Abstract
Background
Hepatotoxicity due to highly active antiretroviral therapy (HAART) has gained prominent attention since it can be affected by many factors. The aim of this study was to determine the prevalence of hepatotoxicity and related risk factors of severe hepatotoxicity following HAART initiation.
Methods
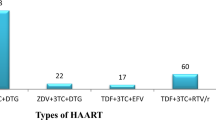
A total of 100 drug-naive patients aged between 18 and 61 years were recruited. They were put on Tenofovir/Lamivudine/Efavirenz [TDF/3TC/EFV] (64), Zidovudine/ Lamivudine/Efavirenz [AZT/3TC/EFV] (22), and Zidovudine/Lamivudine/Nevirapine AZT/3TC/NVP (14) and monitored for 6months and blood samples drawn.Alanine aminotransferases (ALT), aspartate aminotransferases (AST), and alkaline phosphatase (ALP) wereanalyzed by enzymatic methods and used to classify levels of hepatotoxicity.
Results
A total of 37(37%) and 49(49%) patients presented with hepatotoxicity while 15% and 28% had severe hepatotoxicity at 4 and 24 weeks respectively. Serum levels of all enzymes increased significantly (p = 0.001) with increased treatment duration. Univariate analysis revealed that the risk factor of developing severe hepatotoxicity was significantly greater in patients < 30years (p = 0.02), males(p = 0.04), low BMI (p = 0.02), low monthly income (p = 0.01) earners, and patients on AZT + 3TC + NVP regimen (p = 0.01). While multivariate analysis at p < 0.09 showed that age 30–40 years, low BMI, low monthly income, and the use of AZT + 3TC + NVP regimen were independent risk factors.
Conclusions
Low BMI, age group of 30–40years, low monthly income, and the use of AZT + 3TC + NVP regimen identified as risk factors for the development of severe hepatotoxicity should be considered as an important strategy by clinicians in preventing the hepatotoxicity.
Similar content being viewed by others
Introduction
1–3]. However, these HAART have also been reported to induce severe or life-threatening cases of adverse effects such as hepatotoxicity which have been the most important limiting factors to the successful use of HAART [3]. In the era of HAART, hepatotoxicity prevalence range of 1–54.0% and has been attributed to the discontinuation of HAART which has increased the morbidity and mortality rates among patients infected with HIV [2,3,4,5,6,7].
Several studies have been conducted to determine the risk factors of hepatotoxicity in patients with HIV. These factors include age, gender, type of HAART, viral load, hypertryglyceridemia, hyperglycemia, history of tuberculosis therapy and hepatitis B and Hepatitis C co-infection, alcohol abuse, higher baseline ALT or AST [3, 5, 7,8,9,10]. However, these findings cannot be universally applicable based on diverse regions, cultures, and specific human adaptations making it impossible to extrapolate the information from one population to another. Thus this study aimed to determine the prevalence of hepatotoxicity and related risk factors of severe hepatotoxicity among patients infected with HIV on HAART.
Methods
Study design and population
This was a hospital-based longitudinal analytical study conducted at the HIV Outpatient Clinic in Ndop, Santa, Bali, and Bafut District Hospitals and the Regional hospital of Bamenda of the NWR of Cameroon from February 2016 to November 2016. The sampled sites better represent the different backgrounds of individuals from the entire NWR of Cameroon. Bafut, Santa, Bali, and Ndop are in rural areas while Bamenda is from urban settings. The National Ethics Committee of Cameroon approved the study protocols with ethical approval number No 2016/01/689/CE/CNERSH/SP, and all patients gave their written consent before enrolment. The eligibility criteria included a confirmed HIV-1 infection, HAART naïve, negative for viral hepatitis B or C, not on tuberculosis treatment, and willingness to be followed up for at least 24 weeks.
