Abstract
Background
Aorto-esophageal fistula (AEF) caused by foreign bodies ingestion is a rare but devastating disorder. Thoracic endovascular aortic repair (TEVAR) has become a widely accepted intervention for treating aorto-esophageal fistulas. As for post-TEVAR esophageal defect, secondary esophagectomy has been the recommended choice for most of the AEFs, but there is no general consensus with regard to the need of secondary surgeries for patients in the absence of clear signs of reinfection or bleeding. We herein presented a case of an AEF caused by fishbone ingestion, after successful TEVAR, the esophageal lesion was closed endoscopically.
Case presentation
A 38-year-old male presented with esophageal fistula for 4 months. He was diagnosed with AEF because of Chiari's triad after fishbone ingestion 4 months ago. Emergency thoracic aortic stent implantation was done, and given broad spectrum antibiotics and blood transfusion. His symptoms were improved, and discharged with an esophageal fistula left to heal itself. Nevertheless, after 4 months, re-examination of esophago-gastro-duodenoscopy revealed that the diameter of the fistula was changed from 3 to 6 mm. He was then admitted to our hospital for esophageal fistula repair. Laboratory examinations and chest computed tomography showed no signs of active infection, and endoscopic closure of the fistula was achieved with 4 clips. After that, he was discharged and gradually returned to normal diet.
Conclusion
For AEFs in the absence of active infection with repaired aorta but persistent esophageal fistula, endoscopic closure by endoclips might be an effective treatment choice.
Similar content being viewed by others
Background
Aorto-esophageal fistula (AEF) caused by foreign bodies (FB) ingestion is a rare but devastating disorder. On account of initial exsanguination and secondary mediastinal infection and subsequent sepsis, the mortality rate of aorto-esophageal fistula formed by FB ingestion is higher than 90% [1, 2]. The current first-line treatment strategy is to control fatal bleeding by TEVAR in the urgent phase, followed by radically debridement of the contaminated area as well as the aortic/esophageal reconstruction in the semi-urgent phase [3, 4]. However, here is no general consensus with regard to the need for secondary surgeries following the successful use of TEVAR in the absence of clear signs of reinfection or bleeding [5]. We herein presented our successful experience in managing an AEF patient, by describing a combined endovascular and endoscopic approach.
Case presentation
Chief complaints
A 38-year-old male who underwent TEVAR for AEF was admitted to our hospital with esophageal fistula lasting from 4 months.
History of present illness
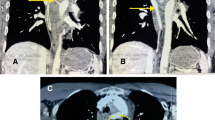
The patient while eating was stuck by a fishbone, and he consciously swallowed it by drinking water 4 months ago. On day 6, he developed chest pain, on day 8 melena, on day 9 fever, and then he was admitted to hospital. Esophagogastroduodenoscopy (EGD) showed an esophageal fistula of 30 cm from the incisors, about 3 mm in diameter with influx of bloody fluid, and accompanied by a hematoma and hyperemic mucosa (Fig. 1a). Chest CT revealed air bubbles in the mediastinum, suggesting esophageal perforation (Fig. 2a). Neither EGD nor CT found any signs of fish bones. Broad spectrum antibiotics and nasojejunal feeding was given. The patient after that developed sudden massive hematemesis and melena in 7 days. Enhanced CT showed formation of mediastinal abscess, pseudoaneurysm and contrast material extravasation from the aorta to esophagus (Fig. 2b–d), confirming the diagnosis of AEF. A thoracic endovascular stent graft (Zenith TX2 TAA ref ZTEG-2PT-32-160; Cook, Denmark) in 32-mm proximal and 28-mm distal diameter and 160-mm length was then implanted. His symptoms were improved, and discharged with a nasojejunal tube, and the esophageal fistula was assumed to heal on its own. About 4 months later, EGD again displayed the esophageal fistula of about 0.6 cm in diameter (Fig. 1b). He was then admitted to our hospital for esophageal repair. In the past 4 months, he ate through nasojejunal tube and lost 6 kg weight, and had no fever, hematemesis or melena.
Endoscopy view: a an esophageal fistula of 30 cm from the incisors, about 3 mm in diameter with influx of bloody fluids, and accompanied with a hematoma and hyperemic mucosa; b after 4 months, the esophageal fistula was about 6 mm in diameter; c endoclips were used to close the fistula; d the fistula was closed at 1-month follow-up; e endoscopy revealed completely healed fistula and fungal esophagitis at 3-months follow-up; f at 1-year follow-up, the fistula was healed with no signs of infection
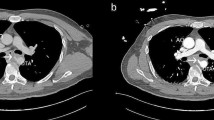
Computed tomography: a air bubbles in the mediastinum (white arrow); b mediastinal abscess in the mediastinum (white arrow); c coronal CT angiography showed contrast leakage from aorta toward esophagus (white arrow); d sagittal CT angiography demonstrated a pseudoaneurysm with a primary entry tear at the thoracic aorta (white arrow); e except for the radiopaque of aortic stent and nasojejunal tube, no signs of infection were found; f sagittal CT angiography did not find any abnormality, except for the radiopaque of aortic stent
History of past illness
The patient had no previous medical history and no specific family history.
