Abstract
Background
Lipid metabolism disorders play a critical role in the progression of non-alcoholic fatty liver disease (NAFLD). However, the number of studies on the relationships among blood lipid-related indexes and NAFLD is limited, and few studies have emphasized the comparison of blood lipid-related indexes in the same population to identify the optimal index for NAFLD screening. This study aimed to investigate the relationships among several blood lipid-related indexes and NAFLD, and to find the index with the best screening value for NAFLD.
Methods
Based on a general health examination at community health service agencies in the Pearl River Delta region of China in 2015, 3239 women were recruited in this cross-sectional study. The relationships among blood lipid-related indexes and NAFLD were assessed separately by constructing multivariate logistic regression models. Receiver operating characteristic analysis was used to evaluate and compare the screening abilities of the indexes for NAFLD. All data analyses were conducted in SPSS and MedCalc software.
Results
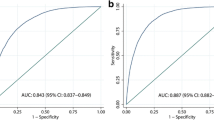
Whether in the crude model or each model adjusted for possible confounding factors, the risk of NAFLD significantly rose with increasing cardiometabolic index (CMI), triglyceride glucose index (TyG), triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) ratio (TG/HDL-C), total cholesterol (TC) to HDL-C ratio (TC/HDL-C) and low-density lipoprotein (LDL-C) to HDL-C ratio (LDL-C/HDL-C). Moreover, the area under the curve (AUC) of CMI was 0.744, which was better than that of TyG (0.725), TG/HDL-C (0.715), TC/HDL-C (0.650), and LDL-C/HDL-C (0.644) (P < 0.001). In addition, the optimal cut-off points were 0.62 for CMI, 8.55 for TyG, 1.15 for TG/HDL-C, 4.17 for TC/HDL-C, and 2.22 for LDL-C/HDL-C.
Conclusions
CMI is easy to obtain, is a recommended index in the screening of NAFLD in women and may be useful for detecting populations that are at high risk of NAFLD.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is considered to be the most common liver disease and is the main cause of chronic liver disease, affecting approximately one-quarter of the general population worldwide [1, 2]. There are regional variations in its prevalence. For example, the prevalence of NAFLD is 27.37% in Asia, 24.13% in North America, the highest prevalence is 31.79% in the Middle East and the lowest prevalence is 13.48% in Africa [3]. Estimates suggest that the prevalence of NAFLD continues to be on the rise globally [4, 5]. The spectrum of NAFLD includes simple steatosis, non-alcoholic steatohepatitis (NASH), hepatic fibrosis, and cirrhosis. Among them, NASH has the potential to progress to cirrhosis and hepatocellular carcinoma (HCC), leading to liver transplantation or death among some patients [6]. In addition to the serious consequences that NAFLD may progress, NAFLD can increase the clinical and economic burden on patients worldwide. It has been reported that NAFLD-related HCC is the most rapidly growing cause of liver transplantation in the USA, and in 2014, the total estimated national hospitalization costs to patients with NAFLD reached $19.9 billion [7, 8]. Additionally, the estimated total charges associated with NASH in 2018 were between £5.6 and £10.5 billion in the UK [9]. In China, NAFLD has become an important public health problem with the rapid increase in the number of patients with NAFLD [4]. Moreover, patients with NAFLD have a high prevalence of various comorbidities (e.g., metabolic syndrome [MS], cardiovascular disease and chronic kidney disease, et al.), which lead to increasing hospitalization charges and decreasing quality of life [5, 10,11,12]. Thus, more attention should be given to NAFLD based on the above characteristics.
The gold standard for the diagnosis of NAFLD is liver biopsy, but it is obviously an expensive and invasive procedure with risks of mortality, bleeding, infection and pain [13]. These issues limit the use of liver biopsy in the screening for NAFLD in the population. As an alternative tool of liver biopsy, liver ultrasonography is widely used for the diagnosis of NAFLD in clinical practice [14, 15]. However, when ultrasound imaging cannot be obtained in some large-scale screening programmes, the use of biomarker panels based on blood samples to identify NAFLD is a good method [16, 17]. Therefore, it is necessary to explore simpler and more effective monitoring indicators as the supplementary tools for ultrasonography, for the risk assessment and early screening of NAFLD.