Data were obtained with the aid of a pre-tested questionnaire and with the consultation of the patient’s record. These include information on; age, sex, weight, level of education, monthly income, history of alcohol and cigarette use, type of HAART, concomitant use of other drugs within 4 weeks before enrolment, WHO clinical staging, and the year the patient was first diagnosed of HIV. Patients routinely visited the HIV clinic every 4 weeks and were either on Tenofovir (TDF) + Lamivudine (3TC) + Efavirenz (EFV) or Zidovudine (AZT) + Lamivudine + Nevirapine (NVP) or AZT + 3TC + EFV according to Cameroonian HIV-1 first-line treatment guidelines. On each of these visits, clinical evaluation and assessment for adherence to HAART using self-report and pill counts were performed.
Laboratory evaluations
About five ml of venous blood was collected from each subject and processed to obtain serum. Measurement of Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) was done using the SPINREACT commercial kits(Ctra Santa Coloma, Spain) as described by manufactures’ manual and guided by the controls using the Urit 3300 machine (Diamond Diagnostics, USA). Case definitions for the various hepatic enzymes were determined based on sex with respect to the reference range considered as up to 40.0 U/L, 38.0 U/L and between 69 and 117.0 U/L for males; and up to 32.0 U/ L, up to 31.0 U/L and between 69 and 117.0 U/L for females; for ALT, AST, and ALP respectively.
Hepatotoxicity was graded using the levels of ALT, AST, and ALP according to classified AIDS Clinical Trials Group classifications as; grade 1 (1.25-2.5xULN), grade 2 (2.51-5.0xULN), Grade 3 (5.1-10xULN ) and, grade 4 (> 10xULN) [11]. Severe hepatotoxicity was defined as Grade 3 or 4. ALT, AST, and ALP grades were discordant. The one with the highest of the three grades was therefore used for classification [9, 12].
Statistical analysis
The clinical assessment and laboratory results were recorded and double-checked using Microsoft Excel database and analyzed using Statistical Package for the Social Sciences version 20. Categorical variables were expressed as frequencies and proportions and compared using the Chi-square test while continuous variables were expressed as means ± standard error of mean (SEM) and compared to the different treatment durations using the unpaired t-test. The fixed covariates considered to be possible risk factors for the liver elevated enzyme (LEE) at baseline parameters were explored using univariate logistic regression with unadjusted odds ratios and adjusted odds ratios for multivariate analyses to identify risk factors associated with severe hepatotoxicity. The level of significance was set at 5% throughout the analyses.
Results
Study population
A total of 100 patients were recruited into the study. Mean (SEM) age of the100 patients with HIV was 36.53(0.56) years and ranged from 18–61 years. The majority 53(53.0%), 43(43.0%), 57(57.0%), and 64(64.0%) were female, within the age range 30–39 years, had CD4+ T cell count of < 200 cells/mm3 and were placed on TDF + 3TC + EFV treatment respectively (Table 1).
Prevalence of hepatotoxicity
All the patients 100 (100%) had no significant LEE at baseline. Using ALT, AST, ALP or a combination of any of them showed that 37(37%) and 49(49%) patients presented with hepatotoxicity at 4 and 24 weeks respectively. This difference was significant (χ2 = 68.18; p = 0.000). Of this, 22% and 21% had mild-to-moderate (Grades 1 and 2) toxicity at 4 and 24 weeks while 15% and 28% had severe hepatotoxicity (Grades 3 and 4) at 4 and 24 weeks respectively as shown in Fig. 1.
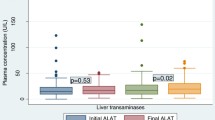
Median ALT, AST and ALP increased significantly with increase in treatment duration at 4weeks and 24weeks; ALT (F = 16.8; p = 0.001), AST (F = 11.3;p = 0.001) and ALP (F = 7.8; p = 0.001) Fig. 2.
Risk factors for severe hepatotoxicity at 24 weeks
The mean (SD) BMI and CD4+T cell count of patients with severe hepatotoxicity was 27.4 (2.4) kg/m2 and 168.0 (25.3) cells/mm3 respectively. From univariate regression analyses, severe hepatotoxicity was significantly (p < 0.05) high in the age group < 30 years, males, patients with low monthly income (< 50,000 FRS), low BMI (> 18.5 Kg/m2), and patients who took AZT + 3TC + NVP treatment. The prevalence of severe hepatotoxicity among those who consumed alcohol and smoked cigarettes or tobacco only showed a trend (p = 0.07) and (p = 0.09) respectively. In addition level of education, year of diagnosis, WHO staging, were not predictive (P > 0.05) for the development of severe hepatotoxicity (Table 2
Socio-demographic variables (age, gender, BMI, monthly income, alcohol intake, cigarette or tobacco intake, CD4+3
Discussion
With the widespread use of HAART and the availability of new ARV medications, hepatotoxicity has gained prominent attention in the management of patients with HIV/AIDS [3, 9, 13].