Physical examination
At admission, the patient’s temperature was 36.5 °C, heart rate was 96 bpm, respiratory rate was 20 breaths per minute, blood pressure was 125/80 mmHg, height was 178 cm, and weight was 57.5 kg. No other special findings were reported.
Laboratory examinations
Routine blood tests, including white blood cell count of 7.56 × 109/L, mainly neutrophils 68.7%, hemoglobin concentration 134 g/L were normal. The blood biochemistries were also normal. The procalcitonin concentration of 0.13 ng/mL was shown to be slightly increased, and fecal occult blood was weakly positive.
Imaging examinations
Except for the radiopaque of well-positioned aortic stent and nasojejunal tube, there were no signs of infection on chest plain CT (Fig. 2e).
Final diagnosis
Aorto-esophageal fistula (after thoracic aortic stent implantation).
Treatment
The patient had no signs of active infection and the aortic stent functioning was well, but the fistula was growing. Hence, 4 endoclips were used to close the fistula (Fig. 1c).
Outcome and follow-up
No postoperative complications occurred. He was discharged from the hospital the next day. At 1-month follow-up, EGD revealed the closure of the fistula (Fig. 1d). At 3-months follow-up, EGD revealed completely healed fistula and suspicious fungal esophagitis (Fig. 1e). Brush biopsy did not find any fungus, and so no treatment was given. The nasojejunal tube was removed and the patient was returned to normal diet. At 12 months, the patient had fever, and blood culture detected streptococcus anginosus. Endoscopic brush biopsy found yeast-like fungus, and routine urine test found yeast cells and routine stool test found fungus. Chest CT and angiography revealed no evidence of mediastinitis, aortic stent-graft infection, or new episodes of gastrointestinal bleeding (Fig. 2f). After combined treatment of aztreonam, vancomycin and voriconazole, the patient showed improvement and was discharged from the hospital. Re-examination by EGD revealed no signs of infection (Fig. 1f). In the last year, the patient gained 7 kg weight. The timeline of this case was shown in Fig. 3.
Discussion and conclusions
In association with life-threatening hemorrhage and infection, AEF is a rare but devastating disorder. Most of the patients present with Chiari's triad, which includes midthoracic pain or dysphagia, a sentinel episode of hematemesis, followed by fatal exsanguination. With the aim to save life, the therapeutic principles of AEF included controlling fatal bleeding, restoring the integrity of aorta and esophagus, treating and preventing infection. In recent years, TEVAR has become a widely accepted intervention for AEFs owing to its definite operative effect in bleeding control and minimal injury in restoring the aortic continuity. However, the use of TEVAR alone for AEF left esophageal perforation untreated, and therefore the patients are at risk of AEF recurrence and/or stent-graft contamination, which is associated with high mid-term mortality [5]. Therefore, TEVAR should be applied as a first life-saving choice for AEF patients, but subsequent treatment is indispensable [3, 4].
In special patients, the esophageal defects were healed by themselves. Tao et al. have reported that a 5 mm esophageal fistula caused by fishbone ingestion is healed on its own after endovascular repair, but the whole process took 1 year and 3 months, another case reported by Shen JY et al. with two esophageal fistulas of 0.2 cm and 0.3 cm in diameter took 6 months to heal by itself [6, 7]. In most of the cases, considering the high risk of infection, most of the authors are in favor of secondary esophagectomy followed by esophageal reconstruction [1, 3, 8]. Due to non-negligible injuries, expenses, and complications associated with open surgery, the pros and cons of esophagectomy should be carefully weighed, especially in patients with the repaired aorta and without any infection or bleeding.
With the development of endoscopy, endoscopic therapy has taken part in the management of AEFs. Luca Mezzetto et al. have reported a case of an AEF who was successfully treated by endoscopic clipping of the esophageal defect before endovascular repair [9]. Esophageal stents have been used for managing AEFs, but the experiences with its use in AEFs are limited as well. A 2017 study summarized the previous application of an esophageal stent in AEF, with 10 cases involved [10]. Compared to esophagectomy, esophageal stent therapy had a more unfavorable prognosis, and so not recommended as a routine for patients with AEF [3]. Meanwhile, esophageal stent placement carries the risks of severe adverse events, including stent migration that requires repositioning or replacement [4]. More importantly, the main purpose of esophageal stent placement in AEF patients involves the prevention of aortic graft contamination as a bridging therapy in the urgent phase [10,11,12]. In our case, his infection and bleeding were controlled, and his main demand was to return to a normal oral diet. At the same time, as we know, endoclip is an effective choice to close the chronic esophageal perforation and can be used even for persistent fistulas [13]. Therefore, rather than esophageal stent, the endoclips therapy was chosen, as it is a one-shot operation, more convenient, targeted, and cost-efficient.