The inner mechanisms underlying NAFLD are not fully understood [18]. However, animal model tests have shown that dyslipidemia can lead to the occurrence and development of hepatic steatosis [19, 20]. Moreover, alterations in plasma lipoproteins are very common in patients with NAFLD and are closely related to the main complication of NAFLD, cardiovascular disease [21, 22]. Paying attention to the relationship between the lipid profile and NAFLD plays an important role in the overall management of patients with NAFLD. Furthermore, lipid levels in blood samples are easy to measure during routine physical examinations. Thus, the lipid profile may be potential indicators of identifying NAFLD. In recent years, studies have found that several indexes related to blood lipids, such as the triglyceride glucose index (TyG) [23, 24], triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) ratio (TG/HDL-C) [25, 26], total cholesterol (TC) to HDL-C ratio (TC/HDL-C) [27] and low-density lipoprotein cholesterol (LDL-C) to HDL-C ratio (LDL-C/HDL-C) [28], have significant predictive value for NAFLD, and these combined indexes of blood lipids are better than an isolated lipoprotein in predicting the risk of NAFLD. Additionally, as a new predictor of obesity and blood lipids, the cardiometabolic index (CMI) has been shown to be associated with diabetes, cardiovascular disease and MS [29,30,31,32]. Both CMI and NAFLD have relationships with these diseases, but there is limited available evidence suggesting an association of CMI with NAFLD. To our knowledge, only one Chinese study has reported that CMI has good predictive value for NAFLD in patients with type 2 diabetes [33].
Here, we performed a cross-sectional study to explore the relationships among the above blood lipid-related indexes (especially CMI) and NAFLD, and to find the index with the best screening value for NAFLD in women based on health examination data of residents in southern China. Studies have typically used sex as an adjustment variable [27, 34, 35], but we were told that the prevalence of obesity among women (5.9%) was higher than that among men (4.8%) in China [36] and that the prevalence of MS among women (36.8%; 35.6%) was higher than that among men (31.0%; 30.3%) [37, 38]. It is known that the incidence and development of NAFLD are closely related to obesity, and NAFLD is considered to be the hepatic manifestation of MS [39, 40]. Thus, based on the contributions of women to the prevalence of obesity and MS, our intent was to focus on women with NAFLD. Our study can provide a simple and reliable index for the early identification of women with NAFLD.
Methods
Participants
In total, 3239 subjects who underwent a general health examination at community health service centers in the Pearl River Delta region of China in 2015 were recruited in this cross-sectional study. The health examination included self-reported medical history, physical examination, instrument-based examination (abdominal ultrasonography, electrocardiogram and chest X-ray) and laboratory examination. Participants eligible for this study were women who were diagnosed with fatty liver by liver ultrasonography. Participants with a history of excessive alcohol consumption (alcohol intake ≥ 20 g/day) or other possible causes of hepatic steatosis (viral or autoimmune) were excluded. In addition, subjects without complete health examination data in Table 1 were also excluded. To protect the confidentiality of personal information of participants, we hid their names and ID card numbers. To protect and promote the health of research subjects, all procedures were performed in accordance with the Declaration of Helsinki, and ethics approval was obtained from the ethics committee of Guangdong Sociological Society. Moreover, informed consent was obtained from all participants.
Data collection, measurements and quality control
Demographic data (sex, age) and information on health-related behaviours (smoking, drinking), history of medical conditions diagnosed by doctors (e.g., hypertension, diabetes, hepatitis), and family history were obtained from self-reported questionnaires completed under the guidance of trained investigators.
The physical examination included measurements of weight, height, waist circumference (WC) and blood pressure. Weight and height were measured by weight and height measuring instruments when participants were dressed in light clothes without shoes. WC was measured at the midpoint between the lowest rib and the iliac crest in the standing position with an inelastic soft ruler as the participant remained relaxed. The subject assumed the sitting position, and the right arm was uncovered or placed in light clothes on the table so that the elbow and heart were at the same level. Then, the examiner used an electronic sphygmomanometer produced by Omron company to measure systolic blood pressure (SBP) and diastolic blood pressure (DBP).
After an overnight fast, venous blood samples were collected by trained medical staff and analyzed with a blood analyzer (Mindray BC-2900, Shenzhen, China) and an automatic biochemical analyzer (Mindray BS-420, Shenzhen, China). The laboratory parameters included in this study were red blood cell (RBC), white blood cell (WBC), hemoglobin (Hb), platelet (PLT), fasting plasma glucose (FPG), glycated hemoglobin (HbAlc), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (GGT), total bilirubin (TBIL), serum creatinine (Scr), blood urea nitrogen (BUN), uric acid (UA), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C).
A reasonable number of medical examiners was arranged, and they were trained in and carried out the standard operating procedure. In addition, there were full-time staff to supervise and verify the authenticity of the data.