Results from this study like in previous studies in other parts of Cameroon and elsewhere have shown a high prevalence of hepatotoxicity among treated patients with HIV/AIDS [6, 9]. HAART-related severe hepatotoxicity results directly either from drug toxicity and/or drug metabolism, hypersensitivity reactions, mitochondrial toxicity, and immune reconstitution inflammatory syndrome [7, 13].
However, in this study, the adverse event did not have a negative impact on the continuation of the treatment. Our data ranged higher than what has been reported from previous studies by Price and Thio (10%) [7], and Sterling et al. (43%) [13]. The observed difference could be associated with differences in the population characteristics, the definition of hepatotoxicity, and the type of HAART being used.
In this study, a decline in the number of cases on Grade 1 and 2 hepatotoxicities with those in Grade 3 and 4, increasing. This change could not be confirmed based of the short period of follow-up. It will therefore be appropriate if a longer period of follow up could be conducted to fully confirm the observed trend on the hepatotoxicity trend [4, 9].
The high prevalence of hepatotoxicity after one month of treatment is a clear indication that some liver diseases such as cytomegalovirus and mycobacterium infections, and AIDS-related neoplasms such as lymphoma and Kaposi’s sarcoma [7] are often associated with HIV infection other than HAART. Of the 38 clients presenting with hepatotoxicity after 4 weeks, 17(44.7%) did not present with hepatotoxicity at 24 weeks. However, it has been reported that the hepatotoxic effects of HAART may resolve with time when the organ gets used to the drug in some patients [14]. Nevertheless, early development of hepatotoxicity can be attributed to mitochondrial toxicity, hypersensitivity reaction, or other opportunistic diseases such as Mycobacterium avium complex, Candidaalbicans, Toxoplasma gondii, Leishmania species, Strongyloidesstercoralis, Cytomegalovirus, etc[7, 10].
On the other hand, since HIV-1 infected patients could be co-infected with other infections, we could not rule out the possibility of these patients having taken othercombinations of non-ART medications orherbal concoctions, which could also have had adverse liver effects either alone or in combination[7]. Secondly, all patients in this study received cotrimoxazole prophylaxis for the treatment of opportunistic infections. All these factors have been reported to cause an increased level of transaminases [4, 5, 7].
Our data reveal that elevated transaminases showed a significant positive linear relationship with an increase in the duration of treatment irrespective of the ARV regimen [5]. Thus, the hepatotoxicity seen in this study was mostly hepatocellular (increases in AST and ALT) than cholestatic (increases in ALP) [13, 14]. The observed severe hepatotoxicity was shown to develop as early as a month after starting treatment [4, 15].
Age was found to be a predictor of severe hepatotoxicity contrary to previous studies, [16]. Our findings showed that severe hepatotoxicity was significantly high among individuals aged < 30 years contrary to previous studies conducted in other regions of Cameroon and elsewhere [5, 9, 13]. The most probable reason for the high prevalence in the group can be attributed to their lifestyle. This group is an active age group in terms of social activities such as high intake of alcohol (63% vs. 53.5% vs. 43.3%) and cigarette smoking (48.1% vs. 30.0% vs. 11.6%)that have been shown to increase the levels of liver enzymes [7, 17, 18]. Secondly, there is a high probability that this group of individuals could also have taken other drugs including herbs to get healed.
Gender was analysed to see if it had an impact on hepatotoxicity. However, in this study, no association was observed according to multivariate analysis. The findings concurred with previous studies [4, 14] but also contrary to other findings elsewhere [8, 13]. Given that the courses of HAART metabolism in humans are not gendered dependent [16], this high prevalence could be associated with other social habits that are common with males rather than females. For instance, more males were found to consume alcohol more (55.4% vs. 44.6%) and cigarettes (62.9% vs. 27.1%) than femalesin this study.
