Abstract
Background
Intraoperative near-infrared fluorescence (NIR) imaging with indocyanine green (ICG) can demonstrate real-time lymphatic drainage and thus improve the accuracy and completeness of lymphadenectomy in colorectal cancer surgery. However, it has not been utilized in the inguinal lymphadenectomy in rectal cancer. This study aimed to describe a case of combined laparoscopic lymphadenectomy of left lateral pelvic and inguinal nodal metastases using NIR imaging with ICG imaging guidance for a rectal cancer patient with left lateral pelvic and inguinal lymph node metastases.
Case presentation
A 26-year-old man presented rectal cancer located 7 cm from the anal verge and enlarged lymph nodes in the left inguinal area. Pretreatment workup revealed rectal cancer with left lateral pelvic and inguinal lymph node metastases. The patient received preoperative chemoradiotherapy (pCRT), including radiation (total dose of 50.4 Gy in 28 fractions) to the whole pelvis and bilateral inguinal regions together with eight cycles of FOLFOX (oxaliplatin, fluoropyrimidine, and leucovorin) and three cycles of bevacizumab targeted chemotherapy. After pCRT, both colonoscopy and MR scan revealed a significant response of the primary tumor to pCRT, while MR scan revealed enlarged left lateral pelvic and inguinal lymph nodes. After four months from the completion of radiation (2 months after the last course of bevacizumab targeted therapy), the patient underwent laparoscopic-assisted ultra-low anterior resection and lymphadenectomy of left lateral pelvic and inguinal nodal metastases using ICG-NIR fluorescence imaging. The combined procedure was performed successfully without perioperative complication. Total operative time was 480 min and estimated blood loss 50 mL. Totally 34 lymph nodes were retrieved.
Conclusions
This is the first report of the safety and feasibility of ICG-NIR fluorescence imaging-guided laparoscopic lymphadenectomy of left lateral pelvic and inguinal nodal metastases in managing low rectal cancer with lateral pelvic and inguinal LNs metastases.
Similar content being viewed by others
Background
Inguinal lymph node metastasis (ILNM) occurs in approximately 1.2%-2.4% of rectal cancer patients with a dismal prognosis [1,2,3]. The TNM 8th edition staging system classifies rectal cancer with ILNM as distant metastasis that heralds a poor prognosis [4]. Inguinal lymph node dissection (ILND) is an integral part of the treatment of rectal cancer with ILNM [5] and can be beneficial for certain patients [6, 7]. Traditional ILND via an open approach is limited by high postoperative complications and impaired quality of life [8]. To minimize surgical morbidities, video endoscopic inguinal lymphadenectomy (VEIL) has been proposed as an alternative to open ILND in urological and gynecological surgeries [9].
Intraoperative near-infrared fluorescence (NIR) imaging with indocyanine green (ICG) can demonstrate real-time lymphatic drainage and thus improve the accuracy and completeness of lymphadenectomy in colorectal cancer surgery [10, 11]. It has also been reported to be a feasible technique to guide lateral pelvic lymph node dissection (LPLND) during rectal cancer surgery, which may help identify metastatic LNs and improve the surgical quality of lymphadenectomy [12, 13]. We hypothesized that ICG fluorescence imaging also has the potential to guide inguinal lymphadenectomy in rectal cancer. However, incorporating NIR/ICG fluorescence imaging guidance in VEIL for rectal cancer with ILNM has not yet been reported.
Herein, we presented a case of combined laparoscopic lymphadenectomy of left lateral pelvic and inguinal nodal metastases using NIR/ICG fluorescence imaging guidance for a rectal cancer patient with left lateral pelvic and inguinal lymph node metastases.
