Abstract
Background
Pneumatosis intestinalis (PI) is a rare condition characterized by gas collection in the intestinal wall. We aimed to determine the etiology and affected segments associated with complications, treatment, and outcome.
Methods
We conducted a multicenter epidemiological survey using a standardized data collection sheet in Japan. Complicating PI was defined as strangulation or bowel necrosis, bowel obstruction, adynamic ileus, sepsis, shock, and massive gastrointestinal bleeding requiring blood transfusion.
Results
We enrolled 167 patients from 48 facilities. Multivariate analysis revealed that older age (adjusted OR, 1.05 and 95% confidence intervals [CI], 1.02–1.09, P = 0.0053) and chronic kidney disease (adjusted OR, 13.19 and 95% CI 1.04–167.62, P = 0.0468) were independent predictors of the small-bowel-involved type. Complicating PI was associated with the small-bowel-involved combined type (adjusted OR, 27.02 and 95% CI 4.80–152.01, P = 0.0002), the small-bowel-only type (adjusted OR, 3.94 and 95% CI 1.02–15.27, P = 0.0472), and symptomatic PI (adjusted OR, 16.24 and 95% CI 1.82–145.24, P = 0.0126). Oxygen therapy was performed in patients with a past history of bowel obstruction (adjusted OR, 13.77 and 95% CI 1.31–144.56, P = 0.0288) and surgery was performed in patients with complicating PI (adjusted OR, 8.93 and 95% CI 1.10–72.78, P = 0.0408). Antihypertensives (adjusted OR, 12.28 and 95% CI 1.07–140.79, P = 0.0439) and complicating PI (adjusted OR, 11.77 and 95% CI 1.053–131.526; P = 0.0453) were associated with exacerbation of PI. The complicating PI was the only indicator of death (adjusted OR, 14.40 and 95% CI 1.09–189.48, P = 0.0425).
Discussion
Small-bowel-involved type and symptomatic PI were associated with complications which were indicators of poor prognosis.
Similar content being viewed by others
Introduction
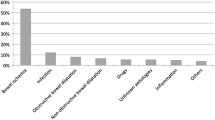
Pneumatosis intestinalis (PI) is a rare condition characterized by the collection of gas, which can have a hydrogen content up to 50%, in the intestinal wall [1, 2]. PI has been classified pathogenically into four categories: bowel necrosis, mucosal disruption, increased mucosal permeability, and pulmonary disease [2]. The relevant literature from Japan has described 12 patients with PI due to chronic occupational exposure to trichloroethylene [3], 14 following treatment of diabetes mellitus with α-glucosidase inhibitors [4], and 39 with systemic sclerosis [5]. An American multicenter retrospective study including 500 PI patients reported that 40% had transmural ischemia or withdrawal of clinical care and subsequent mortality, while 60% had benign diseases that could be observed without any aggressive intervention [6]. In Asia, a Korean retrospective study including 123 PI patients demonstrated that the most common cause was mesenteric vascular ischemia (35.0%), followed by bowel obstruction (13.8%), chemotherapy (10.6%), adynamic ileus (7.3%), post-anastomosis (3.3%), chronic obstructive pulmonary disease (2.4%), and nonspecific enteritis (2.4%) [7].
PI is distributed throughout the digestive tract, especially in the small and large intestine, and PI is occasionally accompanied by pneumoperitoneum and portal venous gas. The clinical significance of PI varies from an incidental finding that resolves spontaneously to death. A multicenter prospective epidemiologic study of the American Association for the Surgery of Trauma including 127 patients with PI recommended that surgical exploration be strongly considered for patients presenting with a blood lactate value greater than 2.0 mmol/L (18 mg/dL) and/or peritonitis; furthermore, the authors of the report suggested strong suspicion for necrosis in those patients and small bowel involvement, ascites, adynamic ileus, anemia, and a high international normalized ratio [8]. The large intestine is affected most commonly, while the small intestine is associated with this complicating pathologic PI [6], the mechanism of which is not fully elucidated. In addition, a large-scale multicenter study of PI has not been conducted in Japan to date. Therefore, we conducted a multicenter epidemiological survey including 167 patients with PI in Japan and determined the etiology and affected segments associated with complications, treatment, and outcome.