Our observations suggest that body weight could bea major factor in determining the serum level of liver enzymes. Low BMI was found to be an independent predictor of severe hepatotoxicity. A finding that confirms that good nutritional status at the start of HAART could be protective against early hepatotoxicity [3, 4]. Studies have shown that an increase in serum ALT and AST levels could be associated with an increase in BMI as a result of anincrease in dietary fat content [19, 20]. There is a high probability that underweight patients usually consume large amounts of dietary fat. A high level of dietary fat causes higher levels of oxidative stress and lipid peroxidation which results in oxidative stress leading to mitochondrial and DNA damage thus causing hepatocellular injury, activation of hepatic stellate cells hence increased levels of liver enzymes [3, 20]. Furthermore, the use of NVP based regimen as recommended for low BMI patients often causes hepatotoxicity [10].
Monthly income has been shown to play an important role in the life of individuals. In this study low monthly income was another significant predictor of severe hepatotoxicity from the multivariate model. This group of persons usually have increased consumption of dietary fat and thus increase in their BMI that accounted for the increase in the liver enzyme. It has been reported that lower monthly income is associated with negative effects on the quality of life such as anxiety and depression symptoms that worsen their clinical conditions as a result making them prefer herbal and cheaper drug concoctions [21, 22].
The prevalence of severe hepatotoxicity associated with NVP (57.1%) based regimen in this study was similar to other studies [7, 10, 13]. Furthermore, we realise that those who took AZT + 3TC + NVP presented with elevated ALP. This confirms the fact that the use of NVP is associated with cholestatic liver enzyme elevations [13].
Alcohol consumption which is associated with elevated liver enzyme levels is most prevalent in most countries [7, 16, 18]. It has been reported that interactions between alcohol and HAART often appear to be crucial in the development of liver disease especially in HIV-1 infected patients [23].Although the prevalence of severe hepatotoxicity was higher among those patients who took alcohol, alcohol intake only showed a trend in the univariate analysis findings that concur with previous findings [3]. This study findings show that alcohol consumption leads to weight gain hence high-level secretions of the liver enzyme especially in men [24, 25]. There were significantly more men who were overweight and had high BMI with the majority of them having a significant high level of severe hepatotoxicity at 24 weeks. Furthermore, since the AST/ALT ratio at week 24 was < 1.5, it is suggestive that, the experienced liver injury could be associated with HAART [25]. That is why we suggest that interpretation of these findings be taken cautiously these findings on the blood alcohols levels were not interpreted beforehand. In addition, the existing inconsistencies regarding the definitions of alcohol consumption and different types of alcohol products being consumed could have misinterpreted the findings.
Cigarette smoking has significantly been associated with increased levels of ALP and to a lesser extent AST and ALT [17, 26,27,28]. In this study, however, no significant effect on the liver enzymes was observed contrary to previous studies [26]. The observed high levels of liver enzymes as associated with hepatocellular damage resulting out of the production of numerous toxins like. nicotine that causes lipid peroxidation propagation which in return damages the biological cell membrane of the liver, or the increased production of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α that results in liver cell injury [28,29,30].Smoking has also been shown to further aggravate the pathogenic effects on theliver during the processing of alcohol and medications [10, 27, 30]. In addition, smoking could decrease CD4+ T cells count and lowering of BMI hence a minor risk to liver toxicity [17, 29].
Interestingly, in our study low CD4+T cell count was a minor risk factor for liver toxicity since low CD4+T cell count showed a trend with hepatotoxicity only in the univariate analysis. Our data also revealed that the high CD4+T cell count after 24 weeks is associated with a higher risk of hepatotoxicity in the adjusted multivariate analysis though it was insignificant. Similar findings have been reported by Price and Thio [7] This result attests to the fact that the use of NVP based regimen and high CD4+ T cell count is a risk factor to the development of severe hepatotoxicity [3, 10, 13].
