Abstract
Background
Gastric epithelial hyper-proliferation was reported in patients with Helicobacter pylori (H. pylori)–infected gastric mucosa with intestinal metaplasia (IM) changes. In patients with gastric ulcer (GU) and IM, the GU may have a different healing rate in comparison to patients without IM. This study aimed to compare the difference in GU healing between H. pylori–infected patients with IM and those without IM.
Methods
We retrospectively analyzed patients at the Keelung Chung Gung Memorial Hospital during the period from March 2005 to January 2011. The inclusion criteria were: 1) endoscopic findings of GU and biopsy histological examination plus rapid urease test indicating H. pylori infection; 2) gastric IM adjacent to a GU but with no atrophic gastritis changes; 3) patients receiving H. pylori eradication triple therapy and 8 weeks of maintenance therapy with a proton pump inhibitor; and 4) patients receiving follow-up endoscopy within the 3rd and the 4th months after treatment.
Results
In total, 327 patients with GU and H. pylori infection (136 with IM and 191 without IM) were included. Patients with IM had a higher GU healing rate than those without IM (91.9% vs. 84.3%, P = 0.040). Multivariate logistical regression analysis revealed that failure of H. pylori eradication (Odds = 4.013, 95% CI: 1.840–8.951, P < 0.001) and gastric IM (Odds = 0.369, 95% CI: 0.168–0.812, P = 0.013) were the predictors of non-healing GU following treatment.
Conclusions
Patient with gastric IM change may have a higher GU healing rate than those without gastric IM. However, successful H. pylori eradication is a more important factor for GU healing than gastric IM.
Similar content being viewed by others
Background
Intestinal metaplasia (IM) is a common finding in patients with Helicobacter pylori (H. pylori) infection. The prevalence of IM in patients with H. pylori infection is 30–40% in patients approximately 50 years old [1, 2]. Some studies revealed that the Wingless-Int (Wnt)/β catenin pathway plays an important role in the progression of H. pylori-related IM [3–6]. The Wnt signal transduction pathway is also important in intestinal epithelial homeostasis, wound repair and epithelial proliferation [7]. Gastric epithelial hyperproliferation has been observed in patients with gastritis and IM caused by H. pylori infection [8–11]. Epithelial cell proliferation is one of the mechanisms governing the repair of a gastric ulcer (GU) [12–14]. Patients with a condition of IM near the GU might have a different outcome of GU healing due to gastric mucosal hyperproliferation. To the best of our knowledge, no study has analyzed whether IM influences GU healing or H. pylori eradication. This study aimed to compare the difference in GU healing between H. pylori–infected patients with and those without IM adjacent to the GU.
Methods
We retrospectively analyzed the clinical presentations, endoscopic findings and pathologic records of all patients treated for peptic ulcers at Keelung Chang Gung Memorial Hospital from March 2005 to January 2011. The inclusion criteria in the study were: patients with esophagogastroduodenoscopy (EGD) evidence of active GU in the gastric antrum or body; patients with histological findings by GU biopsy and rapid urease tests indicating H. pylori infection with or without IM change; patients who received standard triple therapy (including proton pump inhibitor (PPI), lansoprazole 30 mg or esomerpazole 40 mg, 1 g amoxicillin, and 500 mg clarithromycin twice daily for 7 days) and 8 weeks of maintenance PPI therapy; and patients receiving follow-up EGD and undergoing a rapid urease test and a histological study within the 3rd and the 4th month following treatment. The exclusion criteria were patients receiving PPI or antibiotics two weeks before any of the follow-up EGD studies, and patients taking non-steroid anti-inflammatory drugs (NSAIDs) or aspirin during the healing phase.
If a patient had several EGD studies, only the findings of the 1st and 2nd (follow-up) EGD studies were included in the analysis. The exclusion criteria included patients with underlying malignancy, gastric malignancy revealed by GU biopsy or dysplasia change detected via GU biopsy. In some patients, long-term H. pylori infection will induce a progressive gastric atrophy including loss of acid-producing parietal cells. Gastric atrophy leads to lowered gastric acid output which might influence GU healing [15]. Moreover, this study aimed to elucidate the influence of IM adjacent to GU on GU healing and the data of intra-gastric pH could not be available in this retrospective study. Patients with gastric mucosal atrophy according to the results of GU biopsy were also excluded to avoid low gastric acid interfering with GU healing in this study.