With no evidence of mediastinitis and aortic stent-graft infection, we inferred that the patient’s fungal esophagitis, and subsequent streptococcal septicemia and systemic fungal infection might be associated with the previous use of broad-spectrum antibiotics, decreased immunity, and long-term nasojejunal feeding. The patient improved after adequate antibiotic treatment. But he was followed up closely. As recommended by the recent reviews, TEVAR should not be considered as a definitive surgery, and similarly, the secondary surgery of graft replacement and esophagectomy should be planned for complete cure, regardless of the level of surgical invasion [3, 4]. Once the symptoms of infection occur in any patient with a history of AEF, especially those without secondary surgery, they should be highly suspected for the possibility of mediastinitis or stent-graft infection and evaluated for secondary surgeries in the clinical setting. Also, lifelong imaging surveillance is mandatory [7].
The combined strategy of endovascular and endoscopic treatment worked well in our case. Certainly, it can only be considered for selected patients. Also, it should be validated with more persuasive studies like case–control studies, whereas it is difficult to achieve by the nature of the rarity of AEF.
TEVAR has gained recognition in treating AEF due to its critical technique to control exsanguination and restore aorta integrity. As for the management of esophageal lesions after TEVAR, endoscopic therapy might be a minimally invasive alternative to surgery in selected patients with small defects and without active infection. Certainly, careful and comprehensive evaluation before the operation and strict intensive follow-up are required.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AEF:
-
Aorto-esophageal fistula
- FB:
-
Foreign bodies
- TEVAR:
-
Thoracic endovascular aortic repair
- AEFs:
-
Aorto-esophageal fistulas
- EGD:
-
Esophago-gastro-duodenoscopy
- CT:
-
Computed tomography
References
Zhang X, Liu J, Li J, Hu J, Yu F, Li S, Yang X. Diagnosis and treatment of 32 cases with aortoesophageal fistula due to esophageal foreign body. Laryngoscope. 2011;121(2):267–72.
Kelly SL, Peters P, Ogg MJ, Li A, Smithers BM. Successful management of an aortoesophageal fistula caused by a fish bone–case report and review of literature. J Cardiothorac Surg. 2009;4:21.
Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. 2019 (555).
Uno K, Koike T, Takahashi S, Komazawa D, Shimosegawa T. Management of aorto-esophageal fistula secondary after thoracic endovascular aortic repair: a review of literature. Clin J Gastroenterol. 2017;10:393–402.
Akashi H, Kawamoto S, Saiki Y, Sakamoto T, Sawa Y, Tsukube T, Kubota S, Matsui Y, Karube N, Imoto K, et al. Therapeutic strategy for treating aortoesophageal fistulas. Gen Thorac Cardiovasc Surg. 2014;62(10):573–80.
Tao K, Cheng H, Hu Z, Kong M. An aorto-oesophageal fistula treated with endovascular aortic repair: the fate of untreated oesophageal lesion on endoscopic follow-up. Interact Cardiovasc Thorac Surg. 2017;25(6):990–2.
Shen JY, Zhang HW, Fan KJ, Liao H, Zhang EY, Hu J. Aortoesophageal fistula and arch pseudoaneurysm after removing of a swallowed chicken bone: a case report of one-stage hybrid treatment. BMC Surg. 2018;18(1):3.
Kotelis D, Gombert A, Jacobs MJ. Treatment of post-thoracic endovascular aortic repair aorto-esophageal fistula-only radical surgery can be effective: techniques and sequence of treatment. J Thorac Dis. 2018;10(6):3869–73.
Mezzetto L, Treppiedi E, Scorsone L, Giacopuzzi S, Perandini S, Macri M, Veraldi GF. Thoracic aortic pseudoaneurysm after esophageal perforation and mediastinitis caused by accidental ingestion of a mutton bone: a case report on staged endoscopic and endovascular treatments. Ann Vasc Surg. 2016;30(307):e315–e309.
Rodrigues-Pinto E, Pereira P, Vilas-Boas F, Peixoto A, Silva M, Macedo G. Esophageal stents in aortoesophageal fistulas-anecdotal experiences or new armamentarium? Am J Gastroenterol. 2017;112(8):1343–5.
Zuber-Jerger I, Hempel U, Rockmann F, Klebl F. Temporary stent placement in 2 cases of aortoesophageal fistula. Gastrointest Endosc. 2008;68(3):599–602.
Chen X, Li J, Chen J, Zhou Y, Zhang Y, Ding H, Huang S, Zhang Z. A combined minimally invasive approach for the treatment of aortoesophageal fistula caused by the ingestion of a chicken bone: case report and literature review. Clinics (Sao Paulo, Brazil). 2012;67(2):195–7.
Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66(3):605–11.
Acknowledgements
Not applicable.
Funding
This study was funded by 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (20HXFH016).
Author information
Authors and Affiliations
Contributions
ZYY, HB found this patient and conceived the study; HB performed the treatment; ZYY and LS drafted the article; YXL and HB provided critical revision to the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written consent was obtained from the patient for the patient's personal or clinical details along with any identifying images to be published in this study.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, YY., Li, S., Yuan, XL. et al. Aorto-esophageal fistula caused by fishbone ingestion: a case report on staged endovascular and endoscopic treatment. BMC Gastroenterol 21, 46 (2021). https://doi.org/10.1186/s12876-021-01624-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-021-01624-9