Conclusions
Age group, Low baseline BMI, low monthly income, and use of AZT + 3TC + NVP regimen were identified as independent risk factors for developing severe hepatotoxicity. Severe hepatotoxicity may arise more frequently with increased use of HAART. Thus, there is a need to educate the community of the potential risk factors (such as nutritional habits, alcohol, and smoking abstention)associated with severe hepatotoxicity in other to improve patient management and care.
Recommendation
The use of NVP based regimen should not be recommended for low BMI patientsand patients > 40 years. The HAART regimen of those with severe hepatotoxicity should first be altered and followed up before discontinuation.
Limitations
This study had some limitations as follows. The study design did not allow us to establish age, gender, and type of HAART balance ratio at recruitment. In addition, adequate drug adherence assessment and serum drug level estimation could not be performed due to the lack of facilities for such tests. The use of traditional herbs was not considered in this study with concurrent non TB medication. Lastly, liver histology could not be performed in these patients.
Availability of data and materials
The dataset used for analyzed in this study is available from the corresponding author on request.
Abbreviations
- 3TC:
-
Lamivudine
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AZT:
-
Zidovudine
- BMI:
-
Body mass index
- EFV:
-
Efavirenz
- NVP:
-
Nevirapine
- SEM:
-
Standard error of mean
- TDF:
-
Tenofovir
References
USAIDS: Global AIDS Update http://www.unaids.org/en/resources/documents. 2016. Assessed on the 13/01/17.
Osakunor D, Obirikorang C, Fianu V, Asare I, Dakorah M. Hepatic enzyme alterations in HIV patients on antiretroviral therapy: A Case-Control Study in a Hospital Setting in Ghana. PLOS ONE. 2015;10:e0134449.
Wambani JR, Ogola PE, Arika WM, Rachuonyo HO, Kemboi NG, Lihana R, et al. Anti-retroviral drug hepatotoxicity and risk factors in HIV patients with or without hepatitis B and C: a review. J Infect Dis Ther. 2015; 3:258.
Wenderlein D, Scarcella P. Antiretroviral treatment-associated hepatotoxicity and anemia in patients receiving stavudine or zidovudine containing regimens in Sub- Saharan African Settings. J AIDS Clin Res. 2016;07(01).
Kalyesubula R, Kagimu M, Opio KC, Kiguba R, Semitala CF, Schlech WF, et al. Hepatotoxicity from first line antiretroviral therapy: an experience from a resource limited setting. Afr Health Sci. 2011;11:16–23.
Lucien K, Clement A, Fon N, Weledji P, Ndikvu C. The effects of antiretroviral treatment on liver function enzymes among HIV-infected outpatients attending the Central Hospital of Yaounde, Cameroon. Afr. J. Clin. Exper. Microbiol. 2010;11(3).
Price J, Thio C. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–12.
Chu K, Boulle A, Ford N, Goemaere E, Asselman V, Van Cutsem G. Nevirapine-associated early hepatotoxicity: incidence, risk factors, and associated mortality in a Primary Care ART Programme in South Africa. PLoS ONE. 2010;5:e9183.
Fokunang CN, Banin AN, Kouanfack C, Ngogang JY. Evaluation of hepatotoxicity and nephrotoxicity in HIV patients on highly active antiretroviral therapy. J AIDS HIV Res. 2010;2:048–57.
Hamza M, Maifada Y, Mijinyawa M, Muhammad B, Musa B, Nalado A, et al. Prevalence and risk factors for hepatotoxicity among patients with HIV/AIDS on highly active antiretroviral therapy in North-Western Nigeria. Sub-Saharan Afr J Med. 2014;1:175.
AIDS Clinical Trials Group, Division of AIDS for grading the severity of adult and pediatric adverse advents. Rockville (MD): National Institute of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS,1992.
Sulkowski M. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000; 283:74.
Sterling R, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C co-infections. Dig. Dis. Sci. 2007; 53:1375–1382.
Bello S, Onunu A, Erah P. Long-term effect of HAART on biochemical profiles of HIV/AIDS patients in a tertiary health facility in Benin city, Nigeria. Trop J Pharm Res. 2014; 13:1941.