Diagnosis of NAFLD
All participants in this study received abdominal liver ultrasonography (Mindray DC-6, Shenzhen, China) performed by professional ultrasound physicians according to the characteristics of the liver anterior echo enhancement (“bright liver”), far-field echo attenuation and unclear intrahepatic duct structure. Participants who consumed more than 20 g/day of alcohol or were diagnosed with viral or autoimmune hepatitis were excluded [41]. The ultrasonic diagnosis criteria were in accordance with guidelines for the diagnosis and treatment of NAFLD as follows [42]: (1) liver anterior echo enhancement (stronger than that in the kidney and spleen) and far-field echo attenuation; (2) unclear intrahepatic duct structure; (3) mild to moderate hepatomegaly with blunted borders; (4) color Doppler flow imaging showing reduced intrahepatic blood flow signal but normal distribution of blood flow; and (5) unclear or incomplete echo of the capsule of the right lobe of the liver and diaphragm. The diagnostic criteria of mild fatty liver are item 1 and any one of items 2–4; the diagnostic criteria of moderate fatty liver are item 1 and any two items of items 2–4; The diagnostic criteria of severe fatty liver are items 1 and 5 and any two of items 2–4.
Derived variables
Body mass index (BMI) was calculated with the following formula: BMI = weight (kg)/height (m)2.
The waist-to-height ratio (WHtR) was calculated with the following formula: WHtR = WC (cm)/height (cm).
CMI was calculated with the following formula [29]: CMI = TG/HDL-C × WHtR.
TyG was calculated with the following formula [23]: TyG = ln (TG [mg/dL] × FPG [mg/dL]/2).
Statistical analysis
Data analyses were conducted in SPSS version 23.0 (SPSS Inc, Chicago, IL) and MedCalc version 19.4.1 (MedCalc Software, Ostend, Belgium) software. Continuous variables are presented as the mean ± standard deviation (SD) for normal distributions or the median (interquartile range) for non-normal distributions. Categorical variables are expressed as frequencies (percentages). For between-group comparisons, Student’s t-test (normal distribution) or the Mann–Whitney U test (non-normal distribution) was used for continuous data, and the chi-square test was used for categorical data. The relationships among blood lipid-related indexes and the risk of NAFLD were assessed respectively by constructing multivariate logistic regression models with indexes as categorical variables (divided into four groups according to quartiles) and continuous variables (standardized using z-score normalization). The following four models were created: the crude model, with no adjustments; model I, with adjustments for age and medical history (hypertension, diabetes); model II, with adjustments for model I plus anthropometric indexes (SBP, DBP, and BMI); and model III, with adjustments for model II plus laboratory parameters (RBC, WBC, Hb, PLT, FPG, HbA1c, ALT, AST, ALP, GGT, TBIL, Scr, BUN and UA). The odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. The area under the curve (AUC) of the receiver operating characteristic (ROC) analysis was used to evaluate the abilities of blood lipid-related indexes to screen for NAFLD (ideal screening tools should have AUC > 0.7), and the highest Youden’s index (sensitivity + specificity − 1) was used to determine the optimal cut-off point of the index to screen for NAFLD. MedCalc software was used to compare different ROC curves. The statistical significance level was set at α = 0.05 (two-tailed).
Results
Characteristics of participants
The age, self-reported medical history, physical examination and laboratory examination data of participants are summarized in Table 1. A total of 3239 women were included, with ages ranging from 19 to 93 years (57.65 ± 12.53 years), and the prevalence of NAFLD was 22.0% in our study. Compared with the non-NAFLD group, the women with NAFLD were older and had higher SBP, DBP, BMI, RBC, WBC, Hb, PLT, FPG, HbAlc, ALT, AST, ALP, GGT, Scr, BUN, UA, TC, TG, LDL-C, CMI, TyG, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C values (P < 0.05). However, the levels of TBIL and HDL-C were higher in the control group (P < 0.05). The prevalence of NAFLD was higher in the population with diabetes (P < 0.05), but no significant difference was observed in the hypertensive population.