Patients and methods
Study design
This was a retrospective multicenter epidemiological study using a standardized data collection sheet. The diagnosis of PI was made with the identification of characteristic features or gas in the bowel wall by endoscopy, endoscopic ultrasonography, CT, plain abdominal roentgenogram, barium enema roentgenogram, or laparotomy. The aim of this study was to define the clinical features leading to complications and poor prognosis. Complicating PI was defined as having strangulation or bowel necrosis, bowel obstruction, adynamic ileus without mechanical obstruction, sepsis, shock, and massive gastrointestinal bleeding requiring blood transfusion. Benign PI was defined as not having those complications. This study was reviewed and approved by the institutional review board and ethics committee of Fujita Health University Hospital. Informed consent was obtained in the form of opt-out on the web-site. Those who rejected it were excluded. All authors had access to the study data and reviewed and approved the final manuscript.
Survey
The data collection sheet comprised questions on demographics, medical history, medications used, examinations, symptoms, complications, treatment, and outcome, as listed in Additional file 1: Supplementary Table 1. We sent this data sheet to 150 medical facilities in Japan in April 2013 and collected it by August 2013. When some items of the data sheet were unanswered, the numbers of patients subjected to analyses varied among the items.
Statistical analysis
The numbers in the text are expressed as the median (range), and comparisons were analyzed using the Mann–Whitney U test. The proportions of patients among categorical items were compared by Fisher’s exact probability test or the χ2 test. Multivariate analysis was performed using logistic regression analysis for categorical items with P-values less than 0.10 in the univariate analysis. Categorical items were excluded for multivariate analysis unless the number of patients in either of groups exceeded 2. To elucidate the relationship between the segments involved and clinical characteristics, the segments were classified into two types: large bowel-only type and small bowel-involved type. The small-bowel-involved type was classified into the small-bowel-involved combined type with affected segments combining the small bowel and the other gastrointestinal tract, and the small-bowel-only type. Crude odds ratios (ORs) and adjusted ORs with 95% confidence intervals (95% CIs) were computed. Differences were considered significant with P-values less than 0.05.
Results
Clinical characteristics of pneumatosis intestinalis
Standardized data collection sheets were returned from 48 facilities (32%) including 16 secondary and 32 tertiary health care hospitals, and 167 cases were enrolled. The patients’ demographics, comorbidities, past medical histories, and medications used are shown in Table 1. Symptoms, complications, segments involved, diagnostic examinations, treatment, outcome of PI, and prognosis are shown in Table 2.
Segment of bowel involved
A comparison of clinical characteristics between the large-bowel-only type and the small-bowel-involved type is shown in Additional file 2: Supplementary Table 2. Although univariate analysis demonstrated that older age, non-use of α-glucosidase inhibitors, 5-aminosalicylates, salicylazosulfapyridine, or statins/ezetimib/fibrates, negativity for ulcerative colitis, positivity for autoimmune disease, chronic kidney disease, and cancer other than that of the digestive or hematologic system were associated with the small-bowel-involved type, multivariate analysis revealed that older age (adjusted OR, 1.05 and 95% confidence intervals [CI], 1.02–1.09, P = 0.0053) and chronic kidney disease (adjusted OR, 13.19 and 95% CI 1.04–167.62, P = 0.0468) were the only independent predictors (Table 3).
Complicating pneumatosis intestinalis
A comparison of clinical characteristics between benign and complicating PI is shown in Additional file 3: Supplementary Table 3. Symptoms of complicating PI included abdominal pain/distention (n = 25, 96%) and bloody stool (n = 4, 15%) except one asymptomatic patient with hydrocephalus after subarachnoid hemorrhage, while those of benign PI included abdominal pain/distention (n = 49, 35%), diarrhea (n = 17, 12%), constipation (n = 10, 7%), and bloody stool (n = 8, 6%). The multivariate analysis revealed that the small-bowel-involved combined type (adjusted OR, 27.02 and 95% CI 4.80–152.01, P = 0.0002), symptomatic PI (adjusted OR, 16.24 and 95% CI 1.82–145.24, P = 0.0126), and the small-bowel-only type (adjusted OR, 3.94 and 95% CI 1.02–15.27, P = 0.0472) were the only independent predictors (Table 3).