Endoscopic study
Patients who experienced epigastric pain, dyspepsia or acid reflux symptoms received EGD. Wide base ulcer was defined as GU base more than 1.5 cm in size. During the EGD study, GU biopsies (4 specimens from each GU margin mucosa, another specimen from the gastric antrum and one from the incisura angularis of corpus) were obtained except in patients with active ulcer bleeding or NSAID-related shallow ulcers. The rapid urease test (RUT) was administered to confirm the presence of H. pylori infection. Patients with positive results from both the histological examination and RUT test were included. In patients who had completed standard triple therapy for H. pylori eradication and maintenance PPI therapy, EGD was performed between the 3rd and the 4th month after treatment to evaluate the status of gastric ulcer healing and H. pylori eradication success. Therefore, biopsies were repeated for histological analysis and RUT, likewise to the initial EGD. Three stages of GU were defined by endoscopy, based on the cycle of ulcer formation and resolution. Gastric healed ulcer in this study was defined as the regeneration of epithelium that completely covered the floor of the ulcer (scarring status), replacing the white coating ulcer base. Patients with partially healing GU (not scarring status) or active GU detected in the following EGD were recognized as persistent GU in this study.
Histology and immunohistochemical (IHC) stain for H. pylori detection
All patients received GU biopsy for histology (hematoxylin and eosin) and IHC staining (polyclone, Zytomed Systems GmbH, Berlin, Germany) to evaluate H. pylori infection status. Histological sections of all biopsies were routinely examined to determine H. pylori infection, IM, atrophic gastritis and malignancy. Atrophy of the gastric mucosa was defined as loss of glandular tissue and mucosal thinning changes. IM was detected on the basis of the morphological features in the stomach observed by performing H & E and Alcian blue staining [16–18]. This study applied the most widely used classification, in which there are two types of IM:
-
1)
Complete type IM: presence of small intestinal-type mucosa with goblet cells, a brush border and eosinophilic enterocytes.
-
2)
Incomplete type IM: presence of colonic epithelium with multiple, irregular mucin droplets of variable size in the cytoplasm and absence of a brush border.
IM was scored according to the visual analog scale of the updated Sydney classification [16]. The results of the histological analyses were reviewed by a single experienced pathologist (Dr. Chang LC).
Rapid urease test (RUT)
RUT (Pronto dry test; Medical Instruments Corporation, Switzerland) was performed. The sensitivity and specificity of RUT for detecting H. pylori infection were 99 and 96%, respectively [19].
Antigen Ki-67 stain for epithelial cell proliferation
For patients with adequate residual biopsy specimens from GU margin after staining with an H. pylori antibody, an additional Ki-67 IHC stain was applied to assess cell proliferation. Antigen Ki-67 IHC staining was performed using DAKO autostain agent (Cytomation, Carpinteria, CA). REAL EnVision Detection System, Peroxidase/diaminobenzidine (DAB) (K5007, DAKO) was used to visualize the staining.
Digital data analysis
Digital data analysis was performed with computer software to prevent manual or inter-observer bias for the Ki-67 index score counting. The digital data analysis was processed with ImageJ (1.45i) [20]. The color deconvolution plug-in was used to separate the stains into two 8-bit component images: the DAB image and the hematoxylin image. The DAB image was used for density measurement. We calculated the percentage of positively stained nuclei (labeling index) by using a color deconvolution algorithm to separate the staining components and an adaptive threshold for nuclear area segmentation (Fig. 1, right side) [21]. Five pictures from the targeted gastric epithelium adjacent to GU were applied for digital data analysis. The result was recorded as a mean Ki-67 labeling index (%).