Case presentation
A 26-year-old young man with low rectal cancer without family history was admitted in August 2020 with a complaint of rectal bleeding. Physical examination revealed a rectal mass located 7 cm from the anal verge (by digital rectal examination) and enlarged lymph nodes (LNs) in the left groin area. Colonoscopy demonstrated a cauliflower-shaped tumor located 7 cm from the anal verge, confirmed moderately differentiated adenocarcinoma on biopsy. Laboratory findings demonstrated elevated carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA 19–9) levels (92.9 ng/mL and 146.8 U/mL, respectively). Magnetic resonance (MR) scan revealed a rectal tumor with irregularly shaped and enlarged lymph nodes in the left lateral pelvic (1.8 × 0.6 cm) and the left inguinal (2.0 × 1.5 cm) region, as depicted in Fig. 1A, B (MRI sequence can be seen in Additional file 3: Fig. S1). Furthermore, positron emission tomography/computed tomography (PET/CT) scan also demonstrated left lateral pelvic and inguinal lymph node metastasis. According to the multidisciplinary team's decision, the patient received preoperative chemoradiotherapy (pCRT), including radiation (total dose of 50.4 Gy in 28 fractions) to the whole pelvis and bilateral inguinal regions, as well as 8 cycles of FOLFOX (oxaliplatin, fluoropyrimidine, and leucovorin) and 3 cycles of bevacizumab targeted chemotherapy. After pCRT, MR scan revealed a significant response of the primary tumor to CRT, as shown in Fig. 1C, D. However, MR scan revealed enlarged left lateral pelvic (0.7 × 0.5 cm) and inguinal (1.4 × 0.8 cm) lymph nodes, as shown in Fig. 1C, D. PET/CT scan demonstrated hypermetabolism of 18F-fluorodeoxyglucose into both primary tumor (SUV max 1.0) and the left swollen lateral pelvic (SUV max 3.0) and inguinal lymph nodes (SUV max 3.4). In addition, the serum levels of CEA and CA199 have decreased dramatically (8.8 ng/mL and 21.8 U/mL, respectively).
Pelvic MRI findings before and after pCRT in the high-resolution T2w sequences using a 3.0 Tesla Siemens Prisma MRI Scanner (Siemens Healthineers AG, Erlangen, Germany) with a contrast of Gadobutrol Injection (Gadovist). A Pelvic MRI before pCRT showing rectal tumor and left enlarged lateral LNs (axial T2WI sequence, no fat suppression, oblique transverse section, TR 2000, TE 95, FOV 20×20); B Pelvic MRI before pCRT showing left enlarged inguinal LNs (axial T2WI sequence, no fat suppression, oblique transverse section,TR 2000, TE 95, FOV 20×20); C After pCRT the rectal tumor and left enlarged lateral LNs decreased in size (axial T2WI sequence, no fat suppression, oblique transverse section, TR 2000, TE 89, FOV 22×22); D After pCRT the left enlarged inguinal LNs decreased in size (axial T2WI sequence, no fat suppression, oblique transverse section, TR 2000, TE 89, FOV 22×22). MRI: magnetic resonance imaging; LNs: lymph nodes; pCRT: preoperative chemoradiotherapy; T2WI: T2 weighted imaging; TR: repetition time; ET: echo time; FOV: field of view
After four months from the completion of radiation (2 months after the last course of bevacizumab targeted therapy), the patient underwent laparoscopic-assisted ultra-low anterior resection according to the principle of total mesorectal excision (TME). The patient was placed in the Trendelenburg semi-right lateral position under general anesthesia. After a lateral-to-medial approach of sigmoid colon mobilization, the inferior mesenteric artery was highly ligated. The splenic flexure was mobilized to ensure a tension-free anastomosis. Pelvic autonomic nerves were identified and preserved when mobilizing the rectum down to the pelvic floor.
Then, the rectum was transected 2 cm below the lower edge of the tumor. The specimen extraction was extracted via the stoma site. The proximal colon was transected 10 cm proximal to the tumor.
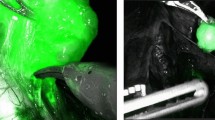
After completion of TME, NIR/ICG fluorescence imaging was used via a near-infrared camera system (KARL STORZ SE & Co. KG, Tuttlingen, Germany) to guide laparoscopic LLND (Additional file 1: Video 1). Two ml of ICG was injected around the tumor transanally 1 day before the operation. Using a fascia priority approach, the ureterohypogastric nerve fascia along the ureter was isolated, and the pelvic plexus was identified and preserved. The vesicohypogastric fascia along the internal iliac artery was isolated, and the parietal pelvic floor, such as the iliopsoas muscle and internal obturator muscle, was exposed. Next, the internal iliac LNs between the ureterohypogastric nerve fascia and the medial aspect of the vesicohypogastric fascia were dissected. An enlarged LN around the external iliac vein found positive in NIR/ICG fluorescence imaging was also separated from the external iliac vein and internal obturator muscle, and the obturator nerve was preserved. The obturator lymph nodes between the vesicohypogastric fascia and external iliac vessels were dissected. The residual soft tissues were examined by NIR/ICG fluorescence imaging to prevent the omission of LNs during LLND. After completion of LPLND, the uterus, the pelvic plexus, the internal and external iliac vessels and their branches, and the obturator nerve remained. Then, an end-to-end anastomosis was constructed using a circular stapler. A diverting ileostomy was performed to protect low rectal anastomosis.