Treatment of pneumatosis intestinalis
The comparison of clinical characteristics in terms of medical treatment, oxygen therapy, endoscopic therapy, and surgery is shown in Additional file 4: Supplementary Table 4. The rates of improvement, exacerbation, recurrence, and death stratified by conservative, oxygen, and surgical treatment were 67.5%, 4.3%, 0.0%, and 5.1%; 80.0%, 2.9%, 2.9%, and 2.9%; and 83.3%, 8.3%, 0.0%, and 8.3%, respectively. Univariate analysis demonstrated the following: non-use of 5-aminosalicylates/salicylazosulfapyridine, a past history of bowel obstruction, autoimmune disease, affected segments other than the large bowel associated with oxygen treatment, use of anticancer agents, the small-bowel-only type and complicating PI associated with surgery. Multivariate analysis demonstrated that oxygen therapy was performed in patients with a past history of bowel obstruction (adjusted OR, 13.77 and 95% CI 1.31–144.56, P = 0.0288); and surgery was performed in patients with complicating PI (adjusted OR, 8.93 and 95% CI 1.10–72.78, P = 0.0408), as shown in Table 3.
Outcome of pneumatosis intestinalis
The comparison of the clinical characteristics among the outcomes of PI, namely, improvement, no change, and exacerbation, is shown in Additional file 5: Supplementary Table 5. Univariate analysis demonstrated that non-use of α-glucosidase inhibitors and use of 5-aminosalicylates/salicylazosulfapyridine associated with no change, use of antihypertensives, chronic kidney disease, and involved segments other than the large bowel were associated with exacerbation. Multivariate analysis demonstrated that the use of antihypertensives (adjusted OR, 12.28 and 95% CI 1.07–140.79, P = 0.0439) and complicating PI (adjusted OR, 11.77 and 95% CI 1.053–131.526; P = 0.0453) were associated with exacerbation of PI, as shown in Table 3.
Death associated with pneumatosis intestinalis
The comparison of clinical characteristics in terms of survival and death associated with PI is shown in Additional file 6: Supplementary Table 6. Although the univariate analysis demonstrated that the use of α-blocker, laxatives, cirrhosis, and chronic kidney disease, the small-bowel-involved type, complicating PI were associated with death, the multivariate analysis revealed that the complicating PI was the only indicator of death (adjusted OR, 14.40 and 95% CI 1.09–189.48, P = 0.0425), as shown in Table 3. Of the eight patients, 6 died from complicating PI (4 with bowel infarction, 1 with septic shock, and 1 with massive gastrointestinal bleeding), and 2 died from severe comorbidities such as liver cirrhosis with chronic kidney disease and acute leukemia followed by graft-versus-host disease.
Discussion
The present multicenter epidemiologic study demonstrated that complicating PI, such as strangulation or bowel necrosis, bowel obstruction, adynamic ileus, sepsis, shock, and massive gastrointestinal bleeding, was significantly associated with the small-bowel-involved combined type, the small-bowel-only type, and symptomatic PI. These results support the findings of another prospective multicenter study including 127 PI patients sponsored by the Association for Surgery of Trauma: the small bowel location of PI, peritonitis, and abnormal laboratory values such as an elevated international normalized ratio, decreased hemoglobin, and lactate values greater than 2.0 mmol/L were predictive of pathologic PI defined as the presence of transmural ischemia during surgical exploration or autopsy [8]. Similarly, the largest-scale retrospective multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma including 500 patients demonstrated that the large bowel disease was the most common site for PI, but the jejunal and ileal pneumatosis locations were most commonly associated with pathologic PI [6]. A retrospective single-center study including 70 patients with PI or portal vein gas-clarified acute mesenteric ischemia was associated with small bowel PI, abdominal pain, elevated lactate, and the calculated vascular disease score [9]. These studies are in line with the present finding, but in a retrospective single-center study including 97 patients with PI (46% colon, 27% stomach, 5% stomach, and 7% both small and large bowel), Morris et al. reported that the location of pneumatosis alone was not predictive of outcome or intervention [10]. The present study has the inherent limitation of its lack of data on blood and physical findings, but a comprehensive diagnosis that includes a physical examination with parameters such as vital and peritoneal signs, laboratory tests, and imaging modalities, is essential to rule out complicating PI. This small-bowel-involved type was shown to be significantly associated with older age and chronic kidney disease in the present study. Among the four cases with chronic kidney disease, 3 were the small-bowel-only type while 1 was the large-bowel-only type, the difference of which seems marginal with P-values of 0.0468. There have been no reports regarding the association between affected segments of PI and kidney disease, but DuBose et al. described that patients with pathologic type were more likely to be older, with a history of enteritis and chronic renal failure [6]. Chronic kidney disease, especially end-stage renal disease increased intestinal permeability[11], which might be associated with PI affecting both the small and large bowels. In contrast to the large-bowel-only type associated with a-glucosidase inhibitors and ulcerative colitis, the small-bowel-involved type associated with older age, autoimmune disease, chronic kidney disease, and cancer can be more intractable and vulnerable to blood perfusion, which leads to the speculation of this type more complicating.
