reported that the primary pCR rates in the NCRT and NCT groups were 14.3% and 11.1%, respectively (P = 0.724). The disease-free survival rate (87.1% vs. 63.9%, P = 0.05) and local tumor-free survival rate (100% vs. 73.3%, P = 0.014) in the NCRT group were better than those in the NCT group. No grade V reaction or perioperative death occurred in either group [20]. This research laid a methodological foundation and provided data to calculate the sample size in this study.
These study results are promising, but one limitation in our study is that we did not employ diagnostic laparoscopy for staging. To solve this problem, two imaging physicians with more than 10 years of working experience in gastrointestinal tumor diagnosis conducted tumor staging before enrollment.
To the best of our knowledge, this is the first attempt to treat GC or EGJ adenocarcinoma patients with NCRT followed by NCCT and surgery. We hope that the results of this promising total neoadjuvant therapy modality may provide a new strategy to further improve patient prognosis.
Trial status
This study was registered online in August 2019. The recruitment started in November 2019 and is still ongoing.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASTRO:
-
American Society for Therapeutic Radiology and Oncology
- BSA:
-
Body surface area
- 4DCT:
-
Four-dimensional computed tomography
- CRF:
-
Case report form
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- CTCAE:
-
Common terminology criteria for adverse events
- CBCT:
-
Cone beam computed tomography
- EGJ:
-
Esophagogastric junction
- ECF/ECX:
-
Stepirubicin, cisplatin and fluorouracil or capecitabine
- FOLFIRINOX:
-
Oxaliplatin, irinotecan, fluorouracil and leucovorin
- FLOT:
-
Oxaliplatin, leucovorin, fluorouracil and docetaxel
- GC:
-
Gastric cancer
- IMRT:
-
Intensity-modulated radiation therapy
- KPS:
-
Karnofsky performance status
- MDT:
-
Multiple disciplinary team
- NCRT:
-
Neoadjuvant chemoradiotherapy
- NCT:
-
Neoadjuvant chemotherapy
- NCCT:
-
Neoadjuvant consolidated chemotherapy
- NCCN:
-
National comprehensive cancer network
- OAR:
-
Organs at risk
- pCR:
-
Pathological complete response
- QA:
-
Quality assurance
- RECIST:
-
Response evaluation criteria in solid tumors
- SOX:
-
Tegafur and oxaliplatin
- TRG:
-
Tumor response grading
- VMAT:
-
Volumetric modulated arc therapy
References
Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64(12):1881–8.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66(2):115–32.
Liu D, Lu M, Li J, et al. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14(1):305–16.
Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–33.
Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial. Ann Oncol. 2020. https://doi.org/10.1016/j.annonc.2020.11.017.
Ahmed S, Fawzy M, Rezk K, et al. Preoperative conformal radiotherapy concurrently with paclitaxel/carboplatin in gastric cancer. J Cancer Ther. 2018;09(06):503–15.
Shapiro J, Van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8.
Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer, V.2.2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
Cats A, Jansen EPM, Van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):616–28.
Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–90.
Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–9.
Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol. 2009;92(2):164–75.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma 3rd English edition. Gastric Cancer. 2011;14(2):101–12.
Stroom JC, Heijmen BJ. Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiother Oncol. 2002;64(1):75–83.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6.
Wang X, Jin J, Zhao DB, et al. A randomized phase II trial of neoadjuvant chemotherapy compared with chemoradiotherapy in locally advanced gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2018;102(3):S29–30.
Tian Y, Wang Q, Wang J, et al. Neoadjuvant chemoradiotherapy combined with surgery versus direct surgery in the treatment of Siewert type II and III adenocarcinomas of the esophagogastric junction: long-term prognostic analysis of a prospective randomized controlled trial]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24(2):128–37.
Liu X, Li G, Long Z, et al. Phase II trial of preoperative chemoradiation plus perioperative SOX chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol. 2018;117(4):692–8.
Kim HS, Koom WS, Baek SE, et al. Phase II trial of preoperative sequential chemotherapy followed by chemoradiotherapy for high-risk gastric cancer. Radiother Oncol. 2019;140:143–9.
Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92.
Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24(8):2252–8.
Liu X, Jin J, Cai H, et al. Study protocol of a randomized phase III trial of comparing preoperative chemoradiation with preoperative chemotherapy in patients with locally advanced gastric cancer or esophagogastric junction adenocarcinoma: PREACT. BMC Cancer. 2019;19(1):606.
Slagter AE, Jansen EPM, Van Laarhoven HWM, et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 2018;18(1):877.
Petrelli F, Ghidini M, Barni S, et al. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer. 2019;22(2):245–54.
Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24(24):3953–8.
Ahmed S, Fawzy M, Rezk K, Elshrif W, Alaa M, Ellah MMHA. Preoperative conformal radiotherapy concurrently with paclitaxel/carboplatin in gastric cancer. J Cancer Ther. 2018;09(06):503–15.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071.
Wo JY, Clark JW, Roeland E, et al. A pilot study of neoadjuvant FOLFIRINOX FOLLOWED by chemoradiation for gastric and gastroesophageal cancer: preliminary results and prognostic implications of ctDNA. Int J Radiat Oncol Biol Phys. 2019;105(1):S85–6.
Wang X, Zhao DB, Jin J, et al. A preliminary study of efficacy of preoperative concurrent chemoradiotherapy for locally advanced gastric cancer. Chin J Radiat Oncol. 2016;25(11):1204–8.
Sah BK, Zhang B, Zhang H, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun. 2020;11(1):6093.
Jiang Z, Sun Y, Zhang W, et al. Comparison of S-1 plus oxaliplatin (SOX) and capecitabine plus oxaliplatin (XELOX) as adjuvant chemotherapies for stage II and III gastric cancer after D2 resection: a single-center retrospective study. Asia Pac J Clin Oncol. 2020;16(3):180–6.
Wang X, Li S, Sun Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer. 2021;21(1):20.
Wang FH, Zhang XT, Li YF, et al. The Chinese society of clinical oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41(8):747–95 (London).
Acknowledgements
We thank all patients and their families participated in this trial sincerely. We thank all centers and investigators contributed to this trial. Thanks for the high-efficient cooperation of the surgery, internal medicine, radiotherapy, imaging, endoscopy, and pathology departments.
Funding
This study is funded by the Beijing Hope Run Special Fund of Cancer Foundation of China (No. LC2018L03). The funding plays no role in the design or execution of the study.
Author information
Authors and Affiliations
Contributions
JMS drafted this manuscript, DBZ made great effort in surgery and recommending patients to the study; JJ made great contribution to design this study and revised the manuscript carefully; NL, YT and JMS contributed to the organization of this trial; LY were mainly responsible for chemotherapy; LMJ assessed the imaging examinations of each patient; As branch-centers partners, ZYL, SXL, JW, WLW, SYZ, JLY participated in designing and conducting the study; SLW, YWS, YPL, HF, NNL, SNQ, BC, YXL are currently involved in study implementation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval was obtained for this trial from the ethical committee of the Chinese Academy of Medical Sciences. The trial is published on ClinicalTrials.gov (number: NCT04062058, https://clinicaltrials.gov/ct2/show/NCT04062058?term=NCT04062058&draw=2&rank=1). Written informed consent is obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, J., Li, N., Tang, Y. et al. Total neoadjuvant therapy for locally advanced gastric cancer and esophagogastric junction adenocarcinoma: study protocol for a prospective, multicenter, single-arm, phase II clinical trial. BMC Gastroenterol 22, 359 (2022). https://doi.org/10.1186/s12876-022-02440-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02440-5