Next, the patient was switched to a supine position with thigh abduction. First, a 1.5 cm incision was made 2 cm below the lower vertex of the femoral triangle. NIR/ICG guided VEIL was performed using three ports, as shown in Fig. 2A. Scissors and digital maneuvers created a working space superficial to Scarpa’s fascia and then insufflated with CO2 at 12 mm Hg to maintain a visualization. The assistant grabbed the skin and lifted it to provide traction (Fig. 2A). As seen in Additional file 2: Video 2, the dissection boundaries of VEIL, including the sartorius muscle (laterally), adductor longus muscle (medially), and the inguinal ligament (superiorly), were well visualized. Then, superficial inguinal LNs were separated from Scarpa’s fascia. The saphenous vein medially and the spermatic cord, and the external inguinal ring superomedially were also identified and preserved. The saphenous vein is spared when superficial inguinal LNs dissection superior to the Scarpa’s fascia. Deep inguinal LNs dissection was then carried inferomedially starting from the transected Saphenofemoral junction around the femoral vein. Then, NIR/ICG fluorescence imaging confirmed that there were no residual LNs. Finally, the resected LNs were packed in a specimen retrieval bag and then extracted through the 10 mm trocar, and a suction drain was placed through the access of the 5 mm trocar. The total operating time was 480 min, and the estimated blood loss was 50 mL.
The surgical specimens showed a 1.2 × 1.5 cm scar after pCRT. The pathology revealed no residual carcinoma in the rectum with no tumor involvement in the perirectal, intermediate, and principal LNs, as shown in Fig. 2B, C. The internal iliac LN showed no tumor involvement, while pathology revealed infiltrated 7 of 11 dissected obturator LNs and 2 of 2 external iliac LNs. Noteworthily, pathology revealed infiltrated 2 of 3 dissected deep inguinal LNs, while the superficial inguinal LN had no tumor involvement. The tumor was classified as ypT0N0M1 according to the TNM classification of the Union for International Cancer Control (UICC). No postoperative incisional and urinary dysfunctions were observed, and the patient was discharged on postoperative day 8. Until now, the patient received FOLFOX chemotherapy for 4 cycles and bevacizumab targeted chemotherapy for 3 cycles. Postoperative surveillance (6 months) revealed no locoregional or systemic tumor relapse.
Discussion and conclusions
Currently, there is no consensus nor guideline regarding the treatment strategy of ILNM from rectal cancer due to the rarity of this disease [14]. However, some studies have demonstrated that isolated ILNM from rectal cancer represents a distinct entity with a favorable prognosis after curative surgical resection [6, 15]. ILNM usually develops in low rectal cancer located below the dentate line [1]. Considering rectal cancer located 6 cm from the anal verge, in this case, ILNM can be explained by retrograde nodal spread owing to the anterograde lymphatic blockade by advanced primary tumors [10]. Pretreatment work-up excluded evidence of distant metastases.
The patient in the present study was classified as rectal cancer with solitary lateral pelvic and inguinal LNs metastases, that is, an oligometastatic state rather than systemic metastasis. In this respect, we proposed that the patient should be treated with curative intent.
According to current guidelines, prophylactical radiation of bilateral inguinal areas is not recommended for all rectal cancer patients, mainly due to significant radiation-induced side effects [16]. In addition, few studies have demonstrated the efficacy of pCRT in ILNM from rectal cancer. In our daily practice, pCRT was indicated from patients with locally advanced rectal cancer. For local control and possible cure, pCRT (radiation dose of 50.4 Gy in 28 fractions with concomitant FOLFOX and bevacizumab chemotherapy) was administered to the patient in this case study. Results from postoperative pathological examination revealed a pathologically complete response of the primary tumor to pCRT, while the effects on lateral pelvic and inguinal LNs were not that pronounced. The discrepancy in treatment response of primary tumor and metastatic lateral pelvic and inguinal LNs suggested the treatment insufficiency of pCRT. It indicated the rationale for performing lymphadenectomy for both lateral pelvic and inguinal LNs metastasis.