Association between blood lipid-related indexes and NAFLD
Table 2 and Fig. 1 present the relationships between NAFLD and blood lipid-related indexes when they were analyzed as categorical variables and continuous variables, respectively. As shown in Table 2, whether in the crude model or any of the models adjusted for possible confounding factors (model I, II or III), the risk of NAFLD significantly rose with increasing CMI quartiles (P < 0.001). Additionally, with increasing TyG, TG/HDL-C, TC/HDL-C or LDL-C/HDL-C quartiles, the corresponding OR value also increased gradually. The relationships between the risk of NAFLD and blood lipid-related indexes were also verified in the above model analyses when these indexes were analyzed as continuous variables. Based on z-score standardization, for each 1-unit increase in SD for CMI, TyG, TG/HDL-C, TC/HDL-C or LDL-C/HDL-C, the risk of NAFLD increased by 31.0% (OR: 1.310, 95% CI 1.198–1.433), 71.6% (OR: 1.716, 95% CI 1.520–1.936), 27.7% (OR: 1.277, 95% CI 1.175–1.389), 26.7% (OR: 1.267, 95% CI 1.133–1.417), and 23.7% (OR: 1.237, 95% CI 1.110–1.378), respectively, in model III (all P < 0.001). The results indicated that CMI, TyG, TG/HDL-C, TC/HDL-C and LDL-C/HDL-C were related to NAFLD.
The relationships between blood lipid-related indexes and the risk of NAFLD. These indexes were regarded as continuous variables in the multivariate logistic regression models in this figure. Because the indexes were converted to z scores in the multivariate logistic regression model, OR increase with each 1-unit increase in SD for every index. Crude model: unadjusted; Model I: adjusted for age, hypertension, diabetes; Model II: adjusted for Model I plus SBP, DBP, and BMI; Model III: adjusted for model II plus RBC, WBC, Hb, PLT, FPG, HbA1c, ALT, AST, ALP, GGT, TBIL, Scr, BUN and UA
The ROC analysis of blood lipid-related indexes in the screening of NAFLD
The ROC curves of blood lipid-related indexes were analyzed and compared to explore the screening value and optimal cut-off points for identifying NAFLD and to determine the most notable screening index. ROC curves are presented in Fig. 2. As shown in Table 3, the AUCs of CMI, TyG, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C were 0.744 (95% CI 0.724–0.763), 0.725 (95% CI 0.705–0.746), 0.715 (95% CI 0.695–0.735), 0.650 (95% CI 0.627–0.672), and 0.644 (95% CI 0.622–0.666) respectively, while the optimal cut-off points were 0.62 (sensitivity = 0.74, specificity = 0.65), 8.55 (sensitivity = 0.75, specificity = 0.58), 1.15 (sensitivity = 0.70, specificity = 0.64), 4.17 (sensitivity = 0.57, specificity = 0.66), and 2.22 (sensitivity = 0.64, specificity = 0.60) respectively for women. The above results suggested that these indexes may be effective in the early screening of NAFLD risk in women, and it is worth noting that the ability of CMI to detect NAFLD was significantly better than that of the other indexes mentioned above (P < 0.001) (Table 4).
Discussion
In recent years, an increasing number of researchers have realized that developing effective, simple and inexpensive tools for identifying NAFLD is a considerably interesting subject [43, 44], but few studies have compared these tools in the same population to identify the best tool for screening of NAFLD. In the current study, several indexes related to blood lipids were analyzed and compared in the screening of NAFLD. We found that CMI, TyG, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C were risk factors for NAFLD in models with or without adjustments. Additionally, ROC curve analysis indicated that CMI, TyG, and TG/HDL-C could be used to screen for NAFLD effectively (AUC > 0.7), but each of the other two indexes had a poor ability to detect NAFLD (0.5 < AUC < 0.7). Compared with TyG, TG/HDL-C, TC/HDL-C, or LDL-C/HDL-C, CMI was the better index for NAFLD screening in women because it had the larger AUC (P < 0.001), which was similar to the results of logistic regression analysis. In the logistic regression models we constructed, CMI had the highest OR values both before and after adjusting for confounding factors when CMI was used as a categorical variable. However, TyG had the highest OR values in the four models when indexes were used as continuous variables.
NAFLD is associated with lipid metabolism disorders [45]. Blood lipid levels can reflect systemic lipid metabolism. Thus, blood lipid-related indexes can be used to screen for NAFLD. The simple and effective lipid profile can be used as "early warning indicators" for the early screening of NAFLD, and people with scores higher than the optimal cut-off point of the indicators (suspected hepatic steatosis disease) can be urged to have a further check-up as soon as possible.