Regarding treatment, oxygen therapy was significantly associated with patients with a past medical history of bowel obstruction, and surgery was significantly associated with complicating PI. Hyperbaric oxygen therapy is a controversial treatment for adhesive postoperative small bowel obstruction, but Fukami et al. described that 143 patients (87.7%) were treated successfully with hyperbaric oxygen therapy without long-tube decompression. This oxygen therapy was associated with earlier resumption of oral intake and a shorter hospital stay, and the rate of operation was 7.4% in the hyperbaric oxygen therapy group and 14.8% in group treated by decompression alone [12]. In this context, patients with PI with a history of bowel obstruction likely underwent oxygen therapy. Duron et al. reported that abdominal distention, peritonitis, and lactic acidemia were predictive of positive intraoperative findings mandating intervention including mesenteric ischemia, an obstruction, or an incarcerated hernia on multivariate analysis in a retrospective multicenter record review of 150 PI patients, 54 (36%) of whom were managed nonoperatively, 72 of whom underwent surgery, and 24 of whom were given comfort measures only [13]. Generally, complicating or pathologic PI is an indication for surgery, as shown in the present study.
The last finding of the present study was that complicating PI was significantly associated with exacerbation of PI and subsequent death, which also makes medical sense. Wiesner et al. reported that of seven patients with infarction limited to one bowel segment (jejunum, ileum, or colon), only one patient (14%) died, whereas of the 10 patients with infarction of two or three bowel segments, eight patients (80%) died. These authors concluded that CT findings of PI and portomesenteric venous gas due to bowel ischemia do not generally allow prediction of transmural bowel infarction because these findings may be observed in patients with only partial ischemic bowel wall damage, and the clinical outcomes of patients with bowel ischemia with these CT findings seem to depend mainly on the severity and extent of their underlying disease [14], which is consistent with our comprehensive finding.
The present study has inherent limitations, including its retrospective design, ethnically homogeneous sample, low response rate to this survey, no laboratory data or images included, no CKD classification obtained, and participation bias in terms of data collection, which was conducted mainly by gastroenterologists and a few surgeons but no radiologists or acute care physicians. Therefore, the proportion of surgery in the treatment was as low as 4.6%.
Conclusions
In conclusion, our study highlights that small-bowel-involved type and symptomatic PI are associated with complications which are indicators of poor prognosis in the largest Asian population ever.
Availability of data and materials
All data generated or analysed during this study are included in this article and its supplementary material tables. Further enquiries can be directed to the corresponding author.
Abbreviations
- PI:
-
Pneumatosis intestinalis
References
Galandiuk S, Fazio VW. Pneumatosis cystoides intestinalis. A review of the literature. Dis Colon Rectum. 1986;29:358–63.
Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188:1604–13.
Yamaguchi K, Shirai T, Shimakura K, Akamatsu T, Nakama H, Kono K, Sakato M, Shigeno T, Furuta S, Nakajima T, et al. Pneumatosis cystoides intestinalis and trichloroethylene exposure. Am J Gastroenterol. 1985;80:753–7.
Tsujimoto T, Shioyama E, Moriya K, Kawaratani H, Shirai Y, Toyohara M, Mitoro A, Yamao J, Fujii H, Fukui H. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: a case report and review of the literature. World J Gastroenterol. 2008;14:6087–92.
Kaneko M, Sasaki S, Teruya S, Ozaki K, Ishimaru K, Terai E, Nakayama H, Watanabe T. Pneumatosis cystoides intestinalis in patients with systemic sclerosis: a case report and review of 39 Japanese cases. Case Rep Gastrointest Med. 2016;2016:2474515.
DuBose JJ, Lissauer M, Maung AA, Piper GL, O’Callaghan TA, Luo-Owen X, Inaba K, Okoye O, Shestopalov A, Fielder WD, Ferrada P, Wilson A, Channel J, Moore FO, Paul DB, Johnson S. Pneumatosis Intestinalis Predictive Evaluation Study (PIPES): a multicenter epidemiologic study of the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2013;75:15–23.
Lee HS, Cho YW, Kim KJ, Lee JS, Lee SS, Yang SK. A simple score for predicting mortality in patients with pneumatosis intestinalis. Eur J Radiol. 2014;83:639–45.