Left side: Photomicrographs of gastric intestinal metaplasia. Gastric mucosa on the left show intestinal metaplasia with histological features similar to the small bowel epithelium. Gastric mucosa lined by foveolar cells at the right upper portion. (Hematoxylin and eosin stain; 100×). Right side: ImmunoRatio was applied to calculate the percentage of positively stained nuclear area (labeling index, DAB: diaminobenzidine area) among the background hematoxylin stain area (Ki-67 index: DAB/nuclear area = 26.6% in this slide)
Statistical methods
To the best of our knowledge, there are no previous reports on peptic ulcer-healing rate or H. pylori eradication rate following PPI administration in patients with GU and IM. Because GU patients with H. pylori infection and IM exhibit gastric epithelial hyperproliferation, the null hypothesis was that patients with IM had a higher rate of GU scarring (prediction: 95%) than patients without IM (80% in our previous study) following standard triple eradication and PPI therapy [18]. To obtain a power of 0.8 at the significant level of 0.05, the minimal required number of patients was 70 for each group. Hence, it was reasonable to enroll at least 140 patients in this study.
Continuous data were expressed as the mean ± standard deviation (SD). Continuous data were evaluated using the paired-t test if the sample size was more than 30 in each group and the Mann–Whitney test was applied if the sample size was less than 30. The chi-square test was used for nominal data. Categorical data were analyzed with the chi-square test or Fisher’s exact test, where appropriate. All statistical tests were 2-tailed. A P-value of <0.05 was considered statistically significant. The correlation coefficients such as Pearson’s correlation coefficient, Point bi-serial correlation coefficient and Spearman’s co-efficiency rho were appropriately chosen based on the data types including numerical, nominal or ordinal data. A multivariate logistic regression analysis was applied for the predictors’ evaluation for non-healing GU and persistent H. pylori infection after treatment.
Statistical analyses were performed using the Statistical Package for the Social Science software version 16.0 for Windows (SPSS, Chicago, IL, USA).
This research was approved by the Institutional Review Board of the Chang-Gung Memorial Hospital (IRB No: 99-2661B). Written informed consent was obtained from all enrolled subjects in this study.
Results
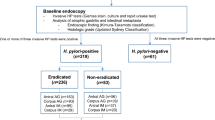
We initially included 3412 cases of GU, which were diagnosed by EGD and pathologic studies. In total, 136 cases of GU with IM (IM group), and 191 cases of GU without IM (no IM group) were enrolled in the final analysis (Fig. 2). Most patients with gastric IM were of the incomplete type (122/136, 89.7%). Because patients with atrophic mucosa in histological findings were excluded in this study, the majority of patients had minimal foci of IM and were scored as 0 (absent) or 1 (mild) (107/136, 78.7%) by using the Sydney system visual analog scale. Only 29 patients with incomplete IM were scored as 2 (moderate). No patient was scored as 3 (marked).
Demographic and other characteristics of the patients are listed in Table 1. No significant inter-group differences were observed in the distribution of gender, symptoms, personal history, co-morbidities, GU size and location. Patients in the IM group were older than those in the no IM group (mean age, 63.5 ± 12.3 years vs. 59.4 ± 13.4 years, P = 0.004). Patients with IM had higher GU-healing rate than those without IM (92.1% vs. 84.3%, P = 0.036). However, there were no significant differences in the H. pylori eradication rates between the two groups [81.6% (IM group) vs. 85.3% (no IM group), P = 0.405]. A subgroup analysis from 274 patients with successful H. pylori eradication was performed and the result revealed no significant difference in GU healing rate between patients with IM (104/111, 93.7%) and those without IM (144/163, 88.3%) (P = 0.125).
The bivariate correlation test revealed that non-healing GU (NHGU) was positively correlated with age and persistent H. pylori infection (failure of H. pylori eradication), but negatively correlated with IM (Table 2). Because patients with IM were older than those without IM in this study, multivariate logistical regression analysis was performed to determine the predictor of GU healing by adjusting the factor of age (Table 3). As positive predictors for NHGU, the analysis revealed age [odds ratio (OR) =1.035, 95% confidence interval (CI) = 1.007–1.064; P = 0.015] and persistent H. pylori infection [OR = 3.924, 95% CI = 1.857–8.294, P < 0.001]. A negative predictor was IM [OR = 0.366, 95% CI = 0.170–0.792, P = 0.011].
For failure of H. pylori eradication, only the factor of NHGU was a predictor. IM was not a predictor and was not correlated with failure of H. pylori eradication (Table 4).
Because this was a retrospective analysis, only adequate GU biopsy specimens from patients before and after H. pylori eradication therapy were used for the IHC stain study. A total of 64 GU biopsy specimens (30 from patients with non-IM, 34 patients with IM) underwent Ki-67 staining (Table 5). There was no significant difference in Ki-67 index between patients with IM and those without IM. The mean Ki-67 index change before or after treatment was only marginally different between patients with IM and those without IM (0.5 ± 1.7 vs. 0.6 ± 1.9, P = 0.06).