Previous studies have demonstrated a survival benefit for rectal cancer surgery with isolated ILNM from rectal cancer [6, 15]. Aiming at decreasing postoperative morbidities of ILND, VEIL has been proposed as an alternative to traditional open ILND in urological and gynecological surgeries. Only one study has reported the utility of VEIL in the management of rectal cancer with ILNM [17]. Herein, the patient experienced an uneventful postoperative course without any complication, such as skin flap necrosis, wound dehiscence, femoral vessel and femoral nerve injury, deep vein thrombosis, and lymphocele [9]. Previous studies have demonstrated similar oncologic outcomes between VEIL and traditional open ILND in genitourinary cancer surgeries. In contrast, the oncological effect of VEIL in rectal cancer with ILNM remains unclear.
Recurrence of pelvic lateral LNs may be related to omission of LNs metastases during LPLND. NIR/ICG fluorescence imaging has been used as a tool to guide LPLND in rectal cancer surgery, which may assist in identifying metastatic LNs and potentially improve the quality of lymphadenectomy [12, 13]. Herein, NIR/ICG fluorescence imaging was used to guide LPLND and utilized to assist lymphadenectomy during VEIL. Our findings demonstrated that NIR/ICG fluorescence imaging-guided laparoscopic LPLND and VEIL was safe and feasible. Besides, ICG has the potential to guided VEIL in rectal cancer.
In conclusion, this study for the first time demonstrated the safety and feasibility of NIR/ICG fluorescence imaging-guided laparoscopic LPLND and VEIL in the management of low rectal cancer with lateral pelvic and inguinal LNs metastasis. NIR/ICG fluorescence imaging-guided laparoscopic LPLND and VEIL is a technically promising technique. Nevertheless, further prospective studies with longer follow-up are warranted to clarify the oncological efficacy of NIR/ICG fluorescence imaging-guided laparoscopic LPLND and VEIL in the treatment of rectal cancer.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to protecting individual patient privacy but are available from the corresponding author on reasonable request.
Abbreviations
- ILNM:
-
Inguinal lymph node metastasis
- ILND:
-
Inguinal lymph node dissection
- VEIL:
-
Video endoscopic inguinal lymphadenectomy
- NIR:
-
Near-infrared fluorescence
- ICG:
-
Indocyanine green
- LPLND:
-
Lateral pelvic lymph node dissection
- LNs:
-
Lymph nodes
- CEA:
-
Carcinoembryonic antigen
- CA 19-9:
-
Carbohydrate antigen 19-9
- PET/CT:
-
Positron emission tomography/computed tomography
- SUV:
-
Standardized uptake value
- pCRT:
-
Preoperative chemoradiotherapy
- FOLFOX:
-
Oxaliplatin, fluoropyrimidine, and leucovorin
- TME:
-
Total mesorectal excision
References
Graham RA, Hohn DC. Management of inguinal lymph node metastases from adenocarcinoma of the rectum. Dis Colon Rectum. 1990;33(3):212–6.
Tocchi A, Lepre L, Costa G, Liotta G, Mazzoni G, Agostini N, Miccini M. Rectal cancer and inguinal metastases: prognostic role and therapeutic indications. Dis Colon Rectum. 1999;42(11):1464–6.
Hamano T, Homma Y, Otsuki Y, Shimizu S, Kobayashi H, Kobayashi Y. Inguinal lymph node metastases are recognized with high frequency in rectal adenocarcinoma invading the dentate line. The histological features at the invasive front may predict inguinal lymph node metastasis. Colorectal Dis. 2010;12(10 Online):e200–5.
Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25(6):1454–5.
Shiratori H, Nozawa H, Kawai K, Hata K, Tanaka T, Kaneko M, Emoto S, Sonoda H, Ishihara S. Risk factors and therapeutic significance of inguinal lymph node metastasis in advanced lower rectal cancer. Int J Colorectal Dis. 2020;35(4):655–64.