Ferrada P, Callcut R, Bauza G, O’Bosky KR, Luo-Owen X, Mansfield NJ, Inaba K, Pasley J, Bugaev N, Pereira B, Moore FO, Han J, Pasley A, DuBose J. Pneumatosis Intestinalis Predictive Evaluation Study: A multicenter epidemiologic study of the American Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:451–60.
Wayne E, Ough M, Wu A, Liao J, Andresen KJ, Kuehn D, Wilkinson N. Management algorithm for pneumatosis intestinalis and portal venous gas: treatment and outcome of 88 consecutive cases. J Gastrointest Surg. 2010;14:437–48.
Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, Schreiber MA. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679–82 (discussion 682–3).
Terpstra ML, Singh R, Geerlings SE, Bemelman FJ. Measurement of the intestinal permeability in chronic kidney disease. World J Nephrol. 2016;5:378–88.
Fukami Y, Kurumiya Y, Mizuno K, Sekoguchi E, Kobayashi S. Clinical effect of hyperbaric oxygen therapy in adhesive postoperative small bowel obstruction. Br J Surg. 2014;101:433–7.
Duron VP, Rutigliano S, Machan JT, Dupuy DE, Mazzaglia PJ. Computed tomographic diagnosis of pneumatosis intestinalis: clinical measures predictive of the need for surgical intervention. Arch Surg. 2011;146:506–10.
Wiesner W, Mortele KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177:1319–23.
Acknowledgements
The authors thank Mrs. Hiromi Yamashita, Mrs. Norimi Shiraishi, and Mrs. Sumie Morishita for technical support.
Intractable Diseases, the Health and Labour Sciences Research Group
Naoki Ohmiya1, Ichiro Hirata1, Hirotsugu Sakamoto2, Toshifumi Morishita3, Eiko Saito4, Katsuyoshi Matsuoka5, Tadanobu Nagaya6, Shinji Nagata7, Miyuki Mukae8, Koji Sano9, Takayoshi Suzuki10, Ken-ichi Tarumi11, Seiji Shimizu12, Kousaku Kawashima13, Toshifumi Hibi14, Akimichi Imamura15, Yohei Minato16, Kazuhiro Matsueda17, Go Kuwata18, Masahiro Sakaguchi19, Daisuke Saito20, Sakae Mikami21, Mitsuhiro Fujishiro22, Shigehiko Fujii23, Junji Umeno24, Kenji Aoi25, Daisuke Nutahara26, Fukunori Kinjo27, Mikihiro Fujiya28, Keita Harada29, Mitsunobu Matsushita30, Toshimi Chiba31, Yutaka Sasaki32, Shinji Tanaka33, Yoshiaki Aomi34, Kunio Kasugai35, Shojiro Yamamoto36, Nobuaki Yagi37, Tomoo Yoshie38, Masaki Yoshida39, Shin Fukudo40, Takanori Yamada41, Kensuke Kitsugi41, Shigeru Kuriyama41, Soichiro Miura42, Yoshiya Fujimoto43, Yasumasa Niwa44, Takashi Nishikawa45, Kiyotaka Okawa46, Makoto Sanomura47, Masanao Nakamura48, Tsutomu Mizoshita49. 1Departments of Gastroenterology and Advanced Endoscopy, Fujita Health University School of Medicine, 1-98 Kutsukake-cho, Toyoake, Aichi 470-1192, Japan; 2Department of Medicine, Division of Gastroenterology, Jichi Medical University, Tochigi, Japan; 3Division of Gastroenterology, Matsuyama Red Cross Hospital, Matsuyama, Japan; 4Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan; 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan; 6Department of Gastroenterology, Shinshu University School of Medicine, Matsumoto, Nagano, Japan; 7Department of Gastroenterology, Hiroshima City Asa Citizens Hospital, Hiroshima, Japan; 8Department of Gastroenterology, Kitasato University, School of Medicine, Kanagawa, Japan; 9Department of Gastroenterology, Osaka City General Hospital, Osaka, Japan; 10Department of Gastroenterology, Tokai University School of Medicine, Kanagawa, Japan; 11Division of Gastroenterology, Department of Internal Medicine, Kawasaki Medical School, Kurashiki, Okayama, Japan; 12Departments of Gastroenterology and Hepatology, Osaka General Hospital of West Japan Railway Company, Osaka, Japan; 13Department of Gastroenterology, Shimane University School of Medicine, Izumo, Shimane, Japan; 14Center for Advanced IBD Research and Treatment, Kitasato Institute Hospital, Kitasato University, Tokyo, Japan; 15Division of Gastroenterology, Sapporo Kosei General Hospital, Sapporo; 16Department of Gastroenterology, NTT Medical Center, Tokyo, Japan; 17Department of Gastroenterology and Hepatology, Kurashiki Central Hospital, Okayama, Japan; 18Department of Gastroenterology, Tokyo Metropolitan Komagome Hospital, Tokyo, Japan; 19Department of Gastroenterology, Moriguchi Keijinkai Hospital, Osaka, Japan; 20Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Japan; 21Department of Gastroenterology and Hepatology, Kobe City Medical Center West Hospital, Kobe, Hyogo, Japan; 22Department of Gastroenterology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; 23Department of Gastroenterology