Discussion
IM is a common pathologic finding in patients with endoscopy-proven GU [22–24]. Previous studies focused on the precancerous condition of IM; however, few studies have investigated the influence of IM on gastric ulcer healing and H. pylori eradication. Factors such as age, smoking and H. pylori eradication were reported as important factors for GU healing [25–27]. In our study, although patients with gastric mucosal IM had a higher GU healing rate than those without IM, the GU healing rates were not significantly different when the focus was on the patients with successful H. pylori eradication. Multivariate logistic regression analyses revealed that failure of H. pylori eradication was a positive predictor (OR = 4.013) and IM was a negative predictor (OR = 0.369) for non-healing GU. Hence, successful H. pylori eradication is a more important predictor than IM for GU healing in this study.
The mechanisms associated with GU healing and repair include epithelial cell proliferation, growth control, epidermal growth factors, angiogenesis and inhibition of acid secretion [12, 13, 28, 29]. Although our original hypothesis was that cell hyperproliferation in IM might influence GU healing, we found that there was no significant difference in cell proliferation Ki-67 stain index between the IM group and the no IM group. Antigen Ki-67 is a nuclear protein that is associated with cellular proliferation and ribosomal RNA transcription. The Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2 and mitosis), but is absent from resting cells (G0). In previous studies, patients with H. pylori infection and chronic gastritis exhibited significantly increased rates of epithelial cell proliferation. Cell proliferation decreases after successful H. pylori eradication [30, 31]. Although the mean Ki-67 index was decreased (decreased cell proliferation) following H. pylori eradication in our study, there was no significant difference in mean Ki-67 index between patients with IM and those without IM. The condition of gastric mucosal cell proliferation might be due to the status of H. pylori infection and chronic gastritis, but not the status of IM [30, 31].
There were two limitations in the present study. First, this study only included patients with GU and IM adjacent to GU, but patients with atrophic gastritis were excluded. It is common to detect atrophic gastritis and IM coexistence in patients with an H. pylori infection [16–18]. Although the intra-gastric juice pH value and serum pepsinogen value are important for the evaluation of atrophic gastritis and GU healing, these data were not available in this retrospective study. It would be difficult to elucidate whether atrophic gastritis or IM has an influence on GU healing when a patient has both pathologic findings of atrophic gastritis and IM in the stomach. Hence, we excluded the patients with atrophic gastritis detected via histological examination in this study. Second, most of the IM types in this study were mild according to the Sydney system. We could not compare differences in GU healing between patients with mild forms of IM and those with marked forms of IM.
Conclusions
Patients with gastric IM may have a higher GU healing rate than those without gastric IM. Age, successful H. pylori eradication and gastric IM were the predictors for GU healing. However, successful H. pylori eradication was a more important factor for GU healing than gastric IM.
Abbreviations
- H. pylori :
-
Helicobacter pylori
- GU:
-
Gastric ulcer
- IM:
-
Intestinal metaplasia
- EGD:
-
Esophagogastroduodenoscopy
- PPI:
-
Proton pump inhibitor
- RUT:
-
Rapid urease test
- DAB:
-
Diaminobenzidine
- IHC:
-
Immunohistochemical
- SD:
-
Standard deviation
References
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–94.
Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294–9.
Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–51.
Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, et al. Helicobacter pylori CagA interacts with Ecadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–26.
Clevers H. Wnt/β-catenis signaling in development and disease. Cell. 2006;127:469–80.
Hung KH, Wu JJ, Yang HB, Su LJ, Sheu BS. Host Wnt/β-catenin pathway triggered by Helicobacter pylori correlates with regression of gastric intestinal metaplasia after H. pylori eradication. J Med Microbiol. 2009;58:567–76.
Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, et al. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–68.
Bechi P, Balzi M, Becciolini A, Maugeri A, Raggi CC, Amorosi A, et al. Helicobacter pylori and cell proliferation of the gastric mucosa: possible implications for gastric carcinogenesis. Am J Gastroenterol. 1996;91:271–6.