CMI was measured as the product of TG/HDL-C and WHtR. TG/HDL-C is a useful alternative indicator of insulin resistance (IR), and IR plays an important role in the progression of NAFLD [46,47,48,49]. IR can promote hepatic lipid deposition by increasing lipolysis of adipocytes and free fatty acids [50]. Regarding TG/HDL-C, our study indicated that it has screening value for NAFLD, and several studies have confirmed the relationship between TG/HDL-C and NAFLD. In a cross-sectional study of 18,061 healthy individuals, researchers have shown that TG/HDL-C may be used as a surrogate for NAFLD [26]. A cohort study of Japanese population revealed that TG/HDL-C could predict the incidence of fatty liver [25]. In addition, studies have shown that obesity, especially abdominal obesity, is associated with NAFLD [39, 51]. WHtR, as an indicator of abdominal obesity, can detect the risk of NAFLD [52, 53]. In previous studies, CMI was associated with a variety of metabolic diseases including obesity and type 2 diabetes, but few studies evaluated the relationship between CMI and the risk of NAFLD [29, 54]. Our study filled this knowledge gap and provided a basis for further research. Our results suggested that the AUC of CMI was 0.744 and that the optimal cut-off point was 0.62. In a study in China [33], the AUC of CMI for screening NAFLD was 0.698, and the optimal cut-off point was 0.694 among women. The small differences may be because the populations were different. They explored the association between CMI and NAFLD in a population of patients with type 2 diabetes, while the subjects in our study were women who underwent health examinations. CMI was better than TG/HDL-C in our study, and another study found that CMI was better than WHtR [33]. CMI takes into account the index of blood lipids and obesity; thus, CMI is reliable and recommended by us as a screening index of NAFLD. It may be beneficial to reduce the CMI of women with NAFLD.
Currently, among many available predictive models for detecting NAFLD, Fatty Liver Index (FLI) is a common and non-invasive score with good accuracy to detect fatty liver, which has been confirmed in many studies [55,56,57]. The use of FLI can help medical service providers screen NAFLD. However, the calculation method of FLI [55] is more complex than that of CMI (the calculation of FLI requires the use of scientific calculators or computers), and it is not easy for the public to calculate FLI. Particularly, in China’s rural areas with severe health workforce challenges and limited use of appropriate care [58] (more than one third of the Chinese population lived in rural areas as of 2020 [59]), the simplicity of surrogate markers are very important to guide the population to early detection of NAFLD by themselves. Thus, based on these, CMI may be easier to apply than FLI.
In addition to CMI and TG/HDL-C, we found that TyG can also be used as a useful screening index of NAFLD. Similar to our study, a retrospective cohort study involving 46,693 elderly individuals in China reported that a higher TyG index was related to a greater risk of NAFLD [23]. Moreover, the screening power of TC/HDL-C and LDL-C/HDL-C for NAFLD was similar; although they all had certain abilities, they were not good indexes in screening for NAFLD. The AUC of TC/HDL-C was 0.650 in women, which was in accordance with previous studies of the association between TC/HDL-C and NAFLD. Research by Wu KT [60] and his colleague showed that adults with high TC/HDL-C had a higher risk for NAFLD, and Ren et al. [27] found that the AUC of TC/HDL-C was 0.645 for predicting NAFLD in a Jinchang cohort study. However, there were no data on the ability of TC/HDL-C to detect NAFLD in women in Ren’s study. The AUC of LDL-C/HDL-C was 0.644 in our study. In a nonobese population based on a 5-year longitudinal cohort study, Zou et al. [28] found that the predictive value of LDL-C/HDL-C for the risk of new-onset NAFLD was 0.671, which was slightly higher than our AUC, possibly due to different study designs and populations. In short, the discrimination abilities of TC/HDL-C and LDL-C/HDL-C were lower than those of CMI, TyG and TG/HDL-C. This finding may be because the abnormal accumulation of TG in the liver was most closely associated with the risk of NAFLD compared with other blood lipid indexes (TC, HDL-C and LDL-C) [61], and TG was a component of CMI, TyG and TG/HDL-C, but was not included in TC/HDL-C or LDL-C/HDL-C.
The strengths of this study are as follows. First, health examinations were carried out by professional medical staff, which helped to reduce error in the examination data. Second, we used rigorous statistical methods, including the construction of different models, to analyze the relationships between blood lipid-related indexes and NAFLD. However, there are several limitations that should be noted. First, this study was a cross-sectional study, and a prospective cohort study is warranted to examine the cause-effect relationship between blood lipid-related indexes and NAFLD. Second, subjects in the present study were from communities in southern China; thus, further in-depth studies should be performed in different countries and regions before applying our findings to people with NAFLD in other countries and regions. Third, we used abdominal liver ultrasonography rather than liver biopsy to diagnose NAFLD. Finally, besides lipid profile, more potentially significant indexes should be explored in future studies to identify the presence of NAFLD, and we also hope that more potential confounding factors (e.g., physical activity and diet) can be adjusted in future longitudinal follow-up data to further verify the relationship between CMI and NAFLD.