Mugusi S, Ngaimisi E, Janabi M, Minzi O, Bakari M, Riedel K, et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS ONE. 2012;7:e40180.
Wondemagegn M, Bokretsion G, Ambahun C, Genetu A, Bayeh A. Hepatotoxicity and associated risk factors in HIV-infected patients receiving antiretroviral therapy at Felegehiwot referral hospital, Bahirdar, Ethiopia. Ethiop J Health Sci. 2013; 23:217–26.
Jang E, Jeong S, Hwang S, Kim H, Ahn S, Lee J, et al. Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross-sectional study. BMC Gastroenterology. 2012;12.
Park E, Lim M, Oh J, Cho H, Bae M, Yun E, et al. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS ONE. 2013;8:e63439.
Qureshi IZ, Shabana A, Fareeha A. Effect of overweight and obesity on liver function in a sample from Pakistani population. Pak J Zool. 2006;38:49–54.
Tasneem R, Angoorbala B, Maheshwari RS, Shweta A, Rai BA. Effect of increasing BMI on enzymes used for assessment of liver function. EJPMR. 2015;2:207–9.
Cho H, Park E. Quality of life of chronic hepatitis C patients and its associated factors. Osong Public Health Res Perspect. 2017;8:124–9.
Oguntibeju O. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV/AIDS - Res Palliative Care. 2012;117.
Barve S, Kapoor R, Moghe A, Julio AR, Eaton JW, Gobejishvili L, et al. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33:229–236.
Suter P, Tremblay A. Is alcohol consumption a risk factor for weight gain and obesity? Crit Rev Clin Lab Sci.2005;42:197–227.
Adams L, Knuiman M, Divitini M, Olynyk J. Body mass index is a stronger predictor of alanine aminotransaminase levels than alcohol consumption. J Gastroenterol Hepatol. 2008; 23:1089–1093.
Whitehead T, Robinson D, Allaway S. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in men. Ann Clin Biochem. 1996;33:530–5.
Wannamethee S, Shaper A. Cigarette smoking and serum liver enzymes: the role of alcohol and inflammation. Ann Clin Biochem. 2010;47: 321–6.
Abdul-Razaq S, Ahmed B. Effect of cigarette smoking on liver function test and some other related parameters. Zanco J Med Sci. 2013;17:556–62.
El-Zayadi A. Heavy smoking and liver. World J Hepatol. 2006;12: 6098.
Elameen MK, Abdrabo AA. Comparative study of liver enzymes activities in smokers and diabetic sudanese patients. AJBPS. 2013;3:39–41.
Acknowledgements
We are grateful to all the hospitals’ management staff for allowing the research to be conducted in their institutions as well as the use of their equipment. We are greatly indebted to the participants in the study. We would like to acknowledge the effort of the laboratory staff during sample collection.
Funding
The study was non-funded by any organization.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: LEA, AKN, FCN, JNT, PO; enrolled the patients: LEA, FCN ,ME, BJ, FH, SB, TCT, ECM and VN; Performed the experiments: LEA; data management and analysis: LEA, NE, ID; Contributed reagents/materials/analysis tools: LEA, NE, ID; prepared the manuscript: LEA, AKN, FCN, JNT, PO, NE, ID; read and approve the manuscripts: LEA, AKN, FCN, JNT, ME, BJ, FH, SB, TCT, ECM, VN and PO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The National Ethics Committee of Cameroon approved the study protocols. Informed written consent for those who could read and write while legal authorization from the next of kin for those who could not read and write was obtained from each participant before enrolment in the study. This study was performed in accordance with the principles of the declaration of Helsinki and its appendices.
Consent for publication
Written informed consent was obtained from patients for publication of the study result as an aggregate data. The data is available to the corresponding author on request.
Competing interests
We declare that we have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abongwa, L.E., Nyamache, A.K., Charles, F. et al. Risk factors of severe hepatotoxicity among HIV-1 infected individuals initiated on highly active antiretroviral therapy in the Northwest Region of Cameroon. BMC Gastroenterol 22, 286 (2022). https://doi.org/10.1186/s12876-022-02305-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02305-x