Adachi T, Hinoi T, Egi H, Ohdan H. Surgical treatment for isolated inguinal lymph node metastasis in lower rectal adenocarcinoma patients improves outcome. Int J Colorectal Dis. 2013;28(12):1675–80.
Tanabe T, Shida D, Komukai S, Nakamura Y, Tsukamoto S, Kanemitsu Y. Long-term outcomes after surgical dissection of inguinal lymph node metastasis from rectal or anal canal adenocarcinoma. BMC Cancer. 2019;19(1):733.
Sharma P, Zargar H, Spiess PE. Surgical advances in inguinal lymph node dissection: optimizing treatment outcomes. Urol Clin N Am. 2016;43(4):457–68.
Nabavizadeh R, Petrinec B, Nabavizadeh B, Singh A, Rawal S, Master V. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol Oncol. 2020. https://doi.org/10.1016/j.urolonc.2020.07.026.
Chand M, Keller DS, Joshi HM, Devoto L, Rodriguez-Justo M, Cohen R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech Coloproctol. 2018;22(4):271–7.
Currie AC, Brigic A, Thomas-Gibson S, Suzuki N, Moorghen M, Jenkins JT, Faiz OD, Kennedy RH. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol. 2017;43(11):2044–51.
Zhou SC, Tian YT, Wang XW, Zhao CD, Ma S, Jiang J, Li EN, Zhou HT, Liu Q, Liang JW, et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. 2019;25(31):4502–11.
Kawada K, Yoshitomi M, Inamoto S, Sakai Y. Indocyanine green fluorescence-guided laparoscopic lateral lymph node dissection for rectal cancer. Dis Colon Rectum. 2019;62(11):1401.
Ueta K, Matsuda T, Yamashita K, Hasegawa H, Mukohyama J, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, et al. Treatment strategy for rectal cancer patients with inguinal lymph node metastasis. Anticancer Res. 2019;39(10):5767–72.
Bardia A, Greeno E, Miller R, Alberts S, Dozois E, Haddock M, Limburg P. Is a solitary inguinal lymph node metastasis from adenocarcinoma of the rectum really a metastasis? Colorectal Dis. 2010;12(4):312–5.
Wo JY, Anker CJ, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT, Dorth J, Garcia-Aguilar J, Goff D, Jacqmin D, et al. Radiation therapy for rectal cancer: executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2021;11(1):13–25.
Zuhdy M, Elbalka SS, Hamdy O, Raafat S, Saleh GA, Abdelazez MA, Roshdy S. A totally laparoendoscopic approach for low rectal cancer with inguinal nodal metastasis. J Laparoendosc Adv Surg Tech A. 2019;29(1):60–4.
Acknowledgements
The authors thank all the staff in the Department of Colorectal Surgery of Fujian Medical University Union Hospital (FMUUH, Fuzhou, China). The authors thank Prof. Zhao Wenxin for establishing a working space during the lymphadenectomy of left ILNM in the Department of Thyroid and Vascular Surgery of Fujian Medical University Union Hospital (FMUUH, Fuzhou, China).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YS and PC designed the study. YS, YL, WJ, and ZL collected the data. Material preparation and the data analysis were performed by YS, YL, WJ, and ZL. The first draft of the manuscript was written by YS, YL, and ZL. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been reviewed by Fujian Medical University Union Hospital (FMUUH, Fuzhou, China). All procedures performed in studies involving human participants were conducted according to the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent for publication of the participant images and clinical details were obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Video 1. V1 LPLND 1.0. Video file of NIR/ICG fluorescence imaging guided laparoscopic LPLND.
Additional file 2: Video 2. V2 VEIL 0.9. Video file of NIR/ICG fluorescence imaging guided laparoscopic VEIL.
Additional file 3: Fig. S1.
Original MRI images of Fig. 1 that contains sequences.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, Y., Lin, Y., Liu, Z. et al. Combined laparoscopic lymphoadenectomy of lateral pelvic and inguinal nodal metastases using indocyanine green fluorescence imaging guidance in low rectal cancer after preoperative chemoradiotherapy: a case report. BMC Gastroenterol 22, 123 (2022). https://doi.org/10.1186/s12876-022-02193-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-022-02193-1