Conclusions
In conclusion, the abilities of CMI, TyG, TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C for detecting NAFLD are different. In our study, CMI was easy to obtain and is a recommended index in the screening of NAFLD in women. Moreover, the optimal cut-off point of CMI was 0.62 in women. Our findings may provide help towards identifying which populations are at high risk of NAFLD, to help medical workers and individuals prevent NAFLD and intervene in its progression as early as possible.
Availability of data and materials
The dataset used during the current study is available from the corresponding author on reasonable request.
Abbreviations
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- HCC:
-
Hepatocellular carcinoma
- TyG:
-
Triglyceride glucose
- TG:
-
Triglycerides
- HDL-C:
-
High-density lipoprotein cholesterol
- TG/HDL-C:
-
Triglycerides to high-density lipoprotein cholesterol ratio
- TC:
-
Total cholesterol
- TC/HDL-C:
-
Total cholesterol to high-density lipoprotein cholesterol ratio
- LDL-C:
-
Low density lipoprotein cholesterol
- LDL-C/HDL-C:
-
Low density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio
- CMI:
-
Cardiometabolic index
- MS:
-
Metabolic syndrome
- WC:
-
Waist circumference
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- RBC:
-
Red blood cell
- WBC:
-
White blood cell
- Hb:
-
Hemoglobin
- PLT:
-
Platelet
- FPG:
-
Fasting plasma glucose
- HbAlc:
-
Glycated hemoglobin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- GGT:
-
γ-Glutamyl transpeptidase
- TBIL:
-
Total bilirubin
- Scr:
-
Serum creatinine
- BUN:
-
Blood urea nitrogen
- UA:
-
Uric acid
- BMI:
-
Body mass index
- WHtR:
-
Waist-to-height ratio
- SD:
-
Standard deviation
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- IR:
-
Insulin resistance
- FLI:
-
Fatty liver index
References
Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–90. https://doi.org/10.1038/nrgastro.2013.171.
Honda Y, Yoneda M, Imajo K, Nakajima A. Elastography techniques for the assessment of liver fibrosis in non-alcoholic fatty liver disease. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21114039.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. https://doi.org/10.1016/j.jhep.2018.05.036.
Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global Perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–82. https://doi.org/10.1002/hep.30251.
Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10(11):656–65. https://doi.org/10.1038/nrgastro.2013.183.
Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019. https://doi.org/10.1016/j.cgh.2018.05.057.
Hirode G, Vittinghoff E, Wong RJ. Increasing clinical and economic burden of nonalcoholic fatty liver disease among hospitalized adults in the United States. J Clin Gastroenterol. 2019;53(10):765–71. https://doi.org/10.1097/MCG.0000000000001229.
Morgan A, Hartmanis S, Tsochatzis E, Newsome PN, Ryder SD, Elliott R, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis (NASH) in the United Kingdom (UK) in 2018. Eur J Health Econ. 2021;22(4):505–18. https://doi.org/10.1007/s10198-020-01256-y.
Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169(9):ITC65–80. https://doi.org/10.7326/AITC201811060.
Yan J, Hou C, Liang Y. The prevalence and risk factors of young male schizophrenics with non-alcoholic fatty liver disease. Neuropsychiatr Dis Treat. 2017;13:1493–8. https://doi.org/10.2147/NDT.S137183.
Heda R, Yazawa M, Shi M, Bhaskaran M, Aloor FZ, Thuluvath PJ, et al. Non-alcoholic fatty liver and chronic kidney disease: retrospect, introspect, and prospect. World J Gastroenterol. 2021;27(17):1864–82. https://doi.org/10.3748/wjg.v27.i17.1864.
Zhou J-H, Cai J-J, She Z-G, Li H-L. Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol. 2019;25(11):1307–26. https://doi.org/10.3748/wjg.v25.i11.1307.
Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32(8):1242–52. https://doi.org/10.1111/j.1478-3231.2012.02804.x.
Siegelman ES, Rosen MA. Imaging of hepatic steatosis. Semin Liver Dis. 2001;21(1):71–80.
Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–72. https://doi.org/10.1053/j.gastro.2009.06.005.
EASL-ALEH Clinical Practice Guidelines. Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. https://doi.org/10.1016/j.jhep.2015.04.006.
Tarantino G, Citro V, Capone D. Nonalcoholic fatty liver disease: a challenge from mechanisms to therapy. J Clin Med. 2019. https://doi.org/10.3390/jcm9010015.