and Hepatology, Kyoto Katsura Hospital, Kyoto, Japan; 24Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; 25Department of Gastrointestinal Oncology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan; 26Department of Gastroenterology and Hepatology, Tokyo Medical University Hachioji Medical Center, Tokyo, Japan; 27Department of Endoscopy, University of the Ryukyus Hospital, Okinawa, Japan; 28Division of Gastroenterology and Hematology/Oncology, Department of Medicine, Asahikawa Medical University, Asahikawa, Japan; 29Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan; 30Third Department of Internal Medicine, Kansai Medical University, Osaka, Japan; 31Division of Gastroenterology, Department of Internal Medicine Iwate Medical University, Morioka, Japan; 32Department of Gastroenterology and Hepatology, Graduate School of Medical Sciences, Kumamoto University, Kumamoto, Japan; 33Department of Endoscopy, Hiroshima University Hospital, Hiroshima, Japan; 34Department of Gastroenterology, Fukuoka University Chikushi Hospital, Fukuoka, Japan; 35Department of Gastroenterology, Aichi Medical University School of Medicine, Nagakute, Aichi, Japan; 36Department of Gastroenterology and Hematology, Faculty of Medicine, University of Miyazaki, Miyazaki, Japan; 37Department of Molecular Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Kyoto, Japan; 38Division of Gastroenterology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan; 39Tohno Kousei Hospital, Mizunami, Gifu, Japan; 40Department of Behavioral Medicine, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan; 41First Department of Medicine, Hamamatsu University School of Medicine, Hamamatsu, Japan; 42Department of Internal Medicine, National Defense Medical College, Saitama, Japan; 43Gastroenterological Center, Department of Gastroenterological Surgery, Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan; 44Department of, Endoscopy, Aichi Cancer Center Hospital, Aichi; 45Nagahama City Hospital, Nagahama, Shiga; 46Department of Gastroenterology, Osaka City Juso Hospital, Osaka, Japan; 47Department of Gastroenterology, Hokusetsu General Hospital, Osaka, Japan; 48Department of Gastroenterology and Hepatology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 49Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
Funding
This work was supported in part by Health and Labour Sciences Grants for research on intractable diseases from Ministry of Health, Labour and Welfare of Japan. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
The study concept and design were developed by NO, IH, and TH. Drafting of the manuscript, critical revision of the manuscript for important intellectual content, analysis and interpretation of data, and statistical analysis were performed by NO. Data from the research group members were managed by NO. Acquisition of the data was performed by NO, HS, TM, ES, KM, TN, SN, MM, KS, TS, KT, SS, KK, and Intractable Diseases, the Health and Labour Sciences Research Group. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was reviewed and approved by the institutional review board and ethics committee of Fujita Health University Hospital (ID: HM20-253), and in accordance with the guidelines of national/international/institutional or Declaration of Helsinki. Informed consent was obtained in the form of opt-out on the web-site. Those who rejected it were excluded. All authors had access to the study data and reviewed and approved the final manuscript.
Consent for publication
Not applicable.
Competing interests
We disclose no reports or publications that contain any materials that appear in the article. The authors have no conflicts of interest to declare including employment, consultancies, honoraria, stock ownership and options, expert testimony, grants or patents received or pending, royalties which took place in the previous three years.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Table 1.
Additional file 2
. Supplementary Table 2.
Additional file 3
. Supplementary Table 3.
Additional file 4
. Supplementary Table 4.
Additional file 5
. Supplementary Table 5.
Additional file 6
. Supplementary Table 6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ohmiya, N., Hirata, I., Sakamoto, H. et al. Multicenter epidemiological survey of pneumatosis intestinalis in Japan. BMC Gastroenterol 22, 272 (2022). https://doi.org/10.1186/s12876-022-02343-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02343-5