Ierardi E, Francavilla R, Panella C. Effect of Helicobacter pylori eradication on intestinal metaplasia and gastric epithelium proliferation. Ital J Gastroenterol Hepatol. 1997;29:470–5.
Peek Jr RM, Moss SF, Tham KT, Pérez-Pérez GI, Wang S, Miller GG, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–8.
Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16–26.
Levi S, Goodlad RA, Lee CY, Walport MJ, Wright NA, Hodgson HJ. Inhibitory effect of non-steroidal anti-inflammatory drugs on mucosal cell proliferation associated with gastric ulcer healing. Lancet. 1990;336:840–3.
Tibble J, Sigthorsson G, Caldwell C, Palmer RH, Bjarnason I. Effects of NSAIDs on cryoprobe-injeced gastric ulcer healing in rats. Aliment Pharmacol Ther. 2001;15:2001–8.
Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11:791–6.
Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–33.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–8.
Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, Leandro G, Price AB, Sipponen P, Solcia E, Watanabe H, Genta RM. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249–59.
Chen LW, Chien RN, Fang KM, Yen CL, Chang JJ, Lee TS, et al. A comparative study on Helicobacter pylori infection in peptic ulcer disease patients with or without previous eradication therapy. Hepatogastroenterology. 2007;54:2209–11.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progresterone receptor (PR), and Ki-67. Breast Cancer Res. 2012;12:R56. doi:10.1186/bcr2615.
Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16–20.
Filipe MI, Muñoz N, Matko I, Kato I, Pompe-Kirn V, Jutersek A, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Solvenia. Int J Cancer. 1994;57:24–9.
Correa P, Piazuelo B, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8.
Arkkila PE, Kokkola A, Seppälä K, Sipponen P. Size of the peptic ulcer in Helicobacter pylori-positive patients: association with the clinical and histological characteristics. Scand J Gastroenterol. 2007;42:695–701.
Graham DY, Lew GM, Klein PD, Evans DG, Evans Jr DJ, Saeed ZA. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer: a randomized, controlled study. Ann Intern Med. 1992;116:705–8.
Labenz J, Borsch G. Evidence for the essential role of Helicobacter pylori in gastric ulcer disease. Gut. 1994;35:19–22.
Konturek PC, Konturek SJ, Brzozowski T, Ernst H. Epidermal growth factor and transforming growth factor-α: Role in protection and healing of gastric mucosal lesions. Eur J Gastroenterol Hepatol. 1995;7:933–7.
Kamada T, Kawano S, Sato N, Fukuda M, Fusamoto H, Abe H. Gastric mucosal blood distribution and its changes in the healing process of gastric ulcer. Gastroenterology. 1983;84:1541–6.
Unger Z, Molnar B, Szaleczky E, Torgyekes E, Muller F, Zagoni T, et al. Effect of Helicobacter pylori infection and eradication on gastric epithelial cell proliferation and apoptosis. J Physiol Paris. 2001;95:355–60.
Szaleczky E, Prónai L, Molnár B, Berczi L, Fehér J, Tulassay Z. Increased cell proliferation in chronic Helicobacter pylori positive gastritis and gastric carcinoma--correlation between immuno-histochemistry and Tv image cytometry. Anal Cell Pathol. 2000;20(2–3):131–9.
Acknowledgments
We acknowledge Miss Chia-Wen Hsieh for help in collecting and analyzing data.
Funding
This study was not supported by any grant.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available because they are from hospital-based data and are not approved for public demonstration by our Institutional Review Board, but these data are available from the corresponding author on reasonable request.
Authors’ contributions
CLW, HBJ, CSW analyzed and interpreted the patient data regarding the gastric ulcers, H. pylori infection and pathologic findings. CLC performed the histological examination of GU and HCC performed the immunohistochemical study. CLW and CRN were the major contributors in writing the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This research was approved by the Institutional Review Board of the Chang-Gung Memorial Hospital (IRB No: 99-2661B). Written informed consent was obtained from all enrolled subjects in this study.
Personal interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, LW., Chang, LC., Hua, CC. et al. Analyzing the influence of gastric intestinal metaplasia on gastric ulcer healing in Helicobacter pylori–infected patients without atrophic gastritis. BMC Gastroenterol 17, 1 (2017). https://doi.org/10.1186/s12876-016-0563-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-016-0563-8