Svop Jensen V, Fledelius C, Max Wulff E, Lykkesfeldt J, Hvid H. Temporal development of dyslipidemia and nonalcoholic fatty liver disease (NAFLD) in syrian hamsters fed a high-fat, high-fructose. High-Cholesterol Diet Nutr. 2021. https://doi.org/10.3390/nu13020604.
Tveden-Nyborg P, Birck MM, Ipsen DH, Thiessen T, Feldmann LdB, Lindblad MM, et al. Diet-induced dyslipidemia leads to nonalcoholic fatty liver disease and oxidative stress in guinea pigs. Transl Res. 2016;168:146–60. https://doi.org/10.1016/j.trsl.2015.10.001.
Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65(8):1109–23. https://doi.org/10.1016/j.metabol.2016.05.003.
Amor AJ, Perea V. Dyslipidemia in nonalcoholic fatty liver disease. Curr Opin Endocrinol Diabetes Obes. 2019;26(2):103–8. https://doi.org/10.1097/MED.0000000000000464.
Huanan C, Sangsang L, Amoah AN, Yacong B, Xuejiao C, Zhan S, et al. Relationship between triglyceride glucose index and the incidence of non-alcoholic fatty liver disease in the elderly: a retrospective cohort study in China. BMJ Open. 2020;10(11):e039804. https://doi.org/10.1136/bmjopen-2020-039804.
Lee SB, Kim MK, Kang S, Park K, Kim JH, Baik SJ, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul). 2019;34(2):179–86. https://doi.org/10.3803/EnM.2019.34.2.179.
Fukuda Y, Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, Ohbora A, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 2016;36(5):713–20. https://doi.org/10.1111/liv.12977.
Fan N, Peng L, Xia Z, Zhang L, Song Z, Wang Y, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. 2019;18(1):39. https://doi.org/10.1186/s12944-019-0986-7.
Ren XY, Shi D, Ding J, Cheng ZY, Li HY, Li JS, et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 2019;18(1):47. https://doi.org/10.1186/s12944-019-0984-9.
Zou Y, Zhong L, Hu C, Zhong M, Peng N, Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. 2021;20(1):28. https://doi.org/10.1186/s12944-021-01457-1.
Wakabayashi I, Daimon T. The, “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. 2015;438:274–8. https://doi.org/10.1016/j.cca.2014.08.042.
Dursun M, Besiroglu H, Otunctemur A, Ozbek E. Association between cardiometabolic index and erectile dysfunction: a new index for predicting cardiovascular disease. Kaohsiung J Med Sci. 2016;32(12):620–3. https://doi.org/10.1016/j.kjms.2016.10.003.
Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–62.
Liu X, Wu Q, Yan G, Duan J, Chen Z, Yang P, et al. Cardiometabolic index: a new tool for screening the metabolically obese normal weight phenotype. J Endocrinol Investig. 2021;44(6):1253–61. https://doi.org/10.1007/s40618-020-01417-z.
Li Y. Relationship between cardiometabolic index and risk of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Jingkou: Jiangsu University; 2020. ((Chinese)).
Hsu C-L, Wu F-Z, Lin K-H, Chen Y-H, Wu P-C, Chen Y-H, et al. Role of fatty liver index and metabolic factors in the prediction of nonalcoholic fatty liver disease in a lean population receiving health checkup. Clin Transl Gastroenterol. 2019;10(5):1–8. https://doi.org/10.14309/ctg.0000000000000042.
Wei F, Li J, Chen C, Zhang K, Cao L, Wang X, et al. Higher serum uric acid level predicts non-alcoholic fatty liver disease: a 4-year prospective cohort study. Front Endocrinol (Lausanne). 2020;11:179. https://doi.org/10.3389/fendo.2020.00179.
Zhang L, Wang Z, Wang X, Chen Z, Shao L, Tian Y, et al. Prevalence of overweight and obesity in China: results from a cross-sectional study of 441 thousand adults, 2012–2015. Obes Res Clin Pract. 2020;14(2):119–26. https://doi.org/10.1016/j.orcp.2020.02.005.
Lu J, Wang L, Li M, Xu Y, Jiang Y, Wang W, et al. Metabolic syndrome among adults in China: The 2010 China noncommunicable disease surveillance. J Clin Endocrinol Metab. 2017;102(2):507–15. https://doi.org/10.1210/jc.2016-2477.
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4. https://doi.org/10.1001/jama.2015.4260.
Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. https://doi.org/10.1016/j.metabol.2018.11.014.
Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–53. https://doi.org/10.1016/j.bpg.2014.07.008.
Fan JG, Wei L, Zhuang H. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20(4):163–73. https://doi.org/10.1111/1751-2980.12685.
Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–12. https://doi.org/10.1111/j.1751-2980.2008.00331.x.
Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–22. https://doi.org/10.1111/apt.12963.
Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441–7. https://doi.org/10.1136/gut.2007.146019.
Narayanan S, Surette FA, Hahn YS. The immune landscape in nonalcoholic steatohepatitis. Immune Netw. 2016;16(3):147–58. https://doi.org/10.4110/in.2016.16.3.147.
Young KA, Maturu A, Lorenzo C, Langefeld CD, Wagenknecht LE, Chen Y-DI, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, β-cell function, and diabetes in Hispanics and African Americans. J Diabetes Complicat. 2019;33(2):118–22. https://doi.org/10.1016/j.jdiacomp.2018.10.018.
Iwani NAKZ, Jalaludin MY, Zin RMWM, Fuziah MZ, Hong JYH, Abqariyah Y, et al. Triglyceride to HDL-C ratio is associated with insulin resistance in overweight and obese children. Sci Rep. 2017;7:40055. https://doi.org/10.1038/srep40055.
Zhou M, Zhu L, Cui X, Feng L, Zhao X, He S, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 2016;15:104. https://doi.org/10.1186/s12944-016-0270-z.
Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–5.
Fujii H, Kawada N, Japan Study Group of Nafld J-N. the role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21113863.
Yoo HJ, Park MS, Lee CH, Yang SJ, Kim TN, Lim KI, et al. Cutoff points of abdominal obesity indices in screening for non-alcoholic fatty liver disease in Asians. Liver Int. 2010;30(8):1189–96. https://doi.org/10.1111/j.1478-3231.2010.02300.x.
Mansour-Ghanaei R, Mansour-Ghanaei F, Naghipour M, Joukar F, Atrkar-Roushan Z, Tabatabaii M, et al. The role of anthropometric indices in the prediction of non-alcoholic fatty liver disease in the PERSIAN Guilan Cohort study (PGCS). J Med Life. 2018;11(3):194–202. https://doi.org/10.25122/jml-2018-0031.
Sheng G, Xie Q, Wang R, Hu C, Zhong M, Zou Y. Waist-to-height ratio and non-alcoholic fatty liver disease in adults. BMC Gastroenterol. 2021;21(1):239. https://doi.org/10.1186/s12876-021-01824-3.
Shi W-R, Wang H-Y, Chen S, Guo X-F, Li Z, Sun Y-X. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: a community-based study. Lipids Health Dis. 2018;17(1):236. https://doi.org/10.1186/s12944-018-0886-2.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Sviklāne L, Olmane E, Dzērve Z, Kupčs K, Pīrāgs V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33(1):270–6. https://doi.org/10.1111/jgh.13814.
Motamed N, Faraji AH, Khonsari MR, Maadi M, Tameshkel FS, Keyvani H, et al. Fatty liver index (FLI) and prediction of new cases of non-alcoholic fatty liver disease: a population-based study of northern Iran. Clin Nutr. 2020;39(2):468–74. https://doi.org/10.1016/j.clnu.2019.02.024.
Yip W, Fu H, Chen AT, Zhai T, Jian W, Xu R, et al. 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet. 2019;394(10204):1192–204. https://doi.org/10.1016/S0140-6736(19)32136-1.
National Bureau of Statistics of China. China statistical yearbook 2021 (Internet). Beijing: China Statistics Press; 2021.
Wu K-T, Kuo P-L, Su S-B, Chen Y-Y, Yeh M-L, Huang C-I, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol. 2016. https://doi.org/10.1016/j.jacl.2015.12.026.
Cohen DE, Fisher EA. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis. 2013;33(4):380–8. https://doi.org/10.1055/s-0033-1358519.
Acknowledgements
We would like to express our gratitude to the medical staff who offered their assistance in the data collection.
Funding
None.
Author information
Authors and Affiliations
Contributions
WJR and WPX contributed to the study concept and design. WJR and SZZ were major contributors in conducting the data analyses and drafted the manuscript. FYJ contributed to statistical analyses and the preparation of tables and figures in this study. XRH, LJM and WPX participated in revising the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures in our study were performed in accordance with the Declaration of Helsinki. Ethics approval was obtained from the ethics committee of Guangdong Sociological Society. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Su, Z., Feng, Y. et al. Comparison of several blood lipid-related indexes in the screening of non-alcoholic fatty liver disease in women: a cross-sectional study in the Pearl River Delta region of southern China. BMC Gastroenterol 21, 482 (2021). https://doi.org/10.1186/s12876-021-02072-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-02072-1